Abstract
Anti-CD3 mAbs may prolong β cell function up to 2 years in patients with new onset Type 1 diabetes (T1DM). A randomized open label trial of anti-CD3 mAb, Teplizumab, in T1DM was stopped after 10 subjects because of increased adverse events than in a previous trial related with higher dosing of drug. Teplizumab caused transient reduction in circulating T cells, but the recovered cells were not new thymic emigrants because T cell receptor excision circles were not increased. There was a trend for reduced loss of C-peptide over 2 yrs with drug treatment (p=0.1), and insulin use was lower (p<0.001). In 4 drug treated subjects followed up to 60 months, C-peptide responses were maintained. We conclude that increased doses of Teplizumab are associated with greater adverse events without improved efficacy. The drug may marginate rather than deplete T cells. C-peptide levels may remain detectable up to 5 yrs after treatment.
Keywords: Type 1 diabetes mellitus, immunotherapy, anti-CD3 monoclonal antibody, T lymphocyte
The goal of newer therapies for treatment of Type 1 diabetes (T1DM) is to arrest the autoimmune destruction of β cells for an extended period of time without the need for continuous immune suppression. FcR non-binding anti-CD3 mAbs are thought to attenuate destruction of β cells in T1DM over the first 18 months to 2 years of the disease when they are administered at diagnosis, but the duration of this effect of a treatment dose and the mechanism of action of anti-CD3 mAb are not known [1; 2][3]. In two previously published randomized trials that enrolled approximately 120 subjects, a single course of treatment with two different FcR non-binding anti-CD3 mAbs was shown to improve insulin production for 18 and 24 months after diagnosis[1; 2][3]. In our previous study, glycated hemoglobin levels were also improved, suggesting a benefit of drug treatment in glucose control whereas in the study of Keymeulen et al, clinical management was aimed at maintaining similar glucose control in the control and drug treated subjects to avoid any differential effect of glycemic control on β cell function[3]. Despite these differences, both studies demonstrated significantly reduced insulin usage with drug treatment.
The mechanism(s) of action of FcR non-binding anti-CD3 mAbs are not clear. It is possible that the mAbs deplete effector T cells, similar to the mechanisms attributed to Rituximab (anti-CD20 mAb) or Alemtuzumab (anti-CD52 mAb), whereas preclinical studies and preliminary human data suggest that immune regulation is involved[4][5][6]. Rather than elimination of circulating T cells, some studies have suggested that the modified anti-CD3 mAbs cause margination rather than depletion of T cells, possibly as a result of T cell activation in vivo, but the source of recovering T cells after anti-CD3 mAb has not been addressed[7][8].
We initiated a trial of anti-CD3 mAb, teplizumab (formerly called hOKT3γ1(Ala-Ala)), for treatment of patients with new onset T1DM, and enrolled 10 subjects – 6 were randomized to drug treatment and 4 to the control group. This trial was subsequently closed to enrollment after the initial 6 drug treated subjects experienced a higher rate of adverse events (AEs) compared to our previous study with the drug[1; 2]. The increased rate of AEs was the result of higher dosing than the previous regimen related to a change in manufacturing. Although no further subjects were enrolled in the study after the initial 10, we followed the drug treated subjects for up to 5 years after the single course of mAb treatment. This inadvertent increased dosing allowed us to determine the impact on safety and possible efficacy as compared to our previous experience with the drug.
We found an increased frequency of AEs with higher doses of the anti-CD3 mAb. The drug caused a reduction in the number of circulating lymphocytes but there was a rapid recovery of cells without an increase in the levels of T cell receptor (TCR) excision circles (TRECs) a marker of recent thymic emigrants[9; 10]. A single course of treatment with anti-CD3 mAb attenuated the rate of loss of insulin production for 2 years after onset of T1DM and there is evidence of continued preservation of insulin secretion for up to 5 years after treatment in the drug treated subjects. Further studies with long term follow up of additional subjects will be important to determine the impact of drug treatment on the natural progression of the disease.
Methods
Study design
10 subjects were recruited within 6 weeks of diagnosis of T1DM for a randomized, open labeled phase IIb trial of teplizumab(NDB01, ITN007AI)(Table 1)[11] [2]. The original study design was a 2:1 drug:control randomization. The drug treated subjects were to receive 3 cycles of teplizumab, 6 months apart, in order to determine whether a 2nd and 3rd course of mAb treatment would improve the duration of effects of mAb treatment on C-peptides responses to a mixed meal at two years. The protocol was approved by the institutional review boards at each participating center. All subjects were either anti-GAD65or anti-ICA512 autoantibody positive. The 6 drug-treated patients were hospitalized for 5 to 14 days. The drug was administered intravenously over 30 minutes using the following dosing scheme: day 1: 460 mcg/m2, day 2: 919 mcg/m2, and days 3-12: 1,818 mcg/m2. All drug-treated subjects were observed with frequent vital signs for 6 hours after each infusion. AEs were scored using the National Cancer Institute Common Terminology Criteria for Adverse Events.
Table 1. Demographics of study subjects.
| Original study cohort | Ad hoc analysis | ||
|---|---|---|---|
| Drug treated | Control | Control | |
| N | 6 | 4 | 12 |
| Age Mean/Median | 15.5±1.8/17.5 | 9.0±0.9/9.0* | 14.9±1.73/14 |
| M/F | 4/2 | 3/1 | 8/4 |
| Insulin dose (U/kg) | 0.38±0.12 | 0.28±0.09 | 0.41±0.05 |
| Baseline C-peptide AUC (nmol/L) | 0.877±.182 | 0.406±0.12 | 0.529±0.053 |
| Baseline HgbA1c | 7.65±0.51 | 6.68±0.18* | 7.88±0.26 |
| HgbA1c at 24 mos | 8.42±0.76 | 6.87±0.45 | 8.05±0.48 |
p<0.05 vs drug treated
The frequency and severity of AEs was greater in the subjects receiving anti-CD3 mAb as compared to that in a previous study of the same mAb[2]. These findings prompted a study hold, and studies of the drug and dosing regimen. The enrolled subjects randomized to drug treatment did not receive subsequent courses of anti-CD3 mAb. The characteristics of the antibody preparation from the first two studies were compared in biochemical analyses, and no significant differences were noted (data not shown). From an extensive review, it was determined that the effective dose given to subjects in this study may have been approximately 40% greater than the dose used in our previous study. The initial drug lots used in the prior study were packaged in snap-off glass vials, and the drug was passed through a filter prior to infusion into the subjects to remove potential shards. However, for this study, drug was placed in vials closed with a rubber seal. Following reconstitution, it was not passed through any filter prior to patient infusion. The drug dose actually administered previously was 40% lower based on measurement of protein concentration by A280. The investigators determined that this change in manufacturing was the likely explanation for the differences in anti-CD3 mAb concentration between the two drug preparations.
The enrolled subjects were followed until the predefined endpoint of 24 months: C-peptide responses during a 4 hour mixed meal tolerance test (MMTT), insulin use, and hemoglobin A1c levels were measured every 6 months. All subjects received intensive diabetes management with glycemic targets as defined by the American Diabetes Association[12]. At month 24, all subjects were offered an extended follow up for up to 5 years - 4 of the 6 drug treated subjects agreed to this extended follow up. One of these subjects withdrew from the follow up after 2 visits.
Mixed meal tolerance tests (MMTT)
The MMTTs were performed as described previously [1]. This test was performed at study entry and every 6 months until 24 months and every 9 months between months 24 and 60.
Subset analysis, coating and modulation of CD3, and drug levels
The coating and modulation of CD3 on circulating CD4+ and CD8+ T cells and serum levels of teplizumab were measured in flow cytometric studies as described[13]. The absolute CD4+ and CD8+ cell counts were determined by multiplying the frequency of CD4+ and CD8+ T cells by flow analysis by the absolute lymphocyte count. The results are expressed relative to the baseline value.
TREC analysis
We measured the copy number of TRECs, relative to genomic DNA in CD4+ and CD8+ T cells isolated from frozen peripheral blood mononuclear cells (PBMC). CD4+ and CD8+ T cell subpopulations were magnetically sorted using Miltenyi beads for negative selection (Miltenyi, Auburn, CA). DNA was extracted using Qiagen QiaAmp DNA extraction protocol (Qiagen, Valencia, CA). Real time PCR was used to determine TREC and genomic actin copy number in duplicate standards, control negative and positive DNA samples and test samples, with the modification that BSA 0.04% (molecular biology grade) was included in the PCR reaction [14]. Quantitation of genomic DNA copy number was considered to be twice the nucleated cell number in each sample; results are expressed as TRECs per million cells in each DNA sample analyzed.
Statistical analysis
The data are presented as mean±SEM unless indicated. All statistical analyses were conducted using SAS® (SAS Institute, Version 8.2, Cary, NC). The total C-peptide AUC was calculated using the trapezoidal rule over the 4-hour period. The total AUC and mean AUC per minute were modified by dividing the above AUC results by the total time of 240 minutes * 0.333 to convert from ng/mL to nmol/L. The predefined outcome variable in the original trial design was a comparison of the change in C-peptide responses in the two groups at 24 months. In addition to this primary analysis, a mixed effects model was used to determine the difference in slopes between treatment groups for mean C-Peptide AUC and daily insulin use from baseline up to Month 24. The mixed effects models include the following explanatory variables: treatment, time as continuous variable, time squared (because the trend of the change was not linear) treatment by time interaction, and treatment by time squared interaction. Log(AUC+1) was used as outcome variables for mean C-Peptide AUC while the actual values are used for insulin use and HbA1c. A p-value of less than 0.05 is considered significant.
Because the baseline C-peptide and hemoglobin A1c values and ages of the drug treated participants differed from those of the controls, we also compared the responses in the drug treated group to matched control subjects selected from our previous study by an external statistical group (PPD) [1]. These 12 controls were selected on the basis of age and baseline C-peptide levels that were similar to those of subjects in the drug treated group. Two of the drug-treated participants had ages that could be matched to the control group, but baseline C-peptide responses that could not. Statistical analyses were performed using SAS (Version 8.2 or higher, SAS Institute, Cary, NC). We also compared the effects of treatment on the initial change in AUC from this study compared to that observed in our previous trial [1]. We used a linear mixed model in the xtmixed function in STATA 10 (Stata Corp, College Station, TX).
Results
Study enrollment, safety, and adverse events
Ten subjects were enrolled in the protocol: 6 were randomized to drug treatment and 4 to the control group (Table 1). The subjects were enrolled on average within 1 month of diagnosis (range 19-54 days). None had a history of ketoacidosis. Subjects randomized to the drug treatment group completed a 12 day course of teplizumab. All of them experienced a mild to moderate transient cytokine release syndrome which occurred earlier during the infusion course and involved higher grade AEs than had been seen previously (Table 2). A total of 202 AEs were reported over the 24 month follow up period in the drug treated compared to 50 in the control group. Of the 202, 67% were judged to be related to the use of the study drug. All drug treated subjects had Grade 2 or 3 lymphopenia during treatment. The majority (72%) of the events were mild (Grade 1), 24% were moderate (Grade 2), and 4% were severe (Grade 3). There were a total of 7 serious AEs reported by 3 participants, 2 in the drug treated and 1 in the control group. One drug treated subject had a prolonged grade 3 CD4 cytopenia, which was downgraded to moderate after 4 months and resolved after 27 months. After 24 months, 6 additional AEs were reported in the drug treated group: there was no follow up of the control group.
Table 2. Adverse events in drug treated subjects.
| Grade | 1 | 2 | 3 | 4 | Total (% of subjects) |
|---|---|---|---|---|---|
| GI disorder | 2 | 4 | 100 | ||
| Fever | 2 | 3 | 1 | 100 | |
| Nausea | 3 | 3 | 100 | ||
| Vomiting | 1 | 3 | 67 | ||
| Rigors | 2 | 3 | 83 | ||
| Headache | 3 | 1 | 67 | ||
| Chest pain | 1 | 17 | |||
| Infections | 4 | 2 | 100 | ||
| Pharyngitis or upper respiratory infections | 6 | 1 | 100 | ||
| Myalgia | 2 | 33 | |||
| Pharyngitis | 2 | 33 | |||
| Rash | 4 | 1 | 83 | ||
| Thrombocytopenia | 2 | 33 | |||
| Lymphadenopathy | 1 | 17 |
In our previous study, only 1/16 (6%) of the AEs, associated with the drug infusion, were grade 2 or above. The finding of additional and higher grade AE's prompted the investigators to curtail enrollment and not to administer the second course of drug treatment as originally planned. An extensive analysis of the drug product and the method of administration indicated that the dose administered may have been 40% greater than the dose used previously based on the protein concentration of the drug product that was administered. No other differences in the product or its administration were identified.
Drug levels and coating and modulation of CD3 on circulating T cells
The trough drug levels were measured for 4 days beginning on day 8 of the 14 day treatment protocol (Table 3). The average drug level was significantly greater than the level that had been measured during the same study days in the previous study (n=35, p<0.0001)[1]. The coating and modulation of CD3 molecules on CD4+ and CD8+ T cells were also higher on CD4+ and CD8+ T cells in this study compared to the previous trial (p<0.0001, Table 3).
Table 3. Coating and modulation of CD3 and drug levels.
| NDB01 (n=6) | Study 1 (n=9) | P value | |
|---|---|---|---|
| Peak CD3 coating on CD4+ cells | 78±2.3% | 65±4.3% | 0.03 |
| Peak CD3 modulation on CD4+ cells | 63.8±2.7% | 50.8±2.2% | 0.002 |
| Peak CD3 coating on CD8+ cells | 78.3±1.6% | 68±4.5% | 0.08 |
| Peak CD3 modulation on CD8+ cells | 65±2.2% | 56±1.9% | 0.009 |
| Trough drug levels (days 9-13)(range) | 1050±73 ng/ml(ND-2823) | 123±16 ng/ml (ND-407) | <0.0001 |
Cell counts and TREC analysis of T cell subsets
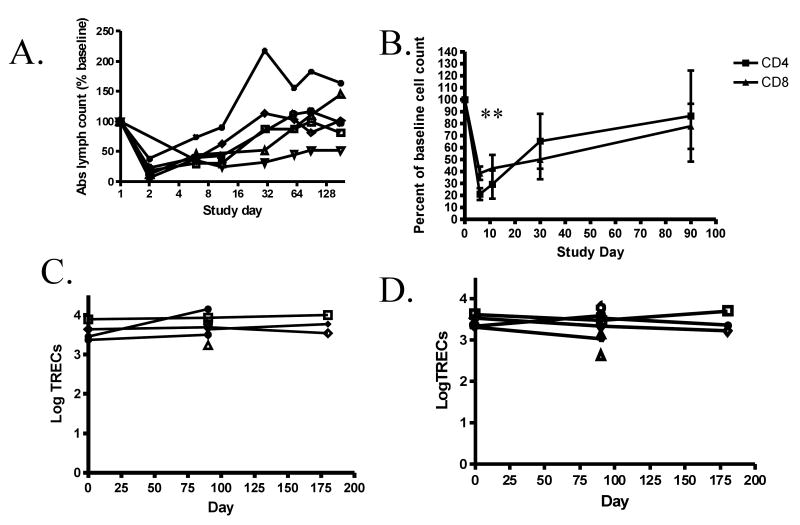
In subjects receiving anti-CD3 mAb, there was a reduction in the number of circulating lymphocytes. On day 3, the day following the first full dose of drug, absolute lymphocyte count was, on average, 24±8.4% of the baseline value (Figure 1A). The number of circulating lymphocytes increased even while the drug was administered so that by day 30, two weeks after the last dose of the drug, the absolute lymphocyte count was 101±33% of the baseline level. The nadir level of CD4+ T cells was significantly lower than CD8+ T cells (p<0.01) but the recovery of CD4+ and CD8+ T cells was similar (Figure 1B).
Figure 1. Effects of teplizumab on circulating T cells.
A: Changes in the absolute lymphocyte count in the peripheral blood during treatment with teplizumab. The number of circulating lymphocytes was calculated from the CBC and differential in subjects in the drug treatment group. The nadir lymphocyte count on Day 2 was 24.4± 8.4 % of the baseline count. Note that the scale on the X axis is logarithmic. The absolute lymphocyte counts in the control subjects were 94% of the baseline value at month 6 (not shown). B: Changes in the absolute number of CD4+ and CD8+ T cells during and after treatment with anti-CD3 mAb. The absolute CD4+ and CD8+ T cell counts (percentage of each subset*absolute lymphocyte count) are expressed as the percentage of the baseline value. The nadir CD4+ T cell count was lower than the nadir CD8+ T cell count (p<0.01). C and D: Copy number of TRECs in CD4+ (C) and CD8+ (D) T cells. The Y axis represents TREC copy number per million cells corrected for actin, as described in Materials and Methods. The symbols used for each subject are consistent in panels A, C, and D.
To determine the mechanisms of changes in T cells, we measured the number of TRECs relative to genomic DNA in CD4+ and CD8+ T cells before and after treatment with the mAb to determine whether the recovering cells had characteristics of recent thymic emigrants. We did not find a significant increase in the levels of TRECs (Figures 1B and C) even though there had been profound changes in the number of lymphocytes found in the peripheral circulation over the same period of time (Figure 1A).
Effects of drug treatment on C-peptide responses and insulin use
The control and drug-treated subjects were followed for 24 months to assess safety and the effect of the drug administration on C-peptide responses (Figure 2A). At 12 months, the C-peptide responses increased in the drug-treated group (from 0.877±0.182 to 0.894±0.245) and fell in the control group (from 0.406±0.119 to 0.189±0.119 nmol/L) but the difference between the groups was not statistically significant (p=0.2). There was not a significant difference in the absolute levels of C-peptide at 24 months between the groups when adjusted for baseline. However, there was a trend for a reduced rate of fall of C-peptide responses was twice in the drug treated group (-0.008 nmol/L/month) compared to the control group (-0.016 nmol/L/month)(p=0.1). Likewise, there was a trend for more of the control subjects (2/4) to reach undetectable C-peptide levels compared to the drug-treated subjects (0/6)(p=0.06), but the control subjects started with a lower C-peptide level (Table 1).
Figure 2. Effects of teplizumab treatment on clinical measures.
A: C-peptide AUC to a mixed meal tolerance test: Data from individuals in the drug treated(open symbols, n=6) and control group (closed symbols, n=4). B: Extended follow up of subjects in the drug treated group (n=4). The average C-peptide responses of the drug treated (open symbol±SEM) and control (solid symbol±SEM) group at 24 months are shown for comparison. C: Insulin use by the study groups: The average (±SEM) insulin use by subjects enrolled in the study (drug treated, n=6, open symbols, and n=4 control, closed symbols, n=4) are shown. There was a significant reduction in the use of insulin in the drug treated group in a mixed effects model (p<0.001, *p<0.05 drug treated vs control). D: Hemoglobin A1c levels in the study groups: The average (±SEM) are shown for the drug treated (open symbol) and control (closed symbol) groups.
There was a trend toward lower insulin usage in the drug-treated group at both 12 months (0.33±0.08U/kg vs 0.62±0.16 U/kg, p=0.11) and 24 months (0.45±0.11 U/kg vs 0.68±0.055 U/kg, p=0.16). In a mixed effects model, there was a significant effect of drug treatment on insulin use (difference in slope = 0.017 U/kg/month, p<0.001, Figure 2C). When data from all of the time points were combined, there was a significant negative correlation between the C-peptide AUC and insulin use (Figure 3, r = -.53, p<0.0001). The glucose control, reflected by hemoglobin A1c, was not significantly different between the groups at the specified time points.
Figure 3. Relationship between C-peptide AUC and insulin use.
Data from all of the time points from all of the 10 enrolled subjects are shown (open squares = drug treated) closed squares = controls) are plotted (R=-.53, p<0.0001).
Extended follow up
Four of the 6 drug-treated subjects (ages 17, 11, 20, and 18 yrs) were followed after the originally scheduled 24-month follow-up, for up to 5 years, to evaluate the effects of the single course of antibody treatment on C-peptide responses over time (Figure 2B). Three of the 4 subjects showed some increase in C-peptide response beyond month 24. All of the subjects had detectable C-peptide responses at month 42 (n=4) and at month 60 (n=3). The average loss of C-peptide at 42 months was 42±12% of the baseline response (n=4) and at month 60, 63±8% (n=3) of the baseline response. The average slope describing the change in C-peptide over time after 24 months was -0.0016± 0.0019 nmol/L/min/mo. At 42 months the subjects (n=4) were taking an average of 0.48±0.19 U/kg of insulin/d.
Ad-hoc analysis
Because of the small size of the enrolled control group and the significant differences in age (p=0.04) and baseline C-peptide responses (p=0.07), we also performed an ad hoc analysis of the outcomes in the drug-treated group using a matched control group from our previous study (Table 1)[1]. The 12 control subjects were selected by independent statisticians on the basis of baseline C-peptide and age. However, two of the drug treated subjects had high baseline C-peptide responses (1.56 and 1.29 nmol/L) which exceeded the range of the baseline C-peptide responses in the previous control group and were therefore matched for age only.
When the responses over 24 months in the drug treated group were compared to the control group in the ad hoc analysis, there was a lower decline in C-peptide responses. This difference between groups was statistically significant using a mixed effects model (p=0.008). At 6 months, the average C-peptide response in the drug treated group increased by 0.081±0.08 but decreased in the control group by 0.14±0.04 (p=0.04, adjusted for baseline), and at 12 months increased by 0.017±0.245 in the drug treatment group but decreased in the control group by 0.238±0.07, but the difference was not statistically significant (p=0.1). The difference between the groups was sustained at 18 months (p<0.05), but tended to decline at 24 months (p= 0.12).
The insulin use by the drug treated group was significantly less in the mixed effects model analysis (p=0.035). At 24 months, the drug-treated group was taking an average of 0.45±0.11U/kg (an increase of 0.07±0.12 U/kg from baseline) compared to 0.79±0.1 U/kg in the control group (p=0.03). The glucose control, reflected by hemoglobin A1c levels was similar in both groups (p=0.273). At 12 and 24 months, the hemoglobin A1c levels in the drug and control groups were 6.78±0.48% and 7.21±0.32% and 8.42±0.76% and 8.05±0.48% respectively.
We also compared the results from this study to the prior experience in the phase 1 / 2 study with this agent to determine if the response to treatment in the two groups was similar, and whether the increased drug dose in this study had any impact on efficacy relative to the previously reported results [1; 2]. This analysis was divided into effects on initial AUC stabilization or increase in the first 6 months following drug therapy, and then the subsequent rate of decline in AUC thereafter. This analysis failed to show a statistical improvement in C-peptide responses over both of these time intervals (p=0.55).
Discussion
Previous studies have shown that a single course of FcR non-binding anti-CD3 mAbs can attenuate the loss of insulin production. In this study, we did not find a statistically significant improvement in C-peptide responses and the responses after 24 months were not different. This result is most likely due to the small number of subjects, in this trial that created an imbalance of baseline C-peptide values (e.g. higher values in the drug treated group). In addition, the repeated dosing of the drug, as was originally intended, was not given. Nonetheless, data from the earlier time points suggest a trend for improved C-peptide responses and the ad hoc analysis using partially matched controls is consistent with the previous findings. Moreover, when subjects were followed for a longer period of time, we found persistent C-peptide responses. Insulin use was significantly reduced in the primary and ad hoc analysis. In addition, we found a significant inverse correlation between C-peptide responses and insulin requirements suggesting that insulin use may be a useful parameter for evaluating residual C-peptide responses. The baseline C-peptide AUC did predict the C-peptide AUC in follow-up but not predict response to drug therapy in this study or in our earlier trial [2]. However, the sample size may be too small to detect a relationship between the baseline response and the effects of treatment.
Our experience herein suggests that AEs with modified anti-CD3 mAbs may be dose-related. Compared to our previous study, we found higher coating and modulation of CD3 on peripheral T cells, and higher trough drug levels, and the AEs were more frequent, of higher grade, and occurred at an earlier time point in this treatment course. Despite the increased rate and severity of AEs, there was no evidence for improved efficacy based on C-peptide responses. Using our prior criteria for a clinical response (i.e. a < 15% decrease in C-peptide response), the response rate at 1 year was not improved with the higher dose of drug (3/6) compared to responses with a lower dose (15/21). Even the improvement in C-peptide responses in the first 6 months after drug treatment was not greater. However, a larger number of subjects are needed to definitively establish the relationship between drug dose and clinical efficacy as well as the long term preservation of C-peptide responses, a possibility raised by the plateau of C-peptide AUC during longer term follow up as shown in Figure 2B.
The mechanism(s) of action of FcR non-binding anti-CD3 mAb remains unknown. Although initial studies with OKT3 and even FcR non-binding anti-CD3 mAbs suggested that anti-CD3 mAbs deplete effector T cells, other preclinical studies and human data were not consistent with this mechanism [15; 16][7; 8]. In murine studies with thymectomized mice Hirsch et al suggested that depletion was not the mechanism by which the mAb induced immunosuppression, and that TCR modulation was associated with disappearance of T cells from the circulation [7]. Indeed, in our clinical study, the appearance of lymphocytes in the periphery coincided with reduced levels of coating and modulation of the TCR. The coating of CD3 fell from a peak of 76% and 75% on CD4 and CD8+ T cells to 13% and 31% by day 30, when T cells reappeared in the circulation. The levels of TRECs, a reflection of new thymic emigrants, in the circulating cells were constant after mAb treatment at the time when the cell counts recovered. Moreover, in a previous study, we did not find an increase in the proportion of CD45RA+ cells after recovery of circulating T cells (60.5±8.8%) compared to before treatment with anti-CD3 mAb (79.1±4.5%, n=7). However, the interpretation of the TREC analysis has certain limitations. For example, homeostatic proliferation of new thymic emigrants possibly after deletion of lymphocytes could have resulted in a rapid decline in TREC levels even though thymic output had increased [10; 17]. Nonetheless, the kinetics of return of circulating lymphocytes was too rapid (2 weeks) to be explained by thymic output, because it has been shown that the absolute production rate of newly divided CD4+ T cells is 10.4±6.5 cells/μl/d and of CD8+ cells 5.9±7.6 cells/μl/d[18]. Depending on the extent of depletion in the secondary lymphoid organs and sites, it would take several months to recover peripheral lymphocytes if new thymic cells were the primary source of new cells. These findings are consistent with the margination or trafficking of T cells after treatment with teplizumab. However, it is also possible that both mechanisms (i.e. recovery from marginated cells and homeostatic proliferation of new thymic emigrants) are operative.
Preclinical studies suggested that the non-FcR binding anti-CD3 mAb may induce regulatory T cells[5]. We had previously reported that there was an increased proportion of CD8+ T cells following treatment with anti-CD3 mAb and increased expression of Foxp3 in CD8+ T cells. We found, in this trial, a relatively greater proportion of CD8+ T cells during drug administration but did not find a difference in recovery of cells at day 90 unlike our previous studies[19]. Further studies will be needed to evaluate the functional changes in the CD8+ subpopulation after mAb treatment.
The duration of the effects on C-peptide responses with mAb treatment is of primary importance for any immune therapy of T1DM. In an extended follow-up of the drug-treated subjects we found detectable insulin production in drug-treated subjects followed for up to 60 months with an increase in insulin production over time in 3/4 subjects followed beyond month 24. In addition, the insulin use 3 ½ yrs after diagnosis was, on average, less than 0.5 U/kg which has been used by some investigators to characterize the “honeymoon” period[20]. Because our sample size is small, we cannot determine whether the persistence of C-peptide responses was significantly different from what might occur in non-treated subjects with T1DM of similar duration. Of note, in the DCCT, only 3% and 8% of subjects < 18 yrs and ≥ 18 yrs respectively had detectable C-peptide levels after 5 years of T1DM: whereas in a more recent analysis, >85% of subjects with T1DM duration up to 4 years had detectable C-peptide. However these studies were cross-sectional and did not evaluate changes in insulin production over time. [21; 22].
In summary, this study supports previous investigations showing that treatment of patients with new onset T1DM with a brief course of teplizumab results in a reduced rate of decline in insulin production and a decrease in insulin requirements for more than 2 years after diagnosis. The rapid return of circulating T cells and the absence of increased levels of TRECs suggests that the drug affects lymphocyte trafficking rather than causes depletion. A higher dose of drug, approximately 40% higher than the dose used previously, resulted in increased AEs. However, the relationship between dose of anti-CD3 mAb the duration of response.merits further investigation.
Acknowledgments
Supported by grants NO1-AI-15416-3700, DK057846, and CTSA grants UL1 RR024139 (Yale Univ), UL1 RR024131 (UCSF), and grants M01-RR-00037 (Univ Wash) and M01-RR00069 (Univ Colo) from National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The authors relied on the expertise of Diana Gonzalez for running the TREC test. JMP received support from the US Immunodeficiency Network (USIDNET). We would like to thank JM McCune, UCSF for helpful discussions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54:1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 3.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 4.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1(Ala-Ala) J Clin Invest. 2003;111:409–18. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 6.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335–40. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch R, Eckhaus M, Auchincloss H, Jr, Sachs DH, Bluestone JA. Effects of in vivo administration of anti-T3 monoclonal antibody on T cell function in mice. I. Immunosuppression of transplantation responses. J Immunol. 1988;140:3766–72. [PubMed] [Google Scholar]
- 8.Hirsch R, Gress RE, Pluznik DH, Eckhaus M, Bluestone JA. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989;142:737–43. [PubMed] [Google Scholar]
- 9.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, Coutinho RA, Lange JM, Rinke de Wit TF, Tsegaye A, van Dongen JJ, Hamann D, de Boer RJ, Miedema F. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 10.Harris JM, Hazenberg MD, Poulin JF, Higuera-Alhino D, Schmidt D, Gotway M, McCune JM. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin Immunol. 2005;115:138–46. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 12.Executive summary - Standards of medical care in diabetes - 2008. Diabetes Care. 2008:S5–S11. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 13.Woodle ES, Xu D, Zivin RA, Auger J, Charette J, O'Laughlin R, Peace D, Jollife LK, Haverty T, Bluestone JA, Thistlethwaite JR., Jr Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 1999;68:608–16. doi: 10.1097/00007890-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–8. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter PA, Pavlovic S, Tso JY, Press OW, Gooley T, Yu XZ, Anasetti C. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J Immunol. 2000;165:6205–13. doi: 10.4049/jimmunol.165.11.6205. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter PA, Tso JY, Press OW, Yu X, Anasetti C. Non-FcR-binding, humanized anti-CD3 antibody Hu291 induces apoptosis of human T cells more effectively than OKT3 and is immunosuppressive in vivo. Transplant Proc. 2000;32:1545–6. doi: 10.1016/s0041-1345(00)01343-9. [DOI] [PubMed] [Google Scholar]
- 17.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, Li K, Killian ML, Bacchetti P, McCune JM. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–98. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 19.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8 T cell population and induces CD8CD25 Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chase HP, MacKenzie TA, Burdick J, Fiallo-Scharer R, Walravens P, Klingensmith G, Rewers M. Redefining the clinical remission period in children with type 1 diabetes. Pediatr Diabetes. 2004;5:16–9. doi: 10.1111/j.1399-543X.2004.00034.x. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53:250–64. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb H. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–71. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]