Abstract
Ozone (O3) is a respiratory irritant that leads to airway inflammation and pulmonary dysfunction. Animal studies show that neonates are more sensitive to O3 inhalation than adults, and children represent a potentially susceptible population. This latter notion is not well established, and biological mechanisms underlying a predisposition to pollution-induced pulmonary effects are unknown. We examined age and strain as interactive factors affecting differential pulmonary responses to inhaled O3. Male and female adult mice (15 weeks old) and neonates (15–16 days old) from eight genetically diverse inbred strains were exposed to 0.8 ppm O3 for 5 h. Pulmonary injury and lung inflammation were quantified as total protein concentration and total polymorphonuclear neutrophil (PMN) number in lavage fluid recovered 24-h postexposure. Dose-response and time-course curves were generated using SJL/J pups, and 18O lung burden dose was assessed in additional mice. Interstrain differences in response to O3 were seen in neonatal mice: Balb/cJ and SJL/J being most sensitive and A/J and 129x1/SvJ most resistant. The PMN response to O3 was greater in neonates than in adults, specifically for SJL/J and C3H/HeJ strains, independent of dose. Small gender differences were also observed in adult mice. Variation in protein concentrations and PMN counts between adults and pups were strain dependent, suggesting that genetic determinants do play a role in age-related sensitivity to O3. Further research will help to determine what genetic factors contribute to these heightened responses, and to quantify the relative contribution of genes vs. environment in O3-induced health effects.
Keywords: children's health, genetic susceptibility, lung inflammation, lung injury, ozone
Air pollution remains an important public health concern throughout the world, especially in industrialized cities. Of the many toxic pollutants comprising air pollution associated with city life, ozone is of particular interest. One of the six “criteria air pollutants,” ambient ozone is formed by the action of sunlight on nitrogen oxides and reactive hydrocarbons, both of which are emitted by motor vehicles and industrial sources (Suh et al., 2000). In addition to being a powerful respiratory irritant in adults and children, studies examining both environmental and chamber exposures in human subjects reveal that ozone leads to airway inflammation via neutrophilia and hyperreactivity (reviewed by Mudway and Kelly, 2000). Furthermore, ozone inhalation can exacerbate symptoms in those with cardiopulmonary disease and asthma (Foster et al., 2000; Romieu et al., 2002). As with other ambient pollutants, the effects of ozone inhalation can be dramatically enhanced in susceptible populations.
There are two central lines of reasoning to explain why children represent a population that may be particularly susceptible to ozone. First, the organ systems of neonates and infants may have less developed protective mechanisms than adults, as lungs do not fully develop until the postnatal stage. Eighty percent of alveoli are formed postnatally and the continued differentiation of critical cells can last throughout the first 6–8 years of childhood (Finkelstein and Johnston, 2004). Second, children have increased exposures to many air pollutants due to their higher minute ventilation and higher levels of physical activity outdoors (Sarangapani et al., 2003). Epidemiological evidence shows that inhalation of air pollutants, such as ozone, by children is associated with wheezing, chest tightness, decrements in pulmonary function and development of respiratory disease (Gent et al., 2003). Moreover, increases in ambient ozone levels, even those below the recently updated National Ambient Air Quality Standard of 0.08 ppm for an 8-h exposure, have been linked to increases in school absences, hospitalizations and emergency room visits (Peel et al., 2005). There is also a small pool of animal studies exploring the sensitivity of young versus adult animals to ozone (Gunnison et al., 1992; Schlesinger et al., 1992), yet the genetic factors governing response to air pollutants at early life stages are unknown.
Genetic variability is becoming increasingly recognized as a significant host factor in environmental disease predisposition. In both human and animal models, evidence supporting a genetic basis for susceptibility to the toxic effects of inhaled pollutants has been well established, and, animal studies show that ozone toxicity is species and strain dependent. In several studies, linkage analyses performed using ozone-resistant and -sensitive mouse strains have identified significant quantitative trait loci (QTLs), along with specific genes within these QTLs, that may contribute to variations in susceptibility (Kleeberger et al., 2000; Prows et al., 1997; Savov et al., 2004). Several genes in humans have also been linked to ozone-induced respiratory effects (reviewed by Yang et al., 2008) as well as to other air pollutants such as diesel exhaust (Gilliland et al., 2004). However, little research has considered the importance of age on genetic susceptibility to disease or the potential interactive effect of these two factors on pollutant susceptibility.
The purpose of the current study was to examine whether there is a genetic basis for an age-dependent differential response to O3 inhalation in mice by examining variable sensitivity to O3 amongst adults and pups from genetically diverse inbred mouse strains. Neonatal and adult male and female mice from eight inbred strains were exposed to 0.8 ppm of O3 for 5 h. Inbred mice are ideal for investigating gene-environment interactions because they share several chromosomal regions of conserved synteny with humans. Interstrain differences in pulmonary injury (as total protein concentration measurement) and lung inflammation (as total PMN [polymorphonuclear neutrophil] count) were examined in bronchoalveolar lavage (BAL) fluid 24 h after exposure to O3. Age-related effects were observed and found to be strain dependent in both endpoints, supporting our hypothesis that multifaceted gene-environment interactions affect ozone sensitivity.
MATERIALS AND METHODS
Animals.
All inbred mouse strains (A/J, AKR/J, C3H/HeJ, BALB/cJ, C57BL/6J, DBA/J, SJL/J, and 129x1/SvJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were acclimated for at least 1 week before exposure, housed in a positive pressure environment with a 12-h light/dark cycle starting at 6:00 A.M., and provided with water and standard laboratory rodent chow (Purina, Indianapolis, IN) ad libitum except during exposure. Mice were handled in accordance with the standards established by the U.S. Animal Welfare Acts set forth in the National Institutes of Health guidelines and by the New York University School of Medicine Division of Laboratory Animal Resources.
Atmosphere generation and characterization.
Mice were exposed to O3 (0.80 ppm) in stainless steel cages inside a 1-m3 stainless steel inhalation chamber. Ozone (0.80 ± 0.02 ppm, mean ± SD) was generated from 10% oxygen (argon balance) using an ultraviolet ozonator (OREC, Phoenix, AZ). Ozone concentration was monitored in the breathing zone of the chamber with a U.S. Environmental Protection Agency calibrated O3 detector (Dasibi Model 1008-PC, Glendale, CA). Air (sham) exposures were also conducted for control purposes. Relative humidity and temperature (T) were monitored hourly and maintained at 50 ± 10% and 70 ± 5 °F, respectively, during exposure using an Omega temperature and humidity monitor.
Experimental design.
To investigate the contribution of age and genetic background to susceptibility to O3, neonatal mice (15–16 days old) from eight inbred strains were exposed to 0.8 ppm O3 for 5 h away from their mothers. Pups included males and females, but sex was not recorded. Susceptibility to O3 was assessed in BAL fluid 24-h postexposure by measuring total protein concentration (a phenotypic marker for pulmonary injury) and total PMN number (a phenotypic marker for lung inflammation). For comparison, lactating (15 weeks old) female mice and age-matched, nonlactating female mice were exposed to 0.8 ppm O3 for 5 h and the same two phenotypic endpoints were recorded. Age-matched, adult males (also 15 weeks old) were exposed to O3 to explore gender differences. Inbred strains with markedly high or low protein concentration and PMN influx in response to O3 exposure were considered as being the most sensitive or most resistant to inhaled O3. Due to high variability between litters in specific exposure groups, additional animals and/or litters were exposed to increase sample size of these particular groups.
As SJL pups appeared to be the most sensitive in terms of lung injury (protein) and lung inflammation (PMNs), pups from this strain were chosen for further studies to determine if their sensitivity would diminish with time postexposure or with decreased O3 concentration. A single litter of SJL pups was exposed to 0.8 ppm O3, and protein concentrations and PMN percentages were recorded in BAL fluid at 24-, 48-, and 72-h postexposure to validate the time course of response. Additional SJL/J pups were exposed to 0.2, 0.4, 0.6, and 0.8 ppm O3 to examine the dose-response curve for the two endpoints. To ensure that the observed differences in O3 response were not due to dose, pups from the most sensitive (Balb/c and SJL) and most resistant (129 and A/J) strains were exposed to O3 generated with 18O, along with strain-matched adults. At zero hour after exposure, the amount of 18O in the lung homogenates was measured to assess O3 dose.
Bronchoalveolar lavage.
At 24-h postexposure to O3 or air, mice were killed by intraperitoneal injections of ketamine HCl (100 mg/kg; Vetalar, Fort Dodge Laboratories, Inc., Fort Dodge, IA) and sodium pentobarbital (175 mg/kg; Sleepaway, Fort Dodge Laboratories, Inc.), and the posterior abdominal aorta was severed. The lungs of each mouse were lavaged twice with 1.2 ml (adult) or 0.26 ml (pup) of Dulbecco's phosphate buffered saline without Ca2+ or Mg2+ (pH 7.2–7.4, 37°C; GibcoBRL, Life Technologies, Grand Island, NY). Recovered BAL fluid was immediately placed on ice (4°C). Total cell count was determined with a hemacytometer. Aliquots (100 μl) of lavage fluid were then cytocentrifuged (Cytospin, Shandon Southern Products, UK), and the cells were stained with Hemacolor (EM Science, Gibbstown, NJ) for differential cell analysis. Differential cell counts were performed by identifying at least 200 cells according to typical cytological procedures. Cell types identified included macrophages (MΦ), PMNs and epithelial cells (Epi). Total BAL cell counts were volume corrected for the amount of lavage fluid recovered/animal and these numbers were used to calculate total PMNs and total Epi (see Tables 1 and 2). (MΦs constituted the remaining percentage of total cells) Lavage fluid was then centrifuged (500 × g, 7 min, 4°C) and the supernatant was decanted. The total protein concentration in the supernatant was measured using a bicinchoninic acid assay kit (Pierce, Rockford, IL).
TABLE 1.
Effects of Ozone Exposure on Lung Injury and Inflammatory Responses in Neonates from Eight Inbred Mouse Strainsa
| Strains | nb | Protein (μg/ml) | Total cellsc (1 × 103) | PMNsd (1 × 102) | Epid (1 × 102) |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Air | |||||
| A/J | 6 | 263.0 (45.8) | 3.92 (0.54) | 2.12 (0.58) | 2.40 (0.41) |
| 129 | 13 | 198.7 (10.3) | 5.71 (0.95) | 0.22 (0.12) | 4.84 (0.08) |
| C3H | 3 | 219.9 (24.4) | 7.09 (2.39) | 1.23 (0.65) | 6.89 (1.63) |
| AKR | 6 | 267.1 (21.9) | 9.54 (3.12) | 0.95 (0.31) | 4.12 (1.20) |
| C57BL6 | 7 | 167.5 (23.6) | 7.83 (1.41) | 3.57 (1.10) | 3.91 (0.86) |
| DBA | 8 | 248.4 (18.6) | 9.04 (1.18) | 0.37 (0.22) | 4.81 (1.73) |
| BALB/c | 10 | 240.6 (22.9) | 8.37 (0.70) | 1.63 (0.54) | 4.78 (0.66) |
| SJL | 21 | 186.3 (7.5) | 5.42 (0.36) | 1.04 (0.23) | 4.90 (0.58) |
| Ozone | |||||
| A/J | 10 | 281.7 (7.5) | 5.95 (0.51) | 3.70 (0.74) | 4.54 (0.46 |
| 129 | 13 | 255.1 (19.6)* | 6.06 (0.81) | 0.90 (0.37) | 5.47 (0.79) |
| C3H | 7 | 298.8 (53.6) | 12.45 (1.97)* | 24.25 (5.13)* | 16.02 (2.83)* |
| AKR | 8 | 338.8 (50.2) | 13.51 (2.16)* | 2.25 (0.81) | 5.85 (0.96) |
| C57BL6 | 10 | 293.4 (46.9)* | 10.51 (1.37) | 15.42 (6.18)* | 4.78 (1.51) |
| DBA | 9 | 444.0 (50.4)* | 9.89 (0.57) | 3.37 (0.59) | 4.84 (1.17) |
| BALB/c | 6 | 420.3 (36.5)* | 14.28 (1.94)* | 25.04 (4.54)* | 13.95 (3.61)* |
| SJL | 16 | 611.9 (63.3)* | 9.86 (1.14)* | 47.46 (7.33)* | 5.76 (0.78) |
Note. *Significantly increased (p < 0.05) from strain-matched, air controls. Epi = epithelial cells.
Means include males and females. Pups were not sexed.
n = mice/strain/exposure group.
Determined as: [total cells/ml] × [vol. BAL fluid recovered (ml)].
Calculated as: [total cells] × [%cell type].
TABLE 2.
Effects of Ozone Exposure on Lung Injury and Inflammatory Responses in Adult Mice from Eight Inbred Strainsa
| Strains | nb | Protein (μg/ml) | Total cellsc (1 × 103) | PMNsd (1 × 102) | Epid (1 × 102) |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Air | |||||
| A/J | 12 | 166.8 (13.1) | 42.39 (4.06) | 5.41 (1.50) | 35.34 (5.9) |
| 129 | 12 | 153.9 (5.9) | 57.07 (4.16) | 1.96 (0.83) | 32.08 (3.83) |
| C3H | 14 | 122.0 (10.1) | 55.02 (5.77) | 13.37 (3.40) | 54.19 (13.30) |
| AKR | 37 | 183.7 (8.3) | 58.37 (3.49) | 13.57 (3.80) | 35.84 (3.42) |
| C57BL6 | 12 | 191.4 (13.5) | 49.28 (2.51) | 2.53 (0.78) | 34.67 (6.93) |
| DBA | 11 | 240.0 (16.6) | 48.91 (5.08) | 6.12 (2.35) | 33.17 (5.00) |
| BALB/c | 19 | 200.7 (9.3) | 67.39 (5.66) | 3.36 (1.07) | 40.03 (5.85) |
| SJL | 12 | 401.9 (39.3) | 58.49 (6.07) | 8.59 (3.61) | 46.73 (6.23) |
| Ozone | |||||
| A/J | 10 | 268.9 (27.7)* | 89.13 (7.50)* | 22.39 (8.64) | 81.50 (12.10)* |
| 129 | 11 | 235.0 (19.0)* | 53.36 (5.90) | 5.01 (1.37) | 38.22 (7.89) |
| C3H | 10 | 246.2 (13.0)* | 66.35 (6.86) | 53.53 (5.40) | 38.42 (7.89) |
| AKR | 46 | 286.3 (12.3)* | 71.81 (3.86)* | 99.06 (14.86)* | 34.80 (3.34) |
| C57BL6 | 12 | 425.4 (24.6)* | 52.25 (3.23) | 17.23 (3.17) | 38.12 (3.29) |
| DBA | 12 | 343.7 (30.0)* | 65.91 (5.67) | 7.10 (2.82) | 40.94 (8.43) |
| BALB/c | 28 | 402.6 (11.8)* | 67.92 (6.80) | 108.53 (24.77)* | 52.15 (10.34) |
| SJL | 11 | 553.7 (35.8)* | 52.98 (5.09) | 17.17 (5.68) | 52.25 (7.88) |
Note. *Significantly increased (p < 0.05) from strain-matched, air controls. Epi = epithelial cells.
Means include males and females.
n = mice/strain/exposure group.
Determined as: [total cells/ml] × [vol. BAL fluid recovered (ml)].
Calculated as: [total cells] × [%cell type].
18O3 generation and exposure.
Mice were exposed to 18O3 in a 1.0- x 3.0-feet plexiglass chamber. The chamber was lined with Teflon to prevent the absorption of 18O to the plexiglass and 18O3 was run into the chamber before mice were loaded to stabilize the O3 concentration. Pups and adults from the selected strains were placed into the chamber and individually separated from each other by stainless steel dividers to ensure an unbiased dose. Also, mice were randomly strain and age distributed amongst the dividers, again, to reduce variability in dose among groups. The mice were exposed to 18O3, diluted to maintain a 0.8 ppm concentration, for 5 h. Chamber flow was maintained at 16.5 liters per minute, which translates to 20.2 air changes/h for the 49-l chamber.
18O analysis.
Following exposure, all animals were sacrificed by intraperitoneal injection of sodium pentobarbital (175 mg/kg; Sleepaway, Fort Dodge Laboratories, Inc.) within 1 h. Lungs were removed and flash frozen with liquid nitrogen for storage at −70°C to be processed within 3 weeks. Lungs were thawed, minced, and homogenized in distilled water. The whole homogenate was freeze dried and stored again at −70°C before being shipped to the Environmental Protection Agency for 18O analysis which follows the analytic protocol described by Hatch et al. (1994). The purpose of this method is to detect reaction product of 18O with the lung tissue, which translates to O3 dose. Plasma samples from exposed animals were also sent to the EPA for baseline measurements, as 18O2 is present at low levels in the normal atmosphere (∼0.2% of atmospheric O2) leading to the same natural abundance of 18O in animal tissues.
Data analysis.
Ozone-induced changes in protein and PMNs in BAL fluid are presented as the mean ± SE. Differences between means were assessed using a two-factor ANOVA with the independent factors of strain and exposure. Age and gender were also considered as independent factors in additional ANOVA tests. The Student-Newman-Keuls and Dunnett's one-tailed a posteriori tests of significance were used to assess differences between means using Prism (Graphpad Software Inc., San Diego, CA) and SuperANOVA (ABACUS Concepts, Berkeley, CA) statistical packages. Statistical significance was accepted at p ≤ 0.05.
RESULTS
Strain
Exposure to O3 induced variable degrees of lung injury and inflammation in both neonatal and adult inbred strains of mice. Protein levels were increased in all neonatal mice exposed to O3 over strain-matched air controls, with increases reaching statistical significance in five strains (p < 0.05; Table 1). Significant increases in PMNs resulting from O3 were seen in neonatal mice from four strains, three of which had significantly higher total cell counts than air controls, and two of which also had a significantly increased number of epithelial cells in their lavage fluid (p < 0.05; Table 1). Three neonatal strains with significant O3-induced increases in both protein and PMNs were considered to be most sensitive, whereas those strains having no difference in total cell, PMN or Epi number were considered to be most resistant. Remaining strains demonstrated an intermediate, yet semicontinuous strain distribution pattern in their PMN counts and/or protein concentrations as a result of O3 exposure.
Focusing on adult mice, significant increases in protein levels in BAL fluid from O3-exposed adults over strain-matched air controls were seen for all strains (p < 0.05; Table 2). However, significant increases in PMNs were recorded in only two of the eight strains (p < 0.05), one of which also experienced a significant increase in total cell number over its corresponding air control. A significant increase in epithelial cell counts was also observed for just one strain, which also had a significantly higher number of total cells than its air-exposed counterpart (Table 2).
Age Analysis
As adult and neonatal mice are vastly different in terms of body weight, lung size, and lung tidal volume, comparisons between adults and pups for our phenotypic endpoints were not based on raw numbers, but on protein (Fig. 1A) and total PMN (Fig. 1B) fold changes (calculated from the total protein concentrations and total PMN counts in O3-exposed animals over the average values of their strain and age-matched air controls). When comparing fold changes in protein in pups versus adults, adult mice in three of the eight strains (A/J, C3H, and BL6) had significantly higher fold changes than strain-matched pups (p < 0.05). In contrast, the protein fold changes for two strains of neonates (DBA and SJL) were significantly increased over strain-matched adults (p < 0.05; Fig. 1A).
FIG. 1.
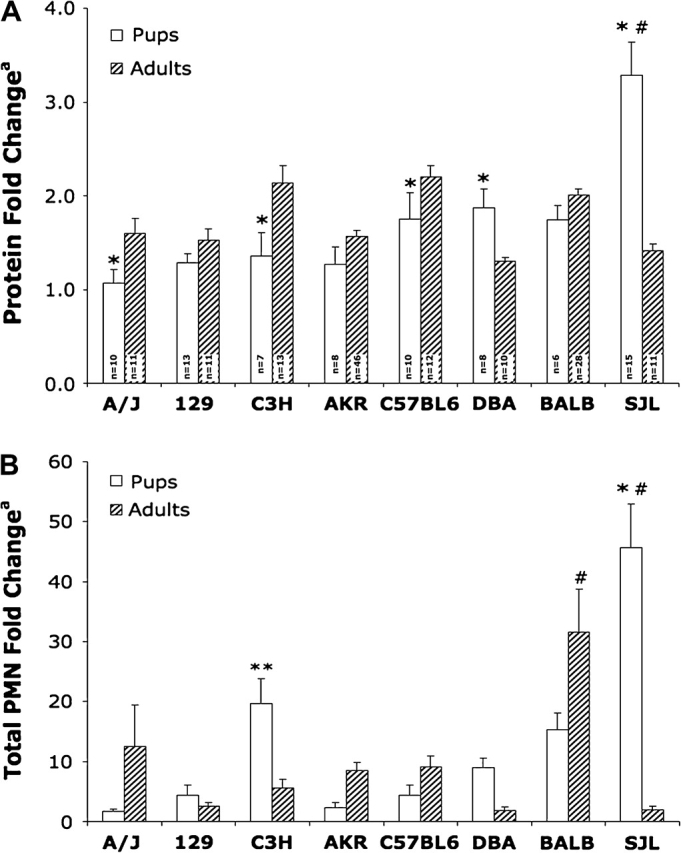
Comparison of lung injury and inflammation in adult and neonatal mice exposed to O3: (A) Protein and (B) PMN influx. Values represent mean ± SE; n = no. of animals per group. Adult bars represent males and females. Total protein and total PMN fold changes were calculated as [total protein concentration (μg/ml) O3]/[average total protein concentration air] and [total PMN count O3]/[average total PMN count air], respectively. *Significantly different (p < 0.05) from strain-matched adults. #Significantly increased (p < 0.5) from all age-matched animals. **Significantly increased (p < 0.5) from age-matched A/J pups.
The total PMN fold change values in neonates were elevated in comparison to adults in four strains, reaching a significant elevation in only the SJL strain (p < 0.05). In addition, this value for SJL pups was significantly larger than the values for pups from all other strains. Although C3H pups did have the next highest total PMN fold change value after SJL pups, it was only considered to be significantly elevated in comparison to A/J pups due to large variability. Adult mice from the remaining four strains tended to have larger total PMN fold change values than strain-matched pups with this difference reaching statistical significance for the Balb/c strain (p < 0.05). The Balb/c adults’ total PMN fold change was also significantly increased in comparison to all other adults (p < 0.05; Fig. 1B).
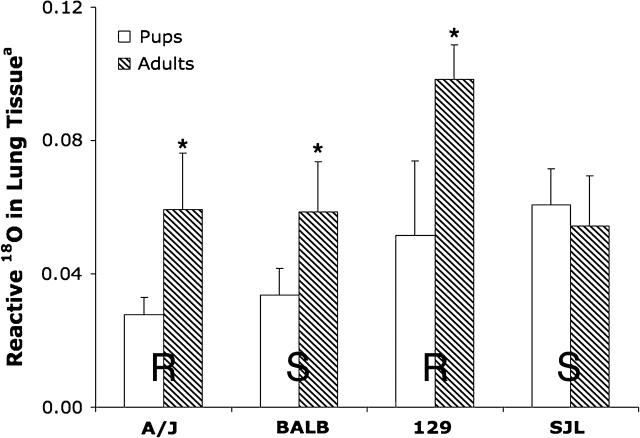
18O Dose Analysis
Ozone dose, as determined by the 18O analysis, for exposed adult females and pups from four strains revealed no significant trends in delivered dose between most resistant (R) and most sensitive (S) neonatal mice. Although adult mice had significantly greater 18O lung burden in three of the four strains (Fig. 2), adult and neonatal SJL mice had comparable 18O dose levels.
FIG. 2.
Comparison of O3 dose as measured by 18O3 lung reaction product in neonatal and adult mice in four strains after exposure to 0.8 ppm 18O3 for 5 h. Values represent mean ± SE; n = 3–4 mice/strain/exposure group. R: resistant strain; S: sensitive strain. Reactive 18O calculated as: ([18O/16O in lung tissue] − [average 18O/16O in plasma blank]) × 1000. *Significantly increased (p < 0.5) from strain-matched pups.
Gender
Nonlactating, adult females had larger O3-induced protein fold change increases over air controls in comparison to males for six of the eight strains, but only reached statistical significance in the C3H strain (p < 0.5; Fig. 3A). Although protein fold changes for BL6 and SJL males were slightly larger than strain-matched females, these values were not significantly different. Furthermore, lactating females seemed to experience the greatest lung injury, having the largest protein fold changes in six of the eight strains with two of these strains being significantly greater than strain-matched, nonlactating females (p < 0.5; Fig. 3A). For lactating females, Figure 3B reveals increases in total PMN fold change in comparison to strain-matched, nonlactating females for five strains, reaching statistical significant level in the BL6 strain (p < 0.05; Fig. 3B). No differences were observed regarding epithelial cell counts (data not shown).
FIG. 3.
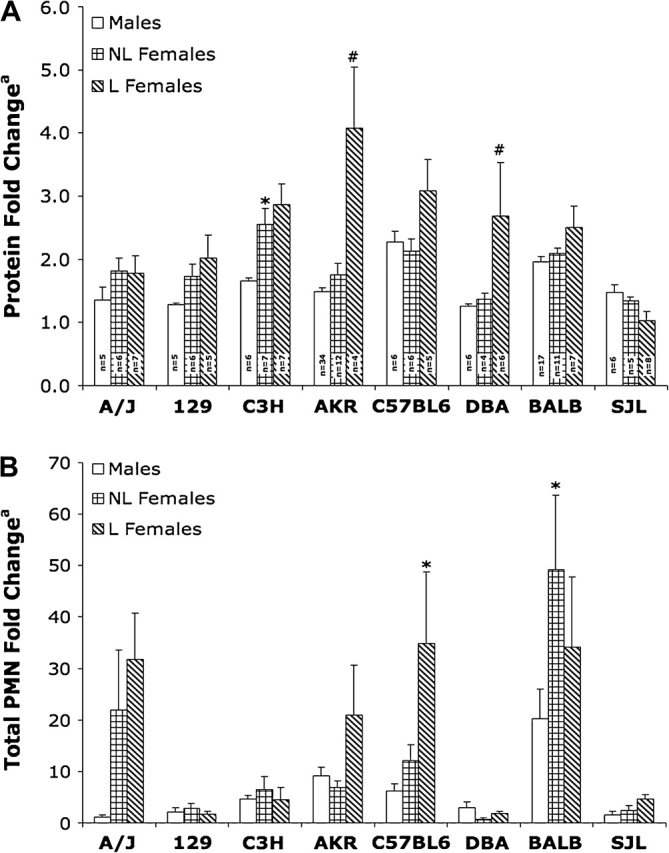
Comparison of lung injury and inflammation in adult male, nonlactating and lactating female mice exposed to O3: (A) Protein fold change and (B) PMN influx. Values represent mean ± SE; n = no. of animals per group. L: lactating, NL: nonlactating. Total protein and total PMN fold changes were calculated as in Fig. 1). *Significantly increased (p < 0.05) from strain-matched males. #Significantly increased (p < 0.05) from strain-matched, nonlactating females.
Along with significant differences in protein and PMN counts resulting from the individual factors of exposure, strain, and age in pups and adults, as well as significant differences resulting from gender and lactation in the adult mice shown above, there were significant interactions overall between strain, exposure and age for total protein and total PMNs in pups as well as significant interactions between gender and exposure for total protein in adults (p < 0.05; Table 3).
TABLE 3.
Statistical Results on the Roles of Age, Strain, Exposure, and Gender (interactions) in Ozone-Induced Lung Injury and Inflammationa
| Protein (μg/ml) | Total PMNs | Total epi cells | |
| Strain | 0.0000001 | 0.0000001 | 0.0000001 |
| Exposure | 0.0000001 | 0.0000001 | 0.72 |
| Age | 0.98 | 0.0003 | 0.87 |
| Genderb | 0.00005 | 0.05 | 0.0000005 |
| Strain:exposure | 0.000001 | 0.0000001 | 0.21 |
| Exposure:age | 0.85 | 0.005 | 0.58 |
| Exposure:genderb | 0.05 | 0.22 | 0.22 |
| Strain:exposure:age | 0.02 | 0.000001 | 0.14 |
| Strain:exposure:genderb | 0.99 | 0.92 | 0.42 |
Values represent p value.
Gender values apply only to adult animals.
Time-Course and Dose-Response Curves
A time-course study using neonatal SJL pups revealed that significant O3-induced increases in BAL protein concentrations were only identifiable 24-h postexposure (p < 0.05); protein concentrations at 48 and 72 h were not different from air control mice (Fig. 4A). Similarly, PMN influx was greatest 24-h postexposure (p < 0.01; Fig. 4B). Although PMN influx was still significantly greater than air controls at 48 h (p < 0.05), it was greatly diminished in comparison to the 24-h time point and was no longer significantly different at 72 h. In contrast, epithelial cell counts were not different from air controls at 24- or 48-h postexposure, yet became significantly increased at 72 h (p < 0.05; Fig. 4B).
FIG. 4.
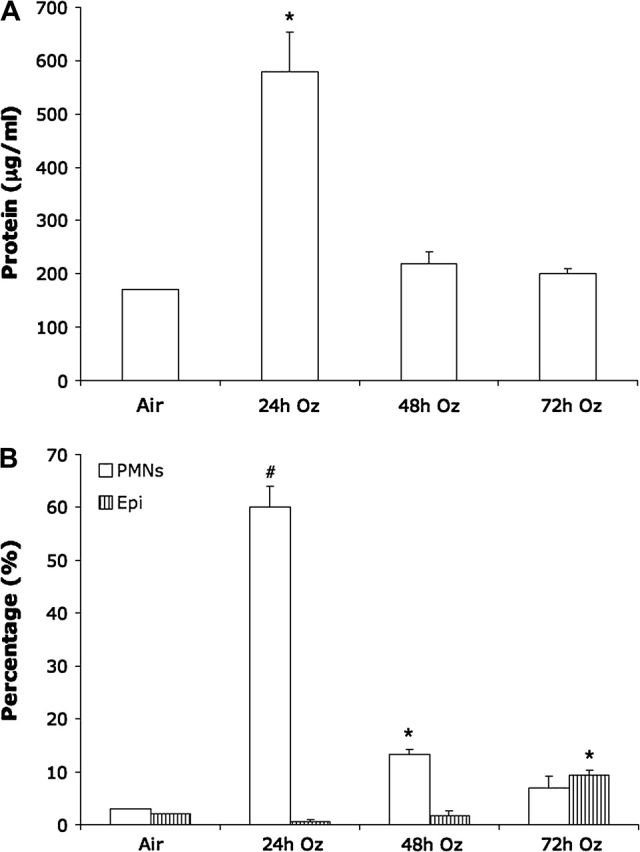
Time-course curves of lung injury and inflammation in SJL/J neonatal mice exposed to 0.8 ppm O3: (A) Protein concentration and (B) PMN and epithelial cell (Epi) influx. Values represent mean ± SE; n = 3 mice per group. *Significantly increased (p < 0.05) from air control. #Significantly increased (p < 0.01) from air control.
In the dose-response study using SJL pups, lung protein concentrations increased in a dose-dependent manner; however, protein concentration only reached a significant level above air controls at the 0.8 ppm O3 concentration (Fig. 5A). PMN counts also increased in a dose-dependent manner, becoming significantly increased at 0.6 ppm (p < 0.05) and 0.8 ppm (p < 0.01; Fig. 5B).
FIG. 5.
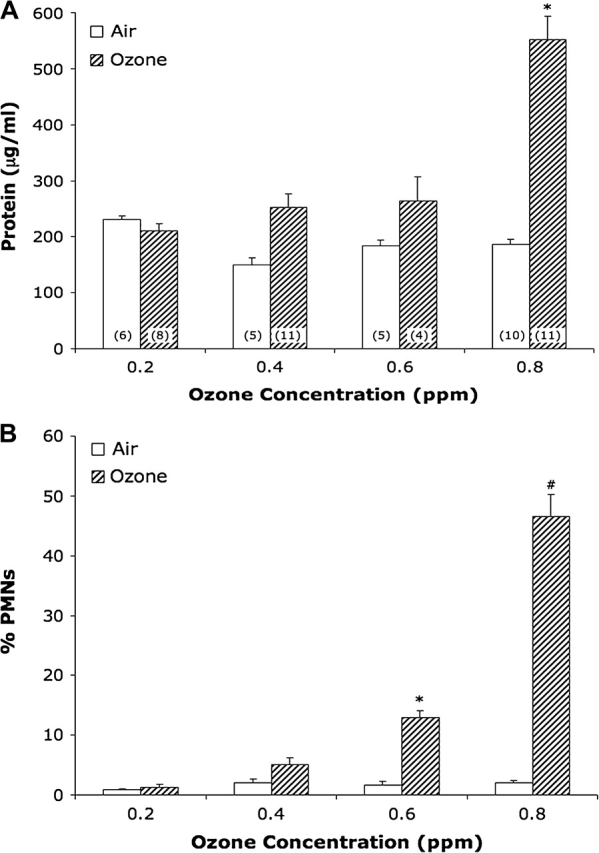
Dose-response curves of lung injury and inflammation in SJL/J neonatal mice exposed to variable O3 concentrations: (A) protein concentration and (B) PMN influx. Values represent mean ± SE; n = no. mice per exposure group. *Significantly increased (p < 0.05) from matched air control. #Significantly increased (p < 0.01) from matched air control.
DISCUSSION
Ozone is a powerful oxidant and respiratory irritant in adults and children, and there is epidemiological evidence that O3 inhalation can lead to airway inflammation and hyperreactivity along with decrements in pulmonary function in healthy individuals (Foster et al., 2000; McDonnell et al., 1999). Ozone inhalation is known to induce lung injuries in rats and guinea pigs as well as airway hyperresponsiveness (Bermudez et al., 1999; Schlesinger et al., 2002). The interaction of O3 exposure, age, and genetics have not been examined together although investigators have shown that the genetic background of inbred adult mice affects the pulmonary response to O3 (Savov et al., 2004; Kleeberger et al., 1997) and two rodent studies have demonstrated age-dependent differences in ozone-induced lung inflammation (Gunnison et al., 1990, 1992). The aims of the current study were to confirm strain, age and gender as important factors in the pulmonary response to O3 as measured by two specific endpoints for lung injury and lung inflammation, and to determine if these factors interact to enhance or diminish O3-induced effects on these endpoints.
Ozone increased inflammation in the lungs, as measured by PMN influx, for all inbred mouse strains in comparison to air controls in the current study, which is consistent with the well-established idea that O3 is an inflammatory stimulant (Mudway and Kelly, 2000). However, only specific strains experienced significant levels of PMN influx, suggesting that genetic background is a major factor in O3-induced pulmonary responses. Furthermore, certain strains, designated as sensitive or resistant based on PMN influx at the adult age, were classified differently at the neonatal age, not only reaffirming the previous findings that age influences inflammation in rodents (Gunnison et al., 1990, 1992), but also suggesting that age and genetic background have an interactive effect on inflammatory responses to O3 inhalation. Statistical analysis of age, strain and exposure interactions on total protein measurement and total PMN counts (p ≤ 0.02, Table 3) further support this idea.
Overall, neonatal mice appeared to be less sensitive than adult mice to O3-induced lung injury as measured by total protein fold change in BAL fluid. SJL pups were the one exception, having a significantly higher protein fold change than SJL adults and all other age-matched strains (discussed below). Although these findings do not support the idea that young mammalian lungs are more sensitive to inhaled oxidants than adult lungs, they are in agreement with an hyperoxia study in mice that reported neonates as being more resistant to hyperoxia-induced lung injury than adults (Choo-Wing et al., 2007). It has been postulated that this tolerance to oxygen toxicity is transient as it takes neonatal lungs a longer time to mount a response possibly because of their delayed respiratory development (Keeney et al., 1995).
Exposure to oxidants during respiratory development does not, however, come without consequences. Oxidant exposure early in life can lead to respiratory remodeling and impairment later on. Studies have shown that premature infants requiring vigorous hyperoxic respiratory support soon after birth frequently develop bronchopulmonary dysplasia (Frank et al., 1991; Jobe, 1999). Mice exposed to 65% oxygen for their first postnatal month of life have fewer and larger alveoli along with greater respiratory system compliance than air-exposed mice when they reach 7–8 months of age (Dauger et al., 2003). Regarding O3 exposure, a study examining lung morphogenesis and function of 1-month-old rhesus monkey demonstrated that 5 months of episodic exposure to 0.5 ppm of O3 compromised lung development (Fanucchi et al., 2006). Similarly, Plopper et al. (2007) reported that ambient O3 alters lung development in children including reduced airway number, hyperplasia of bronchial epithelium and reorganization of the airway vascular and immune system. In the current study, epithelial cell number within the BAL of neonatal SJL/J pups increased significantly 72 h after exposure, suggesting delayed damage and alteration of the lung epithelium.
Furthermore, the mammalian respiratory and immune systems are complex with a portion of their development occurring postnatally. Both organ systems require a series of carefully coordinated events to complete development starting during embryogenesis and ending in childhood (Holladay and Smialowicz, 2000). Alterations to normal development in either of these systems, due to environmental insult or innate genetic changes, may affect organ function and individual susceptibility. For example, the extent of inflammatory responses to oxidant gases is related to the production of cytokines and chemokines by the immune system. After exposure to 95% oxygen, adult mice demonstrate little change in cytokine mRNA expression until lethality is imminent (78 h); whereas, neonates experience a delayed (7 days), yet acute, induction of tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6, important mediators of pulmonary inflammation (Johnston et al., 1997). Consequently, age-dependent, inflammatory variations seen among inbred mouse strains in our study may be associated with differential expression of genes related to immune system maturation and function.
The differences in O3 sensitivity between neonatal mice in our study were not the result of dose of O3 delivered to the lung, as the 18O data revealed similar ozone lung burden in the most sensitive and most resistant neonatal strains. When comparing dose in neonatal lungs to dose in adults, adult mice from three of the four strains examined absorbed more 18O than corresponding pups. Because pups were separated from their mothers and littermates during the O3 exposures in this study, it is possible that their breathing rates decreased due to a loss in body heat (Tattersall and Milsom, 2003), resulting in a lower absorption of 18O in pups in comparison to adults. However, it is not clear whether they lost significant body heat during the few hours of separation from their mother and littermates in this study as the 15- to 16-day-old pups had a thin fur coating.
The SJL strain was, again, the exception with neonates and adults absorbing similar amounts of 18O. The reasoning for this deviation in SJL mice is unclear. Genetic factors may control anatomical, physiological, or behavioral patterns that affect inhaled dose. We maintain, however, that dose is not a contributing factor to O3 sensitivity because there was no consistent pattern of ozone dose for resistance versus sensitive mouse strains. For example, similar levels of 18O were measured in the lungs of SJL pups (a sensitive strain) and 129 pups (a resistant strain). Likewise, the more resistant adult SJL mice had a much lower amount of absorbed 18O in comparison to equally resistant 129 adults, again, suggesting that dose is not a major factor in responses to inhaled O3.
In terms of gender, some epidemiological studies suggest that males are more susceptible to lung damage, experiencing much higher incidence rates for both postinjury and community-acquired pneumonia than females (Gannon et al., 2004; Gutierrez et al., 2006). Similarly, male guinea pigs exposed to O3 have more responsive airways than females (Schlesinger et al., 2002). Still other studies demonstrate that young women are more responsive to O3 than young men (Messineo and Adams, 1990), whereas O3 chamber studies with human subjects as well as chronic ambient O3 exposure studies reveal no significant gender differences in O3-exposed subjects, with males and females having similar decrements in pulmonary function postexposure (Ratto et al., 2006; Tager et al., 2005). Contrasting these studies, we found that adult female mice were generally more susceptible to O3-induced lung injury as well as lung inflammation. Further, lactating females experienced even greater responses for several strains. Interestingly, the sensitivity to O3 inhalation was strain dependent, and the specific strains that did experience increases in protein (AKR and DBA) were different than those with significantly larger total PMN counts (BL6 and Balb). Also, ANOVA tests determined that gender and exposure did interact to significantly alter total protein measurements (p ≤0.05; Table 3). Thus, although our data indicate a statistically significant but small strain-dependent gender effect in the response of inbred mice to O3-induced protein, our findings are not consistent with the literature on the role of gender in the pulmonary response to O3 inhalation.
Hormones can play a pivotal role in gender-dependent lung injury and inflammation. It is well known that estrogen may act to stimulate inflammation and that lactation is associated with proinflammatory hormone surges, like increased prolactin (Laycock and Wise, 1996) which might make the lactating female more responsive to inflammatory agents. This idea is supported by studies showing an increase in lung inflammation and injury in pregnant or lactating rats exposed to O3 (Gunnison and Hatch, 1999). Because increases in lung injury and inflammation were not observed in the present study for all inbred strains, our results suggest that there might be a gene-gender interaction.
As our two measured endpoints representing lung injury (protein) and lung inflammation (PMNs) were differentially altered by O3 inhalation from strain to strain, and from pup to adult within the same strain, we conclude that age-related differences in responses to O3 between strains are rooted in genetic background and may involve multiple genes in a complex matrix. Furthermore, linkage analyses in adult mice have identified significant QTLs on several chromosomes related to O3-induced inflammation, hyperpermeability, and acute lung injury (reviewed by Bauer et al., 2004) along with crucial candidate genes within these regions, such as TLR4, nuclear factor kappa B, TNF-α, and GSTM1 (Hollingsworth et al., 2004; Kleeberger et al., 2000; Prows et al., 1997).
As this project is the first to report exposure-, strain-, and age-dependent interactions between adult and neonatal responses to acute O3 exposure for our two endpoints, a deeper investigation must be performed to identify genes, define mechanisms, and examine signal transduction pathways relating the factors involved. Using O3-resistant and -sensitive strains identified in this study, linkage analysis using F2 populations of neonatal mice will allow us to verify previously identified QTLs and to potentially locate new QTLs associated with the age-specific pulmonary response to O3.
FUNDING
Environmental Protection Agency Ozone Grant (R830755); Science To Achieve Results (STAR) Graduate Fellowship Grant (FP916537) to E.V.; and New York University's National Institute of Environmental Health Sciences Center Grant (ES00260).
Acknowledgments
We would like to thank Kathy Seymour and Lori Horton for technical assistance.
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, USEPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and the policies of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Bauer AK, Malkinson AM, Kleeberger SR. Susceptibility to neoplastic and non-neoplastic pulmonary diseases in mice: Genetic similarities. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L685–L703. doi: 10.1152/ajplung.00223.2003. [DOI] [PubMed] [Google Scholar]
- Bermudez E, Ferng SF, Castro CE, Mustafa MG. DNA strand breaks caused by exposure to ozone and nitrogen dioxide. Environ. Res. 1999;81:72–80. doi: 10.1006/enrs.1999.3955. [DOI] [PubMed] [Google Scholar]
- Choo-Wing R, Nedrelow J, Homer R, Elias J, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L142–L150. doi: 10.1152/ajplung.00434.2006. [DOI] [PubMed] [Google Scholar]
- Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% Oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- Finkelstein JN, Johnston CJ. Enhanced sensitivity of the postnatal lung to environmental insults and oxidant stress. Pediatrics. 2004;113(Suppl. 4):1092–1096. [PubMed] [Google Scholar]
- Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J. Appl. Physiol. 2000;89:1804–1810. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- Frank L. Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med. 1991;11:463–494. doi: 10.1016/0891-5849(91)90062-8. [DOI] [PubMed] [Google Scholar]
- Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: Randomised, placebo-controlled crossover study. Lancet. 2004;363:119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- Gunnison AF, Hatch GE. O3-induced inflammation in prepregnant, pregnant, and lactating rats correlates with O3 dose estimated by 18O. Am. J. Physiol. 1999;276:L332–L340. doi: 10.1152/ajplung.1999.276.2.L332. [DOI] [PubMed] [Google Scholar]
- Gunnison AF, Finkelstein I, Weideman P, Su WY, Sobo M, Schlesinger RB. Age-dependent effect of ozone on pulmonary eicosanoid metabolism in rabbits and rats. Fundam. Appl. Toxicol. 1990;15:779–790. doi: 10.1016/0272-0590(90)90194-o. [DOI] [PubMed] [Google Scholar]
- Gunnison AF, Weideman PA, Sobo M, Koenig KL, Chen LC. Age-dependence of responses to acute ozone exposure in rats. Fundam. Appl. Toxicol. 1992;18:360–369. doi: 10.1016/0272-0590(92)90134-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, Hernandez I, Royo G, Martin-Hidalgo A. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J. Infect. 2006;53:166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000;108:463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth JW, II, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am. J. Respir. Crit. Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- Jobe AH. The new BPD: An arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Wright TW, Reed CK, Finkelstein JN. Comparison of adult and newborn pulmonary cytokine mRNA expression after hyperoxia. Exp. Lung Res. 1997;23:537–552. doi: 10.3109/01902149709039242. [DOI] [PubMed] [Google Scholar]
- Keeney S, Mathews M, Haque A, Schmalstieg F. Comparison of pulmonary neutrophils in the adult and neonatal rat after hyperoxia. Pediatr. Res. 1995;38:857–863. doi: 10.1203/00006450-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat. Genet. 1997;17:475–478. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR, Reddy S, Zhang LY, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: Role of toll-like receptor 4. Am. J. Respir. Cell. Mol. Biol. 2000;22:620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- Laycock JF, Wise PH. Essential Endocrinology. 3rd edn. New York: UP Oxford; 1996. [Google Scholar]
- McDonnell WF, Stewart PW, Smith MV, Pan WK, Pan J. Ozone-induced respiratory symptoms: Exposure-response models and association with lung function. Eur. Respir. J. 1999;14:845–853. doi: 10.1034/j.1399-3003.1999.14d21.x. [DOI] [PubMed] [Google Scholar]
- Messineo TD, Adams WC. Ozone inhalation effects in females varying widely in lung size: Comparison with males. J. Appl. Physiol. 1990;69:96–103. doi: 10.1152/jappl.1990.69.1.96. [DOI] [PubMed] [Google Scholar]
- Mudway I, Kelly F. Ozone and the lung: A sensitive issue. Mol. Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Ambient air pollution and respiratory emergency department visits. Epidemiology (Cambridge, Mass.) 2005;16:164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Smiley-Jewell SM, Miller LA, Fanucchi MV, Evans MJ, Buckpitt AR, Avdalovic M, Gershwin LJ, Joad JP, Kajekar R, et al. Asthma/allergic airways disease: Does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol. Pathol. 2007;35:97–110. doi: 10.1080/01926230601132030. [DOI] [PubMed] [Google Scholar]
- Prows DR, Shertzer HG, Daly MJ, Sidman CL, Leikauf GD. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997;17:471–474. doi: 10.1038/ng1297-471. [DOI] [PubMed] [Google Scholar]
- Ratto J, Wong H, Liu J, Fahy J, Boushey H, Solomon C, Balmes J. Effects of multiday exposure to ozone on airway inflammation as determined using sputum induction. Environ. Health Perspect. 2006;114:209–212. doi: 10.1289/ehp.8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, del Rio-Navarro BE, Ruiz-Navarro MX, Hatch G, Slade R, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am. J. Respir. Crit. Care Med. 2002;166:703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- Sarangapani R, Gentry PR, Covington TR, Teeguarden JG, Clewell HJ., III Evaluation of the potential impact of age- and gender-specific lung morphology and ventilation rate on the dosimetry of vapors. Inhal. Toxicol. 2003;15:987–1016. doi: 10.1080/08958370390226350. [DOI] [PubMed] [Google Scholar]
- Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am. J. Respir. Cell Mol. Biol. Off. 2004;31:69–77. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, Cohen M, Gordon T, Nadziejko C, Zelikoff JT, Sisco M, Regal JF, Menache MG. Ozone-induced modulation of airway hyperresponsiveness in guinea pigs. Res. Rep. (Health Effects Institute) 2002;109:1–40. discussion 41–51. [PubMed] [Google Scholar]
- Suh H, Bahadori T, Vallarino J, Spengler J. Criteria air pollutants and toxic air pollutants. Environ. Health Perspect. 2000;108(Suppl. 4):625–633. doi: 10.1289/ehp.00108s4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Kunzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology. 2005;16:751–759. doi: 10.1097/01.ede.0000183166.68809.b0. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, Milsom WK. Hypothermia-induced respiratory arrest and recovery in neonatal rats. Respir. Physiol. Neurobiol. 2003;137:29–40. doi: 10.1016/s1569-9048(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Yang IA, Fong KM, Zimmerman PV, Holgate ST, Holloway JW. Genetic susceptibility to the respiratory effects of air pollution. Thorax. 2008;63:555–563. doi: 10.1136/thx.2007.079426. [DOI] [PubMed] [Google Scholar]