Abstract
Manganese (Mn) is a redox-active element, and whereas its uptake, disposition, and toxicity in mammals may depend in part on its oxidation state, the proteins affecting manganese oxidation state and speciation in vivo are not well known. Studies have suggested that the oxidase protein ceruloplasmin (Cp) mediates iron and manganese oxidation and loading onto plasma transferrin (Tf), as well as cellular iron efflux. We hypothesized that ceruloplasmin may also affect the tissue distribution and eventual neurotoxicity of manganese. To test this, aceruloplasminemic versus wild-type mice were treated with a single i.p. 54Mn tracer dose, or elevated levels of manganese subchronically (0, 7.5, or 15 mg Mn/kg s.c., three doses per week for 4 weeks), and evaluated for transferrin-bound manganese, blood manganese partitioning, tissue manganese disposition, and levels of brain glutathione, thiobarbituric acid reactive substances (TBARS), and protein carbonyls as measures of oxidative stress, and open arena activity. Results show that ceruloplasmin does not play a role in the loading of manganese onto plasma transferrin in vivo, or in the partitioning of manganese between the plasma and cellular fractions of whole blood. Ceruloplasmin did, however, affect the retention of manganese in blood and its distribution to tissues, most notably kidney and to a lesser extent brain and lung. Results also indicate that ceruloplasmin interacted with chronic elevated manganese exposures to produce greater levels of brain oxidative stress. These results provide evidence that metal oxidase proteins play an important role in altering neurotoxicity arising from elevated manganese exposures.
Keywords: manganese, ceruloplasmin, transferrin, oxidation state, neurotoxicity
Manganese is an essential nutrient and serves numerous functions, including cofactor for a number of enzymes (Finley and Davis, 1999; Keen et al., 1999). However, epidemiologic studies have shown that elevated occupational exposures to manganese are associated with increased risk for Parkinsonian disturbances in adults (Barbeau, 1984; Corrigan et al., 1998; Gorell et al., 1997; Rybicki et al., 1993; Yamada et al., 1986), and that elevated exposures from environmental or dietary sources are associated with memory, learning, or behavioral impairment in young children (Bouchard et al., 2007; Collipp et al., 1983; Ericson et al., 2007; Takser et al., 2003; Wasserman et al., 2006; Woolf et al., 2002; Wright et al., 2006).
Manganese(II) exhibits chemistry similar to Ca(II) (Andersson et al., 1997) and Mg(II) (Vermote and Halford, 1992), whereas Mn(III) is similar to Fe(III) (Silva and Williams, 1991). The similarity between manganese and iron bioinorganic chemistry has been suggested to partly explain some mechanisms affecting the partitioning, transport, and toxicity of manganese in mammals (Abe et al., 2008; Crooks et al., 2007; Dickinson et al., 1996; Kwik-Uribe and Smith, 2006; Reaney and Smith, 2005). The Mn(III) oxidation state has been shown to act as a powerful pro-oxidant in vitro (Archibald and Tyree, 1987; HaMai and Bondy, 2004b), though the presence of significant amounts of Mn(III) in vivo and its role as a pro-oxidant in vivo has been difficult to detect (Gunter et al., 2005, 2006). Still, studies comparing the effects of Mn(II) versus Mn(III) exposures in cell and animal models have shown significant differences in both cell/tissue uptake of manganese and its toxicity, depending on the oxidation state of exposure (Reaney and Smith, 2005; Reaney et al., 2002).
The processes affecting manganese speciation in vivo are poorly known. It has been proposed that like iron, manganese in plasma is oxidized from the (II) to the (III) valence state by the oxidase protein ceruloplasmin (Cp) for loading onto plasma transferrin and transport to tissues (Davidsson et al., 1989; Gibbons et al., 1976). Ceruloplasmin, an abundant plasma protein that as holoceruloplasmin contains six copper atoms, has been shown to oxidize both iron and copper (Stoj and Kosman, 2003), as well as a variety of organic substrates (Frieden and Hsieh, 1976) in the process reducing dioxygen to water. Ceruloplasmin plays an important role in iron mobilization, including cellular iron uptake (Mukhopadhyay et al., 1998) and efflux (Jeong and David, 2003; Sarkar et al., 2003), though it is also well known as an acute phase protein (Cousins and Swerdel, 1985; Gitlin, 1988), and is generally considered to have antioxidant properties (Halliwell and Gutteridge, 1990; Oide et al., 2006).
A glycosylphosphatidylinositol (GPI)-anchored isoform of ceruloplasmin, which is produced by alternative splicing of the ceruloplasmin mRNA (Patel et al., 2000), occurs primarily in the central nervous system and kidney (Patel and David, 1997). Although the specific function of GPI-linked ceruloplasmin in these tissues is unclear, aceruplasminemic humans who are unable to make functional ceruloplasmin are known to accumulate iron in the liver and brain and exhibit neurodegeneration in the basal ganglia by age 45–55 (Xu et al., 2004). Studies in an aceruloplasminemic mouse model have also reported abnormal iron metabolism and neurotoxicity in aged animals (Harris et al., 1999; Patel et al., 2002).
In light of the similar bioinorganic chemistry of Mn(III) and Fe(III), the proposed role of plasma ceruloplasmin in mediating the oxidation of manganese and loading onto plasma transferrin (Davidsson et al., 1989; Gibbons et al., 1976), and the gross similarities in neurodegenerative conditions resulting from elevated manganese exposures and aceruloplasminia, we hypothesized that ceruloplasmin may play an important role in tissue manganese distribution and neurotoxicity. To test this hypothesis, we utilized an aceruloplasminemic mouse model to investigate the effect of ceruloplasmin on the partitioning of manganese in blood and its distribution to tissues, following either a single 54Mn tracer dose or subchronic (4 weeks) treatment with elevated levels of stable manganese. We also investigated the interaction of manganese and ceruloplasmin in the production of neurotoxic oxidative stress outcomes. Taken together, our findings indicate that circulating (plasma) ceruloplasmin does not play a role in the loading of manganese onto plasma transferrin, or in the general partitioning of manganese in whole blood. However, our results suggest that ceruloplasmin affects the tissue distribution of manganese and increases oxidative stress in the brain, though the exact mechanism(s) through which this occurs requires further elucidation.
MATERIALS AND METHODS
Experimental Design
Two studies were conducted: (1) Role of ceruloplasmin in 54Mn radiotracer partitioning and distribution. For this, 54Mn was administered to Cp−/− and Cp+/+ mice (n = 7–8 per genotype) to determine the role of ceruloplasmin on manganese loading onto plasma transferrin, and the partitioning of a single manganese dose in blood and its distribution to tissues; (2) Role of ceruloplasmin in the partitioning, distribution, and toxicity of subchronic elevated manganese exposures. For this, Cp−/− and Cp+/+ mice were exposed to different levels of stable manganese for 4 weeks (n = 5–7 per treatment) to determine the role of ceruloplasmin on the accumulation and toxicity of subchronic manganese exposure in brain and other tissues.
Animals
Mice with a targeted deletion in the ceruloplasmin gene were a generous gift of Z. L. Harris (Johns Hopkins University), and were generated as previously described (Harris et al., 1999). Cp−/− and Cp+/+ littermates that were the progeny of Cp+/− crosses were used in all experiments. The genetic identity of the mice was determined by amplification of tail DNA by PCR using the REDExtract-N-Amp tissue PCR kit (Sigma Aldrich, St Louis, MO). Oligonucleotide primers were used corresponding to exon 16 and either the end of exon 17 or the Neo gene. Mice were maintained in standard mouse chow and water ad libitum throughout the study. All procedures related to animal care conformed to the guidelines set forth in the Guide for the Care and Use of Laboratory (NRC, 1996) animals.
Procedures
Role of ceruloplasmin in 54Mn radiotracer partitioning and distribution.
54MnCl2(Perkin-Elmer/New England Nuclear, Boston, MA) was diluted to 100 μCi/ml (0.0129 μg Mn/ml) in sterile phosphate-buffered saline (PBS) immediately before injection, and 250 μCi/kg body weight (bw) was given via i.p. injection in a volume of 2.5 ml/kg bw. Mice were sacrificed by cervical dislocation 1-h postinjection. The 1-h period between 54Mn administration and sacrifice was selected because it was deemed sufficiently long for 54Mn to reach steady state regarding changes in redox state and ligand-speciation, which occurs within seconds to minutes of dosing (Reaney et al., 2002; Smith et al., 2007), whereas also providing sufficient time for plasma clearance of 54Mn and distribution to tissues.
Following decapitation, whole blood was collected into a heparinized container, followed by collection of lung, kidney, liver, spleen, and brain. Plasma for high-performance liquid chromatography (HPLC) analysis was separated from fresh whole blood by centrifugation (5000 × g for 10 min), followed by dialysis (Slyde-A-Lyzer, 10,000 molecular weight cut-off, Pierce, Rockford, IL) against HPLC buffer A (20mM tris, pH 8.6) for 1 h at room temperature. Blood collection, plasma separation, and dialysis were conducted under hypoxic conditions in an argon-purged glove bag to guard against postsacrifice oxidation of manganese and changes in its biomolecular speciation (Reaney et al., 2002).
Dialyzed plasma was separated by anion-exchange HPLC (Shodex QA-825 column, 8 × 75 mm, 12 μm particles) using a three-step linear salt gradient (30 ml of 0–0.08M NaCl, 30 ml of 0.08–0.10M NaCl, 30 ml of 0.10–0.50M NaCl in 20mM tris, pH 8.6) at a flow rate of 1 ml/min. Fractions corresponding to peaks and interpeak regions were collected, concentrated by lyophilization, and reconstituted in 1 ml of ultrapure water for radioactivity analysis. Sample radioactivity was measured by gamma counter (Beckman Gamma 4000, Beckman Instruments, Inc., Fullerton, CA).
Aliquots of collected HPLC fractions of separated plasma samples were prepared for gel electrophoresis by mixing with 4× NuPAGE sample-loading buffer and reduction with 10% (vol/vol) 0.75M dithiothreitol. Samples were separated on NuPAGE 10% bis-tris gels and bands were visualized by Coomassie blue staining.
Role of ceruloplasmin in the partitioning, distribution, and toxicity of subchronic elevated manganese exposures.
A 2 × 3 (genotype × Mn dose) design was used, in which Cp+/+ and Cp−/− mice (age 12–20 weeks) were treated with MnCl2 at nominal doses of 0, 7.5, or 15 mg Mn/kg bw/dose via subcutaneous injection, delivered three times a week for 4 weeks for cumulative doses of 0, 90, or 180 mg Mn/kg bw (n = 5–7 per treatment). Control mice (no Mn) were injected with equivalent volumes of vehicle saline. Mice were sacrificed one week after the last manganese dose via cervical dislocation and decapitation. Whole blood was collected into heparinized containers, and an aliquot taken for plasma separation as noted above. Liver, kidney, and brain were collected, and brain was dissected into cerebellum and remaining brain; the selection of cerebellum is substantiated by data showing that the cerebellum is affected by Mn exposure (Chen et al., 2002; Erikson et al., 2004). All samples were immediately frozen on dry ice, and stored at −70°C until further processing.
Blood and tissue manganese levels following subchronic exposure.
Total manganese concentrations were measured in collected tissues and total iron was measured in brain tissue only using trace metal clean methods and Zeeman graphite furnace atomic absorption spectrometry, as described previously (Witholt et al., 2000). Briefly, ∼100 mg of wet tissue or blood were dried and digested with ultrapure 16N HNO3 (Optima grade, Fisher Scientific, Fairlawn, NJ), evaporated, and redissolved in 1% HNO3. Manganese levels were determined using a Perkin-Elmer 4100ZL Zeeman graphite furnace AAS, with external standardization using certified SPEX standards. National Institutes of Standards and Technology SRM1577b (bovine liver) was used to evaluate procedural accuracy. The analytical detection limit for manganese and iron was 0.1 ng/ml.
Oxidative stress assays.
Brain oxidative stress was evaluated using tissue glutathione, lipid peroxidation (malondialdehyde, MDA), and protein oxidation (protein carbonyl) assays. Cerebellum and remaining brain samples were thawed and homogenized using a Teflon pestle in 10 volumes of ice-cold PBS containing 1mM ethylenediaminetetraacetic acid and 0.1% sodium dodecyl sulfate (SDS), followed by sonication (5- x 1-s pulses at 25 Hz, 3 mm probe) on ice. Homogenate was centrifuged (10,000 × g for 10 min) and the supernatant was aliquoted for protein (Bradford method), lipid peroxidation, and glutathione assays. For the latter, the aliquot was deproteinated by addition of 50 μl of homogenate to 100 μl of 10% trichloroacetic acid, followed by centrifugation (10,000 × g for 5 min.). All samples were stored frozen at −70°C until analysis.
Lipid peroxidation was assayed using a thiobarbituric acid (TBA)–based method. For this, 100 μl of homogenate was added to 500 μl of TBA reagent (0.5% TBA, 0.5% SDS, 10% acetic acid in ultrapure water), and the mixture was heated to 95°C for 1 h, cooled to room temperature, and centrifuged at 10,000 × g for 5 min. The absorbance of the supernatant was read at 532 nm and compared with a series of standard solutions of 1,1,3,3-tetramethoxypropane treated identically. Results were expressed as nmol MDA equivalents/mg tissue.
Total glutathione (oxidized and reduced, excluding nonprotein thiols) was determined using a 5,5′-dithiobis[2-nitrobenzoic acid] (Ellman's reagent) kinetic assay kit from Northwest Life Science Specialties (Vancouver, WA). The assay was performed following the manufacturer's instructions.
Tissue protein oxidation was determined by visualization of an antibody specific to 2,4-dinitrophenylhydrazone (DNPH) derivatized carbonyls, using a kit from Chemicon International (Temecula, CA). The assay was performed following the manufacturer's instructions, with the following modifications. After derivitization, water was added to normalize protein content across samples. Five microliters of sample solution containing 3.65 μg protein was dot-blotted onto a PVDF membrane, dried, and the membrane was incubated for 1 min in 50 ml of 1:1 ethanol/ethyl acetate to remove unreacted DNPH. The membrane was treated with primary and secondary antibodies provided by the manufacturer. Immunoreactive protein carbonyls were visualized using an ECL Plus detection kit (Amersham Pharmacia, Piscataway, NJ) and Typhoon fluorescence scanner (Amersham Biosciences), and quantified using ImageQuant software (Amersham Biosciences).
Open Arena Activity
Total ambulatory activity in an open enclosure was assessed 6 days following the final subchronic manganese dose using an automated video tracking system from San Diego Instruments (SMART System, San Diego, CA). Animals were placed individually in 60 × 60 x 30 cm open enclosure arenas in a darkened testing room and their movement was video-tracked for 40 min using a digital video camera under infrared light.
Statistics
Summary data are expressed as mean ± SE. The effect of ceruloplasmin genotype in the 54Mn radiotracer study was determined using t-tests. For the subchronic manganese exposure study, treatment and pair-wise comparisons were performed using two-way ANOVA and Fisher's least significant difference post hoc comparison tests, with ceruloplasmin genotype and manganese exposure as the main factors. Because exploratory statistical analyses revealed that manganese treatment effects in the subchronic manganese study differed by gender for some outcomes, ANOVA analyses were performed separately within gender. All data met the assumptions of normality and equality of variances across treatments. A p value of ≤ 0.05 was considered statistically significant for all tests. Analyses were conducted using JMP (Version 7, SAS Institute, Cary, NC) and SYSTAT (SPSS, Chicago, IL, 2000, 10th edition).
RESULTS
Ceruloplasmin Does Not Affect 54Mn Partitioning in Plasma
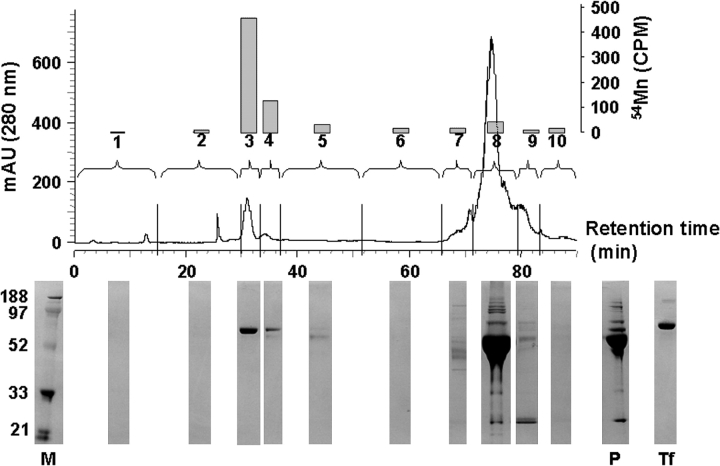
To determine whether plasma ceruloplasmin plays a role in the loading of manganese onto plasma transferrin, as is commonly believed (Critchfield and Keen, 1992; Davidsson et al., 1989; Gibbons et al., 1976), 54Mn was injected i.p. into wild-type (Cp+/+) and ceruloplasmin null (Cp−/−) mice, and plasma was collected at sacrifice 1 h later for HPLC analysis. Ten HPLC fractions were collected corresponding to detectable 280-nm absorbance peaks. A significant majority (∼70%) of the collected 54Mn radioactivity was associated with fractions containing only plasma transferrin (fractions 3 and 4, Fig. 1), whereas relatively little 54Mn was associated with the large plasma albumin fraction (fraction 8, Fig. 1a, b). Plasma transferrin eluted from the HPLC column in a major and minor peak (Fig. 1a), consistent with reports of HPLC separation of two sialated isoforms of transferrin (Turpeinen et al., 2001). The identity of plasma transferrin was verified by HPLC analysis of transferrin standards under identical conditions (data not shown), and by comparison with purified transferrin standard by SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 1).
FIG. 1.
Seventy percent of the collected 54Mn activity was associated with transferrin in HPLC fractions 3 and 4 (transferrin identified by comparison with transferrin standards analyzed by HPLC and SDS-PAGE). Shown is a representative anion-exchange HPLC chromatogram of dialyzed Cp+/+ mouse plasma. Collected fractions (#1–10) indicated by vertical hash marks on x-axis. 54Mn activity within collected fractions (total cpm, right y-axis) indicated as bars above the fraction number. HPLC used a three-step linear salt gradient (30 ml of 0–0.08M NaCl, 30 ml of 0.08–0.10M NaCl, 30 ml of 0.10–0.50M NaCl in 20mM tris, pH 8.65) at a flow rate of 1 ml/min, and absorbance was monitored at 280 nm. Below each fraction is the corresponding analyses with SDS-PAGE with Coomassie stain. Molecular weight marker (M), purified human transferrin (Tf), and undialyzed plasma (P) are also shown.
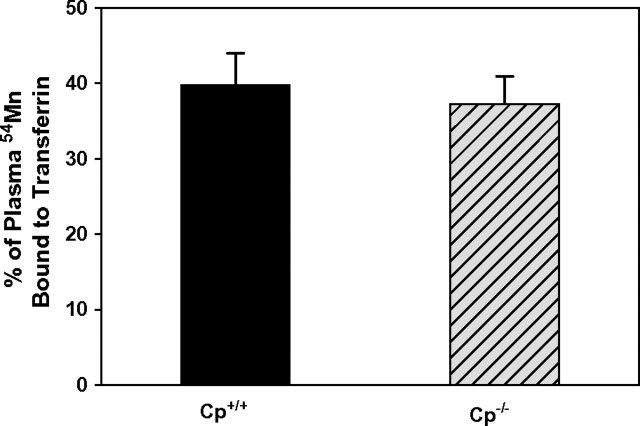
There was no difference between Cp+/+ and Cp−/− mice in the relative amount of plasma 54Mn bound to transferrin (Fig. 2). To determine this, the 54Mn bound to plasma transferrin (fractions 3 and 4, Fig. 1) was normalized to the 54Mn in plasma for Cp+/+ and Cp−/− mice. In Cp+/+ mice 37.3 ± 3.6% (mean ± SE, n = 7) of plasma 54Mn was associated with transferrin, and in Cp−/− mice it was 39.7 ± 4.2% (n = 7) (Fig. 2). Also, ceruloplasmin did not affect the relative amount of manganese bound to low molecular weight species in plasma (i.e., < 10 kDa), based on plasma 54Mn activity before and after dialysis through a 10 kDa molecular weight cut-off preceding HPLC analysis. Approximately 90% of plasma 54Mn was retained in the plasma after dialysis in both Cp+/+ and Cp−/− mice (Cp+/+ 90.3 ± 10.6%, n = 6; Cp−/− 93.4 ± 7.0%, n = 6). These data indicate that ceruloplasmin does not play a significant role in the loading of manganese onto plasma transferrin in vivo.
FIG. 2.
There is no difference (F1,13 = 0.20, p = 0.67) in transferrin-bound manganese between Cp+/+ and Cp−/− mice (mean ± SE, n = 7 per group). Data reflect cpm of transferrin-containing HPLC fractions (fractions 3 and 4 in Fig. 1) as a percent of cpm in all HPLC fractions collected.
Finally, to determine whether Cp+/+ and Cp−/− mice contained different amounts of plasma transferrin that may have confounded detecting differences in 54Mn-bound transferrin, if present, relative amounts of plasma transferrin in both genotypes were evaluated by SDS-PAGE of plasma and ImageQuant quantitation of Coomassie stained bands. Plasma transferrin levels were essentially identical in Cp+/+ and Cp−/− mice (Cp+/+ 0.62 ± 0.04 μg Tf/10 μg plasma protein, n = 6; Cp−/− 0.64 ± 0.07 μg Tf/10 μg plasma protein, n = 6). This result is consistent with previous reports that total plasma iron binding capacity (TIBC) is not significantly different between Cp+/+ and Cp−/− mice (Harris et al., 1999).
Cp−/− Mice Distribute 54Mn Differently than Cp+/+ Mice
Although the above results show that ceruloplasmin did not affect the binding of 54Mn onto plasma transferrin, it is possible that plasma (circulating) and GPI-anchored tissue ceruloplasmin might still affect the partitioning of manganese in whole blood (i.e., plasma versus cellular fractions) and the tissue distribution/retention of manganese. To test this hypothesis, blood (plasma and cellular fractions), liver, kidney, spleen, lungs, and brain were collected for analyses following 54Mn injection.
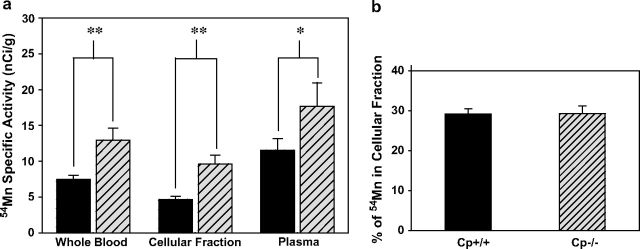
Results show that whole blood of Cp−/− mice contained nearly 75% more 54Mn specific activity compared with Cp+/+ mice (F1,11 = 11.2, p < 0.01), with similar 54Mn increases in independently measured cellular and plasma fractions of whole blood (plasma F1,11 = 5.47, p < 0.05; cellular fraction F1,12 = 14.8, p < 0.01) (Fig. 3a). However, Cp−/− mice also had significantly lower hematocrit values compared with wild-type mice (41 ± 2%, n = 5 vs. 47 ± 2%, n = 4, respectively; p < 0.05), consistent with findings of Cherukuri et al. (2004), who attributed it to lower red blood cell (RBC) volume rather than RBC number in ceruloplasmin null mice. To determine whether ceruloplasmin genotype affected the relative partitioning of manganese in blood, that is, the relative (%) of whole blood 54Mn contained in the cellular or plasma fractions, the 54Mn specific activity in each animal's cellular fraction of blood was adjusted by its hematocrit value and then normalized to the 54Mn activity in whole blood. The results show that the relative partitioning of 54Mn between plasma and cellular fractions of blood was identical in Cp+/+ and Cp−/− mice (cellular fraction 54Mn in Cp+/+ 29 ± 2%, n = 8; Cp−/− 29 ± 1%, n = 6) (Fig. 3b). Thus, although Cp−/− mice contained significantly more 54Mn activity in their blood, the partitioning of 54Mn between cellular and plasma fractions was not different between genotypes.
FIG. 3.
(a) Cp−/− mice (hatched bars) have more 54Mn than Cp+/+ mice (solid bars) in whole blood, cellular fraction, and plasma (mean ± SE, n = 6–7, *p < 0.05, **p < 0.01). Blood was collected and separated into plasma and cellular fractions 1 h after i.p. injection of 250 μCi 54Mn/kg bw. (b) There is no ceruloplasmin genotype difference in 54Mn partitioning between plasma and cellular fractions (mean ± SE, n = 6–8, p = 0.96) (% of Mn in the cellular fraction was calculated as hematocrit × specific activity of cellular fraction/specific activity of whole blood).
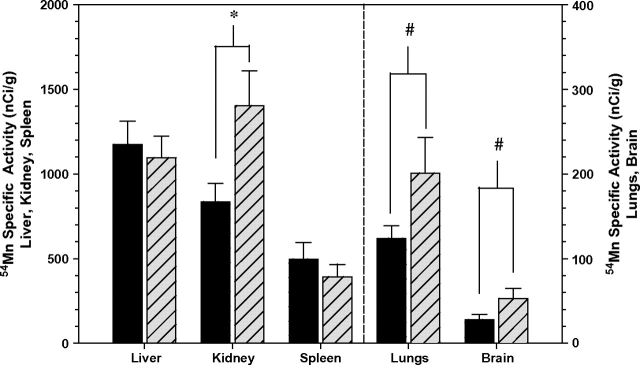
There was a marked range in 54Mn uptake among the measured tissues in both genotypes, in the order liver > kidney > spleen > lung > brain. In addition, Cp−/− mice accumulated a significant 38% more 54Mn activity in kidney (F1,12 = 5.95, p < 0.05), and marginally insignificantly more in lungs (34%, p < 0.10) and brain (52%, p < 0.10) than did Cp+/+ mice (Fig. 4, Table 1). The measured tissues accounted for 25% of the 54Mn injected in both Cp+/+ and Cp−/− mice.
FIG. 4.
54Mn in tissues of Cp+/+ mice (solid bars) and Cp−/− mice (hatched bars) (mean ± SE, n = 7, *p < 0.05, #p < 0.10). Cp−/− mice contained significantly more 54Mn in kidneys than Cp+/+, and Cp−/− mice trended toward more 54Mn in lungs and brain than Cp+/+ mice. Tissues were collected 1 h after i.p. injection of 250 μCi 54Mn/kg bw.
Cp+/+ and Cp−/− Mice Have Different Manganese Tissue Levels after Subchronic Manganese Treatment
The tissue partitioning and accumulation of 54Mn following a single i.p. dose shows that ceruloplasmin plays a role in manganese uptake/retention in blood and kidney, and to a lesser extent in brain and lung tissues. Because the tissue uptake/retention of manganese from prolonged elevated exposures might involve different processes and mechanisms than those affecting a single exposure to a radio-manganese tracer, we investigated whether ceruloplasmin mediates tissue accumulation of manganese in mice subchronically exposed to cumulative manganese doses of 0, 90, and 180 mg/kg given over 4 weeks (three doses per week via s.c. injection).
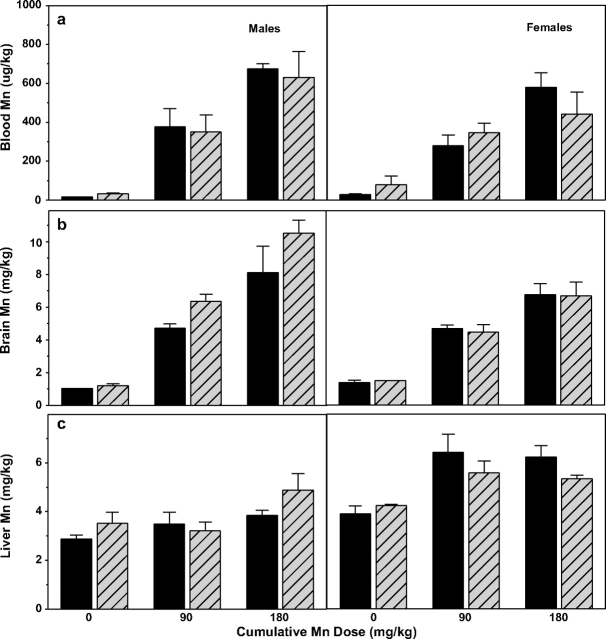
Both Cp+/+ and Cp−/− mice showed a clear dose-response increase in blood manganese levels (males and females), though there was no effect of ceruloplasmin genotype in either gender (Fig. 5a). For blood manganese, two-way ANOVA analyses of genotype × treatment (combined gender) showed a highly significant manganese treatment effect (F2,34 = 66.06, p < 0.001), but no ceruloplasmin genotype effect (F1,34 = 0.06, p = 0.81) or interaction (F2,34 = 0.51, p = 0.61). Similarly, when analyzed by treatment × gender, there remained a highly significant treatment effect (F2,34 = 73.05, p < 0.001), but no gender effect (F1,34 = 1.48, p = 0.23) or interaction (F2,34 = 1.21, p = 0.31).
FIG. 5.
Tissue manganese concentrations after 4 weeks of manganese treatment in Cp+/+ (solid bars) and Cp−/− mice (hatched bars); males (left panels) and females (right panels) are shown separately. (a) Blood manganese levels (μg Mn/kg wet weight) increase with manganese treatment (p < 0.001), but there are no significant differences between ceruloplasmin genotypes (p = 0.81) or genders (p = 0.23). (b) Brain manganese levels (mg Mn/kg dry weight) increase with treatment (p < 0.001), and are higher in male mice (p < 0.05), but there is no difference between ceruloplasmin genotypes (p = 0.17). (c) Liver manganese levels (mg Mn/kg dry weight) increase with treatment (p < 0.001), and are higher in females than males (p < 0.001); there is not a significant ceruloplasmin genotype effect (p = 0.60). All bars are mean ± SE (n = 3–4).
Brain manganese levels showed similar treatment-dependent increases (Fig. 5b), but brain manganese levels differed somewhat by gender (with males higher) and ceruloplasmin genotype (with Cp−/− male mice higher than Cp+/+). Two-way ANOVA analysis (treatment × gender) showed highly significant manganese treatment (F2,30 = 69.93, p < 0.001) and gender effects (F1,30 = 6.20, p < 0.05, males higher), and a borderline insignificant interaction (F2,30 = 3.12, p = 0.06). Male mice also showed a borderline insignificant genotype effect (F1,15 = 3.09, p = 0.10, Cp−/− higher). Comparisons across gender and ceruloplasmin genotype show that the gender difference is driven by the Cp−/− mice, because both male and female Cp+/+ mice possessed comparable brain levels. Notably, this borderline insignificant genotype effect on brain manganese levels in males is consistent with the trend for Cp−/− mice to uptake/retain more 54Mn tracer in brain, noted above.
We also measured brain iron levels to determine whether ceruloplasmin genotype or manganese treatment altered brain iron, which could serve as a confounder to manganese neurotoxicity. Results indicate that there was no difference in brain iron levels due to manganese treatment, genotype, or gender; in all cases brain iron levels were ∼50 ± 5 mg/kg (data not shown). This is consistent with results from Meyer et al. (2001), who reported no difference in brain iron levels in 12-week-old Cp−/− mice compared with Cp+/+. There are reports, however, that older (age 16 months) ceruloplasmin null mice manifest increased iron levels in the cerebellum and brainstem (Patel et al., 2002).
Mice across both genotypes showed some treatment-based increases in liver manganese levels, although the relative increases were notably smaller than observed for blood and brain (Fig. 4c). Two-way ANOVA analysis crossing gender and treatment showed a significant manganese treatment effect (F2,34 = 11.19, p < 0.001), gender effect (F1,34 = 37.88, p < 0.001), and interaction (F2,34 = 3.58, p < 0.05), with males having lower liver manganese levels than females in the manganese-treated groups. There was no significant ceruloplasmin genotype effect in either males or females. Unfortunately, kidney tissues from animals subchronically exposed to manganese were unavailable for comparative analysis.
Manganese Produces Oxidative Stress in Brain Tissue in Cp+/+ Mice, but not in Cp−/− Mice
It has been proposed that the pro-oxidant action of manganese is a primary mechanism of toxicity in the central nervous system (CNS) (Desole et al., 1994, 1995, 1997; Erikson et al., 2004; Taylor et al., 2006), and this effect likely depends upon levels of redox-active manganese in the brain (HaMai and Bondy, 2004b; Reaney et al., 2006). In light of suggestions that GPI-anchored ceruloplasmin in the brain may affect brain iron activity (Patel et al., 2002), we reasoned that the oxidative activity of manganese in the CNS also could be mediated by its interaction with ceruloplasmin.
To test the hypothesis that ceruloplasmin mediates the pro-oxidant action of manganese in brain, cerebellum and remaining brain tissue of Cp+/+ and Cp−/− mice subchronically treated with manganese was tested for three markers of oxidative stress: antioxidant levels in the form of glutathione, lipid peroxidation in the form of TBARS, and protein oxidation in the form of protein carbonyls. Given the above reported differences in tissue manganese levels between genders, and the well-established effect of gender on oxidative stress (C57BL6 mice studied in Ali et al., 2006; reviewed in Vina et al., 2003), the male and female mice were treated as separate groups. In all three assays of oxidative stress females showed no manganese treatment or ceruloplasmin genotype effects, whereas effects were observed in males. Therefore, only the males are shown in Figure 6.
FIG. 6.
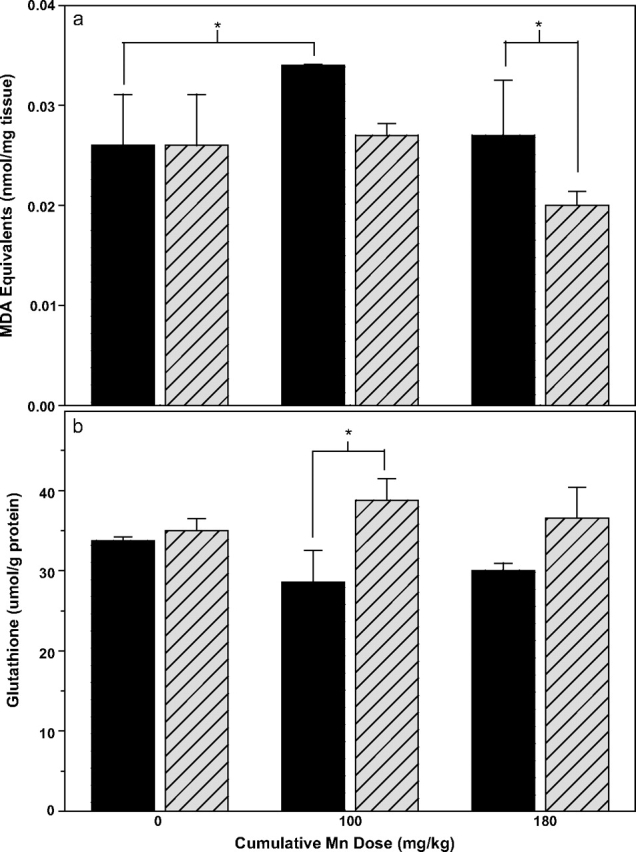
Lipid oxidation (a) and total (oxidized + reduced) glutathione levels (b) in cerebellum of male Cp+/+ (solid bars) and Cp−/− mice (hatched bars) after 4 weeks of manganese treatment (cumulative manganese doses shown on x-axis; mean ± SE, n = 3–4). (a) Two-way ANOVA analysis shows significant manganese treatment (p < 0.05) and ceruloplasmin genotype effects in lipid oxidation (p < 0.05), with a significant difference between genotypes for the 180 mg Mn/kg dose, and a significant difference between the 0 and 90 mg Mn/kg treatment groups for Cp+/+ mice (*p < 0.05, Fisher LSD post hoc test applied to Mn treatments within genotypes and to genotypes within Mn treatments). (b) Two-way ANOVA analysis shows a significant overall genotype effect (p < 0.05), but not an overall manganese treatment effect (p = 0.92). There was a significant difference between genotypes within the 90 mg Mn/kg treatment group (p < 0.05, Fisher LSD post hoc test).
There was a significant ceruloplasmin genotype effect on TBARS levels in the cerebellum of male mice (F1,15 = 6.34, p < 0.05), with Cp+/+ mice having higher TBARS than Cp−/− mice (Fig. 6a). There was also a significant manganese treatment effect (F2,15 = 4.19, p < 0.05), with the low manganese treatment group having higher TBARS than the other groups, but not a significant genotype × treatment interaction (F2,15 = 1.80, p = 0.22). The higher TBARS in cerebellum of Cp+/+ compared with Cp−/− male mice is most pronounced in the manganese-treated groups, as evidenced by the significant genotype effect in the 180 mg Mn/kg treatment group (p < 0.05, LSD post hoc test).
There was a significant effect of ceruloplasmin genotype (F1,17 = 6.49, p < 0.05), but not manganese treatment (F2,17 = 0.09, p = 0.92) on glutathione levels in the cerebellum, with male Cp+/+ mice showing lower glutathione levels than their Cp−/− counterparts in the presence of manganese treatment (Fig. 6b). Although there was not a significant genotype-treatment interaction (F2,17 = 1.29, p = 0.31), the genotype difference was particularly pronounced in the manganese-treated groups, with Fisher's LSD post hoc test showing Cp+/+ mice to have significantly lower glutathione levels than their Cp−/− counterparts (statistical significance reached in the 90 mg Mn/kg group).
There were no significant effects of ceruloplasmin genotype or manganese treatment on TBARS levels in remaining brain. Finally, there were no measurable effects of ceruloplasmin genotype or manganese treatment on cerebellum or remaining brain protein carbonyl levels (data not shown).
Open Arena Activity is Reduced by Manganese Treatment, but not Altered by Ceruloplasmin Genotype
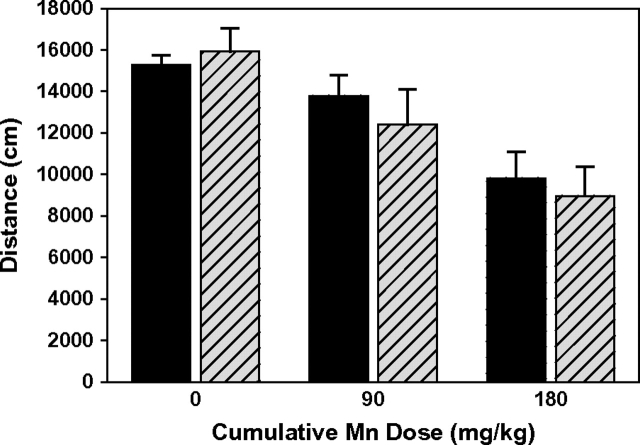
To test the hypothesis that ceruloplasmin mediates gross functional effects of manganese toxicity, the open arena activity of Cp+/+ and Cp−/− mice was measured a week after the final subchronic manganese dose. Previous studies have shown altered activity levels following elevated manganese exposures in adult and early postweanling rodents, and in nonhuman primates (Brenneman et al., 1999; Chandra et al., 1979; Golub et al., 2006; Tran et al., 2002). Here, the open arena activity of the male mice decreased with manganese treatment, whereas there was no effect of treatment in female mice (two-way ANOVA with treatment and gender: treatment F2,33 = 4.96, p < 0.05, gender F1,33 = 4.37, p < 0.05, interaction F2,33 = 4.91, p < 0.05). In male mice analyzed for manganese treatment and ceruloplasmin genotype effects, there was a significant treatment effect as noted above (F2,18 = 13.21, p < 0.001), but no genotype effect (F1,18 = 0.28, p = 0.60) or interaction (F2,18 = 0.39, p = 0.68) (Fig. 7).
FIG. 7.
Open arena activity of male Cp+/+ (solid bars) and Cp−/− mice (hatched bars) after 4 weeks of manganese treatment (mean ± SE, n = 3–4). Activity was measured 6 days after the final manganese treatment, and is reported as distance traveled over 35 min (from 5 to 40 min). There was a significant manganese treatment effect (p < 0.001), but no genotype effect (p = 0.60), based on two-way ANOVA.
DISCUSSION
Plasma ceruloplasmin has been suggested to play a major role in the oxidation of plasma manganese and loading onto plasma transferrin (Davidsson et al., 1989; Gibbons et al., 1976). As a multifunctional oxidase, ceruloplasmin has been shown capable in vitro of oxidizing a variety of substrates, e.g., Fe and Cu, as well as organic substrates, (Frieden and Hsieh, 1976; Stoj and Kosman, 2003), consistent with the potential for ceruloplasmin to oxidize Mn(II) to Mn(III) for loading onto transferrin. However, our results using 54Mn radiotracer indicate that ceruloplasmin is not necessary for the effective loading of manganese onto plasma transferrin in vivo. We observed that the transferrin-containing HPLC fraction contained ∼70% of the total 54Mn collected in the HPLC separated plasma fractions (Fig. 1), and ∼40% of the total 54Mn contained in the plasma fraction of whole blood prior to HPLC separation, with no difference between Cp+/+ and Cp−/− mice (Fig. 2).
Our results are consistent with in vitro studies suggesting that transferrin is the major transport ligand of manganese in plasma (Aschner and Aschner, 1990; Critchfield and Keen, 1992; Davidsson et al., 1989; Gibbons et al., 1976; Scheuhammer and Cherrian, 1985), though they contradict previous suggestions that ceruloplasmin facilitates the oxidation and binding of manganese to plasma transferrin (Davidsson et al., 1989; Gibbons et al., 1976). The study of Gibbons et al. (1976) concluded that ceruloplasmin mediated the loading of manganese onto plasma transferrin, based on the relatively slow loading of manganese onto transferrin in plasma in vitro, no measurable loading of manganese onto purified plasma transferrin, and slow loading of manganese onto purified transferrin in the presence of added ceruloplasmin in vitro. Manganese bound with transferrin is in the Mn(III) state, which forms a stable and much stronger complex with transferrin than Mn(II) (Aisen et al., 1969; Harris and Chen, 1994). Thus, our results suggest that the oxidation of Mn(II) in the absence of ceruloplasmin may occur spontaneously at the neutral pH of plasma (Reaney et al., 2002), and/or be due to the action of redundant oxidases; for example, haephestin expressed in enterocytes, kidney, spleen, and other tissues is in the same protein family and exhibits similar oxidase activity as ceruloplasmin (Petrak and Vyoral, 2005).
Though ceruloplasmin did not play a role in the binding of 54Mn to plasma transferrin, it did significantly affect the levels of 54Mn tracer contained in blood and its distribution to peripheral tissues. Ceruloplasmin null (Cp−/−) mice contained 75% more 54Mn in whole blood than their Cp+/+ counterparts (Fig. 3a). The increased blood 54Mn in Cp−/− mice was not due to differences in blood manganese partitioning in Cp−/− mice, because the partitioning of 54Mn between the cellular and plasma fractions was identical in both genotypes (70% in plasma, 30% in cells, Fig. 3b). This is consistent with the identical manganese-transferrin binding in Cp−/− and Cp+/+ mice, and further indicates that plasma ceruloplasmin does not affect the behavior of manganese within blood. It is also unlikely that metabolic iron status or iron deficiency in Cp−/− mice contributed to their increased blood 54Mn levels, because a number of studies have reported identical TIBC in Cp+/+ and Cp−/− mice (Cherukuri et al., 2004; Harris et al., 1999; Patel et al., 2002). This suggestion is further supported by our observation that Cp+/+ and Cp−/− mice contained comparable levels of plasma transferrin levels.
In contrast to the significant ceruloplasmin effect on blood 54Mn tracer levels measured closely following (1 h) exposure, we did not observe a ceruloplasmin genotype effect on total blood manganese concentrations in our subchronic manganese exposure study (Fig. 5). We attribute this difference to the fact that blood collection in the subchronic study occurred a week after the final exposure dose. These results suggest that the effect of ceruloplasmin to cause increased manganese retention in blood may only be evident when exposures are current or very recent, and not after sufficient time has elapsed because exposure to allow clearance of manganese from blood (Smith et al., 2007).
The greater 54Mn in kidneys but not livers of Cp−/− mice suggests a tissue-specific ceruloplasmin-related mechanism for manganese retention in kidney, rather than simply a reflection of greater 54Mn in blood (Fig. 4). Although the liver produces ceruloplasmin for secretion into the plasma, it has never been shown that the liver possesses tissue-resident GPI-linked ceruloplasmin. However, kidney epithelial cells lining the glomerulus and tubules have been shown to express GPI-linked ceruloplasmin, where it has been suggested to play a protective role as an antioxidant protein, oxidizing toxic ferrous iron in the renal filtrate to the nontoxic ferric form (Wiggins et al., 2006). It is also possible that kidney ceruloplasmin may reduce the reuptake of divalent transition metals such as iron and manganese, both of which are known to gain entry into cells via the divalent cation metal transporter (DMT1). Supporting this suggestion, studies with Belgrade rats, which are deficient in DMT1, have shown a marked decrease in kidney manganese (as opposed to an increase in liver manganese) compared with DMT1-competent Wistar rats following manganese exposure via intravenous injection (Chua and Morgan, 1997) or intratracheal installation (Heileg et al., 2006). We speculate that kidney ceruloplasmin oxidizes filterable Mn(II) to Mn(III) to reduce reuptake by the nephron tubules, and with our observation of increased kidney 54Mn of of Cp−/− mice. Unfortunately, kidney tissues from animals subchronically exposed to manganese were unavailable for comparative analysis.
The marginal increase in brain manganese in Cp−/− compared with Cp+/+ mice in both the 54Mn and subchronic manganese exposure studies (Figs. 4, 5) indicates a role of ceruloplasmin in the CNS disposition of manganese. It has been proposed that ceruloplasmin in the brain facilitates cellular iron efflux by oxidizing Fe(II) upon transport out of CNS cells through ferroportin (Harris et al., 1999; DeDomenico et al., 2007). Consistent with this suggestion, brain tissue of aceruloplasminemic mice and humans shows evidence of cellular iron accumulation with age (Patel et al., 2002; Xu et al., 2004). It is plausible that brain ceruloplasmin plays a similar role in the management of manganese in the CNS, accounting for the marginally increased brain manganese levels observed in this study. However, the fact that aceruloplasminemic humans and mice also accumulate high levels of iron in their livers, whereas there was no ceruloplasmin genotype effect on liver manganese levels in either the 54Mn or subchronic manganese exposure studies here, may underscore the importance of cell/tissue-specific differences in the management of iron and manganese (Review of systemic Fe homeostasis; Raoult, 2001; reviews of systemic manganese homeostasis; Keen et al., 2000; Roth, 2006).
We used several complimentary markers of tissue oxidative stress, glutathione levels, lipid peroxidation reflected in TBARS, and total protein carbonyl levels to determine whether ceruloplasmin modified oxidative stress resulting from subchronic manganese exposure. Our results indicate that ceruloplasmin interacted with elevated manganese exposures to produce heightened oxidative stress in the cerebellum, based on depletion of glutathione and a significant increase in lipid oxidation (TBARS) in manganese-exposed male Cp+/+ mice (Fig. 6). This effect was evident only in comparing ceruloplasmin genotypes within a manganese exposure group; there were no differences in these oxidative stress markers due to manganese exposure alone (across manganese exposures within the wild-type Cp+/+ groups), or due to ceruloplasmin genotype alone (control manganese Cp+/+ versus Cp−/− groups). Thus, subchronic manganese exposures produced oxidative stress in the cerebellum only in the presence of ceruloplasmin, and not in its absence. The lack of a standard dose-response relationship between oxidative stress outcomes and manganese treatment has been observed previously (Taylor et al., 2006), and could be explained by compensatory mechanisms operating at higher manganese doses.
The association of elevated manganese exposure with increased oxidative stress is well established from both in vitro and in vivo studies (Ali et al., 1995; Brouillet et al., 1993; Brown and Taylor, 1999; Dobson et al., 2004; Galvani et al., 1995; HaMai and Bondy, 2004a; Verity, 1999; Zheng and Zhao, 2001). Few studies, however, have been able to tease out the role of manganese redox activity in this effect, due to the challenges with evaluating it in biological systems in vivo (Gunter et al., 2005, 2006; Reaney et al., 2002). Our data suggest that the heightened cerebellar oxidative stress resulting from co-occuring ceruloplasmin and elevated manganese is produced via an extracellular ceruloplasmin—manganese redox mechanism, rather than simply the presence of elevated tissue manganese per se. This is supported by the fact that Cp+/+ mice exhibited higher oxidative stress in the cerebellum, but accumulated marginally lower brain manganese levels relative to their Cp−/− counterparts. Tissue iron also does not appear to have played a role in this effect, because there were no differences in brain iron levels between any of the ceruloplasmin genotype or manganese treatment groups.
In summary, our findings indicate that circulating (plasma) ceruloplasmin does not play a role in the loading of manganese onto plasma transferrin, or in the plasma/cellular partitioning of manganese in whole blood. However, our results suggest that ceruloplasmin does affect the tissue distribution of Mn and increase oxidative stress in the brain, though the exact mechanism(s) through which this occurs requires further elucidation. This suggestion may be contrary to the normally protective role of metal oxidase and reductase proteins in the metabolism of redox-active metals such as iron and copper under normal physiological conditions (Silva and Williams, 1991; Wiggins et al., 2006). Our results are consistent with previous studies (Chen et al., 2001; HaMai and Bondy, 2004; Reaney and Smith, 2005; Reaney et al., 2006) indicating that the oxidation state of manganese is an important mediator of its toxicity, and provide clear evidence that metal oxidase proteins play an important role in mediating neurotoxicity arising from elevated manganese exposures.
FUNDING
National Institute of Environmental Health Sciences grant (#010788).
Acknowledgments
We would like to thank Leah Harris for the generous availability of the mouse model, and William Mobley and Janice Valletta for assistance in 54Mn measurements. We would also like to thank Roberto Gwiazda and Cynthia Kern for their assistance.
References
- Abe K, Chiba Y, Nishida Y. Facile uptake of manganese(III) by apotransferrin: Possible origin of manganism. Z. Naturforsch. 2008;63:154–156. doi: 10.1515/znc-2008-1-228. [DOI] [PubMed] [Google Scholar]
- Aisen P, Aasa R, Redfield AG. The chromium, manganese, and cobalt complexes of transferrin. J. Biol. Chem. 1969;244:4628–4633. [PubMed] [Google Scholar]
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W., Jr Manganese-induced reactive oxygen species: Comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4:329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: Shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5:565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Andersson M, Malmendal A, Linse S, Ivarsson I, Forsen S, Svensson LA. Structural basis for the negative allostery between Ca(2+)- and Mg(2+)-binding in the intracellular Ca(2+)-receptor calbindin D9k. Protein Sci. 1997;6:1139–1147. doi: 10.1002/pro.5560060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch. Biochem. Biophys. 1987;256:638–650. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese transport across the blood-brain barrier: Relationship to iron homeostasis. Brain Res. Bull. 1990;24:857–860. doi: 10.1016/0361-9230(90)90152-p. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: Is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–487. [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Brown S, Taylor NL. Could mitochondrial dysfunction play a role in manganese toxicity? Environ. Toxicol. Pharmacol. 1999;7:49–57. doi: 10.1016/s1382-6689(98)00054-4. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Saxena DK. Manganese-induced behavioral dysfunction and its neurochemical mechanism in growing mice. J. Neurochem. 1979;33:1217–1221. doi: 10.1111/j.1471-4159.1979.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): Special reference to mitochondrial [Fe-S] containing enzymes. Toxicol. Appl. Pharmacol. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MT, Yiin SJ, Sheu JY, Huang YL. Brain lipid peroxidation and changes of trace metals in rats following chronic manganese chloride exposure. J. Toxicol. Environ. Health A. 2002;65:305–316. doi: 10.1080/15287390252800882. [DOI] [PubMed] [Google Scholar]
- Cherukuri S, Tripoulas NA, Nurko S, Fox PL. Anemia and impaired stress-induced erythropoiesis in aceruloplasminemic mice. Blood Cells Mol. Dis. 2004;33:346–355. doi: 10.1016/j.bcmd.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J. Comp. Physiol. B. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann. Nutr. Metab. 1983;27:488–494. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp. Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Cousins RJ, Swerdel MR. Ceruloplasmin and metallothionein induction by zinc and 13-cis-retinoic acid in rats with adjuvant inflammation. Proc. Soc. Exp. Biol. Med. 1985;179:168–172. doi: 10.3181/00379727-179-42080. [DOI] [PubMed] [Google Scholar]
- Critchfield JW, Keen CL. Manganese + 2 exhibits dynamic binding to multiple ligands in human plasma. Metabolism. 1992;41:1087–1092. doi: 10.1016/0026-0495(92)90290-q. [DOI] [PubMed] [Google Scholar]
- Crooks DR, Ghosh MC, Braun-Sommargren M, Rouault TA, Smith DR. Manganese targets m-aconitase and activates iron regulatory protein 2 in AF5 GABAergic cells. J. Neurosci. Res. 2007;85:1797–1809. doi: 10.1002/jnr.21321. [DOI] [PubMed] [Google Scholar]
- Davidsson L, Lonnerdal B, Sandstrom B, Kunz C, Keen CL. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J. Nutr. 1989;119:1461–1464. doi: 10.1093/jn/119.10.1461. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desole MS, Esposito G, Migheli R, Fresu L, Sircana S, Zangani D, Miele M, Miele E. Cellular defence mechanisms in the striatum of young and aged rats subchronically exposed to manganese. Neuropharmacology. 1995;34:289–295. doi: 10.1016/0028-3908(94)00140-n. [DOI] [PubMed] [Google Scholar]
- Desole MS, Esposito G, Migheli R, Sircana S, Delogu MR, Fresu L, Miele M, de Natale G, Miele E. Glutathione deficiency potentiates manganese toxicity in rat striatum and brainstem and in PC12 cells. Pharmacol. Res. 1997;36:285–292. doi: 10.1006/phrs.1997.0197. [DOI] [PubMed] [Google Scholar]
- Desole MS, Miele M, Esposito G, Migheli R, Fresu L, De Natale G, Miele E. Dopaminergic system activity and cellular defense mechanisms in the striatum and striatal synaptosomes of the rat subchronically exposed to manganese. Arch. Toxicol. 1994;68:566–570. doi: 10.1007/s002040050115. [DOI] [PubMed] [Google Scholar]
- Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J. Lab. Clin. Med. 1996;128:270–278. doi: 10.1016/s0022-2143(96)90028-1. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci. Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW, Davis CD. Manganese deficiency and toxicity: Are high or low dietary amounts of manganese cause for concern? Biofactors. 1999;10:15–24. doi: 10.1002/biof.5520100102. [DOI] [PubMed] [Google Scholar]
- Frieden E, Hsieh HS. The biological role of ceruloplasmin and its oxidase activity. Adv. Exp. Med. Biol. 1976;74:505–529. doi: 10.1007/978-1-4684-3270-1_43. [DOI] [PubMed] [Google Scholar]
- Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharmacol. 1995;293:377–383. doi: 10.1016/0926-6917(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Gibbons RA, Dixon SN, Hallis K, Russell AM, Sansom BF, Symonds HW. Manganese metabolism in cows and goats. Biochim. Biophys. Acta. 1976;444:1–10. doi: 10.1016/0304-4165(76)90218-x. [DOI] [PubMed] [Google Scholar]
- Gitlin JD. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J. Biol. Chem. 1988;263:6281–6287. [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol. Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gunter KK, Aschner M, Miller LM, Eliseev R, Salter J, Anderson K, Hammond S, Gunter TE. Determining the oxidation states of manganese in PC12 and nerve growth factor-induced PC12 cells. Free Radic. Biol. Med. 2005;39:164–181. doi: 10.1016/j.freeradbiomed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: A search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Pro- or anti-oxidant manganese: A suggested mechanism for reconciliation. Neurochem. Int. 2004;44:223–229. doi: 10.1016/s0197-0186(03)00152-9. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann. N. Y. Acad. Sci. 2004a;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Pro- or anti-oxidant manganese: A suggested mechanism for reconciliation. Neurochem. Int. 2004b;44:223–229. doi: 10.1016/s0197-0186(03)00152-9. [DOI] [PubMed] [Google Scholar]
- Harris WR, Chen Y. Electron paramagnetic resonance and difference ultraviolet studies of Mn2+ binding to serum transferrin. J. Inorg. Biochem. 1994;54:1–19. doi: 10.1016/0162-0134(94)85119-0. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1247–L1259. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- Jeong SY, David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J. Biol. Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Clegg MS. Manganese metabolism in animals and humans including the toxicity of manganese. Met. Ions Biol. Syst. 2000;37:89–121. [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20:213–223. [PubMed] [Google Scholar]
- Kwik-Uribe C, Smith DR. Temporal responses in the disruption of iron regulation by manganese. J. Neurosci. Res. 2006;83:1601–1610. doi: 10.1002/jnr.20836. [DOI] [PubMed] [Google Scholar]
- Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J. Biol. Chem. 2001;276:36857–36861. doi: 10.1074/jbc.M105361200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay CK, Attieh ZK, Fox PL. Role of ceruloplasmin in cellular iron uptake. Science. 1998;279:714–717. doi: 10.1126/science.279.5351.714. [DOI] [PubMed] [Google Scholar]
- NRC. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Research Council, National Academy Press; 1996. [Google Scholar]
- Oide T, Yoshida K, Kaneko K, Ohta M, Arima K. Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol. Appl. Neurobiol. 2006;32:170–176. doi: 10.1111/j.1365-2990.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J. Biol. Chem. 2000;275:4305–4310. doi: 10.1074/jbc.275.6.4305. [DOI] [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak J, Vyoral D. Hephaestin—A ferroxidase of cellular iron export. Int. J. Biochem. Cell. Biol. 2005;37:1173–1178. doi: 10.1016/j.biocel.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol. Sci. 2006;93:114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Kwik-Uribe CL, Smith DR. Manganese oxidation state and its implications for toxicity. Chem. Res. Toxicol. 2002;15:1119–1126. doi: 10.1021/tx025525e. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Smith DR. Manganese oxidation state mediates toxicity in PC12 cells. Toxicol. Appl. Pharmacol. 2005;205:271–281. doi: 10.1016/j.taap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- Rouault TA. Systemic iron metabolism: A review and implications for brain iron metabolism. Pediatr. Neurol. 2001;25:130–137. doi: 10.1016/s0887-8994(01)00260-0. [DOI] [PubMed] [Google Scholar]
- Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinsons disease mortality and the industrial use of heavy metals in Michigan. Mov. Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Seshadri V, Tripoulas NA, Ketterer ME, Fox PL. Role of ceruloplasmin in macrophage iron efflux during hypoxia. J. Biol. Chem. 2003;278:44018–44024. doi: 10.1074/jbc.M304926200. [DOI] [PubMed] [Google Scholar]
- Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim. Biophys. Acta. 1985;840:163–169. doi: 10.1016/0304-4165(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Silva JRRF d., Williams RJP. The Biological Chemistry of the Elements: the Inorganic Chemistry of Life. New York: Oxford University Press; 1991. [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, Lucchini R. Biomarkers of Mn exposure in humans. Am. J. Ind. Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Stoj C, Kosman DJ. Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: Implication for function. FEBS Lett. 2003;554:422–426. doi: 10.1016/s0014-5793(03)01218-3. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Erikson KM, Dobson AW, Fitsanakis VA, Dorman DC, Aschner M. Effects of inhaled manganese on biomarkers of oxidative stress in the rat brain. Neurotoxicology. 2006;27:788–797. doi: 10.1016/j.neuro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Lonnerdal B, Le L, Parker M, Chicz-Demet A, Crinella FM. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology. 2002;23:645–651. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Turpeinen U, Methuen T, Alfthan H, Laitinen K, Salaspuro M, Stenman UH. Comparison of HPLC and small column (CDTect) methods for disialotransferrin. Clin. Chem. 2001;47:1782–1787. [PubMed] [Google Scholar]
- Verity MA. Manganese neurotoxicity: A mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- Vermote CL, Halford SE. EcoRV restriction endonuclease: Communication between catalytic metal ions and DNA recognition. Biochemistry. 1992;31:6082–6089. doi: 10.1021/bi00141a018. [DOI] [PubMed] [Google Scholar]
- Vina J, Sastre J, Pallardo F, Borras C. Mitochondrial theory of aging: Importance to explain why females live longer than males. Antioxid. Redox Signal. 2003;5:549–556. doi: 10.1089/152308603770310194. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JE, Goyal M, Wharram BL, Wiggins RC. Antioxidant ceruloplasmin is expressed by glomerular parietal epithelial cells and secreted into urine in association with glomerular aging and high-calorie diet. J. Am. Soc. Nephrol. 2006;17:1382–1387. doi: 10.1681/ASN.2005111239. [DOI] [PubMed] [Google Scholar]
- Witholt R, Gwiazda RH, Smith DR. The neurobehavioral effects of subchronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol. Teratol. 2000;22:851–861. doi: 10.1016/s0892-0362(00)00108-2. [DOI] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Xu X, Pin S, Gathinji M, Fuchs R, Harris ZL. Aceruloplasminemia: An inherited neurodegenerative disease with impairment of iron homeostasis. Ann. N. Y. Acad. Sci. 2004;1012:299–305. doi: 10.1196/annals.1306.024. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]