Abstract
OBJECTIVE: To evaluate the independent and joint associations among cardiorespiratory fitness (CRF), body mass index, and risk of mortality from any cause among women with impaired fasting glucose (IFG) or undiagnosed diabetes mellitus (DM).
PATIENTS AND METHODS: Female patients (N=3044; mean age, 47.4 years) with IFG or undiagnosed DM completed a maximal exercise treadmill test (between January 26, 1971, and March 21, 2001). The women had no history of a cardiovascular disease event or diagnosed DM at baseline. Cardiorespiratory fitness was defined categorically as low (bottom 20%), moderate (middle 40%), or high (upper 40%) according to previously published Aerobics Center Longitudinal Study guidelines. Body mass index was calculated as the weight in kilograms divided by the height in meters squared (kg/m2).
RESULTS: During a 16-year follow-up period, 171 deaths occurred. There was an inverse association between CRF and all-cause mortality risk. Women with moderate or high CRF were at lower risk of mortality (moderate CRF, 35% lower; high CRF, 36% lower; Ptrend=.03) than those with low CRF. An exercise capacity lower than 7 metabolic equivalents was associated with a 1.5-fold higher risk of death than an exercise capacity of 9 metabolic equivalents or higher (Ptrend=.05). The multivariate adjusted hazard ratios (HRs), including adjustments for CRF, were higher for heavier patients than for patients of normal weight (overweight patients: HR, 0.86; 95% confidence interval, 0.57-1.30; obese patients: HR, 1.19; 95% confidence interval, 0.70-2.03; Ptrend=.84). Combined analyses showed that women who were overweight or obese and unfit (low CRF) were at more than twice the risk of death than women who were of normal weight and fit (moderate or high CRF).
CONCLUSION: Cardiorespiratory fitness, not body mass index, is a significant predictor of all-cause mortality among women with IFG or undiagnosed DM. Assessing CRF levels provides important prognostic information independent of traditional risk factors.
Cardiorespiratory fitness, not body mass index, is a significant predictor of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus; assessing cardiorespiratory fitness levels provides important prognostic information independent of traditional risk factors. An exercise capacity lower than 7 metabolic equivalents was associated with a 1.5-fold higher risk of death.
ACLS = Aerobics Center Longitudinal Study; BMI = body mass index; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; DM = diabetes mellitus; HR = hazard ratio; IFG = impaired fasting glucose; MET = metabolic equivalent
Impaired fasting glucose (IFG) has been associated with an elevated risk of premature mortality.1,2 Recently, greater attention has been directed toward IFG in efforts to reduce the development of diabetes mellitus (DM) and cardiovascular disease (CVD). In 2003, the American Diabetes Association recommended that the definition of IFG be expanded to include fasting glucose concentrations between 100 mg/dL and 125 mg/dL (to convert to mmol/L, multiply by 0.0555), as opposed to the previous concentrations of 110 mg/dL to 125 mg/dL.3 In 2002, 54 million people in the United States aged 20 years or older had IFG, representing 26% of the total US population; this number would be higher if the more stringent definition were used.4 In 2005, 20.8 million people (7% of the total US population) in the same age group had DM.4 Half of these cases of DM were expected to be undiagnosed and asymptomatic.5 The risk of premature CVD and death is 2 to 4 times higher for persons with DM than for those of equivalent age without DM.6,7 Therefore, the detection and treatment of CVD risk factors may be particularly important for preventing CVD among persons with asymptomatic IFG and DM.
A low cardiorespiratory fitness (CRF) level is a predictor of all-cause mortality among men with type 2 DM.8 Recent studies9,10 have also reported a strong and independent association between CRF and mortality among men with DM in all body mass index (BMI) and body fatness groups.11 However, most previous studies have focused on the association between CRF and mortality among either men only or men and women who are sedentary but apparently healthy.8,9,12
For editorial comment, see page 771
There is a strong independent association between obesity and all-cause mortality among both men and women.13-17 Most studies have found that this association is J-shaped or U-shaped. Like CRF studies, most obesity studies have focused on the association between obesity and mortality in women, men, or both men and women in the general population, regardless of any disease state.13-16 Few studies have focused on persons with IFG and undiagnosed DM, especially women. To our knowledge, no previous study has concurrently evaluated the association between CRF, overweight or obesity status as reflected by BMI, and death among women with IFG. Therefore, the primary aim of this study was to evaluate the association between CRF and risk of all-cause mortality among women with IFG or undiagnosed DM.
PATIENTS AND METHODS
The Aerobics Center Longitudinal Study (ACLS) is a prospective epidemiological investigation of patients who underwent an extensive clinical examination. We reviewed the records of 3044 female patients aged 20 to 79 years (mean, 47.4 years) who had IFG or undiagnosed DM and had completed a preventive medical evaluation for determination of baseline values between January 26, 1971, and March 21, 2001. Impaired fasting glucose was defined as a fasting glucose concentration of 100 mg/dL to 125.9 mg/dL,18 and undiagnosed DM was defined as a fasting glucose concentration of 126 mg/dL or higher among patients with no history of DM and no current therapy with hypoglycemic medication. At baseline, no patients had experienced CVD (myocardial infarction or stroke), and all were able to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate. Details of the study design and the characteristics of the cohort have been reported previously.6,9,19 Most patients were white, well-educated, and from the middle or upper socioeconomic strata. The Institutional Review Board of the Cooper Institute reviewed and approved the study protocol annually.
Clinical Examination
After giving written informed consent, patients underwent a baseline clinical examination that included analyses of fasting blood chemistry, elicitation of personal and family health history, anthropometry, determination of resting blood pressure, and a maximal graded exercise test. Technicians administered all procedures according to a standard manual of operations. Height was measured with a stadiometer, and weight was measured with a standard physician's scale. Body mass index was calculated as weight in kilograms divided by height in meters squared (kg/m2). Patients who were underweight (BMI <18.5 kg/m2) were excluded from the study. Patients were placed into BMI groups according to standard clinical definitions: normal weight, less than 25.0 kg/m2; overweight, 25.0 to 29.9 kg/m2; and obese, 30.0 kg/m2 or more. Resting blood pressure was determined by auscultation and was recorded as the first and fifth Korotkoff sounds.20 Patients were considered to have hypertension if they had a history of this diagnosis by a physician or if they had a resting systolic pressure of 140 mm Hg or higher or a diastolic pressure of 90 mm Hg or higher. Standardized automated bioassays were used to determine serum concentrations of lipids and glucose. Hypercholesterolemia was defined as a total cholesterol concentration of 240 mg/dL (to convert to mmol/L, multiply by 0.0259) or higher. Information about smoking habits (current smoker or not), alcohol intake (number of drinks per week), and family history of DM was obtained with a standardized questionnaire.
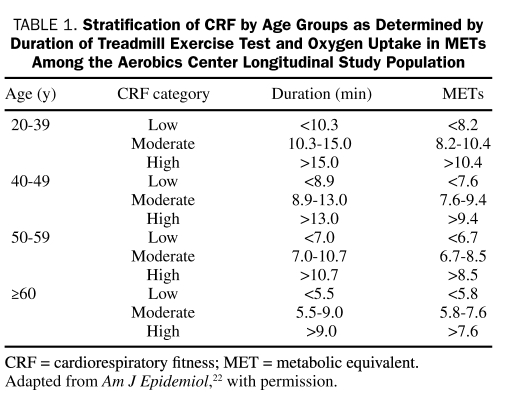
Cardiorespiratory fitness was defined as low, moderate, or high according to previously published age-specific distributions of maximal exercise duration from the ACLS population21,22 (Table 1). These categories are determined by the time (in minutes) on a treadmill exercise test and by oxygen uptake (in metabolic equivalents [METs]). The test of symptom-limited maximal treadmill exercise was performed according to a modified Balke protocol.21,23 Patients began walking at a speed of 88 m/min without elevation. After the first minute, the elevation was increased to 2%; thereafter, the elevation was increased by 1% per minute until the 25th minute. After 25 minutes, the elevation did not change but the speed was increased by 5.4 m/min each minute until the test end point. Patients were encouraged to give maximal effort. The test end point was volitional exhaustion or termination by the physician for medical reasons. Exercise duration on this protocol is strongly positively correlated (r=0.94) with measured maximal oxygen uptake.24 One MET is equal to an uptake of 3.5 mL of oxygen per kilogram of body weight per minute, as calculated from the final treadmill speed and grade.25
TABLE 1.
Stratification of CRF by Age Groups as Determined by Duration of Treadmill Exercise Test and Oxygen Uptake in METs Among the Aerobics Center Longitudinal Study Population
A Framingham risk score was computed for each patient by using the sex-specific algorithm for total cholesterol concentration.26 Risk points were assigned to risk factor categories and were then summed. The point total was used to assign 10-year probabilities of primary CVD events; the probabilities were lower than 10% for the low-risk group, 10% to 19% for the intermediate-risk group, and 20% or higher for the high-risk group.27
Mortality Surveillance
Patients were followed up from the date of their baseline examination to the date of their death or, for survivors, until December 31, 2003. Vital status was ascertained primarily by using the National Death Index. When this method is used, more than 95% of mortality follow-up is complete. The underlying cause of death was coded according to the International Classification of Diseases, Ninth Revision (CVD, 390-449.9) before 1999 and according to the Tenth Revision (CVD, I00-I78) from 1999 to 2003. We restricted the analyses to women who had been followed up for least 1 year so that we could eliminate any possibility of increased risk due to undetected disease at baseline.
Statistical Analyses
Descriptive statistics were used to summarize baseline characteristics on the basis of vital status. Cox proportional hazards regression analysis was used to estimate rates (per 10,000 woman-years), hazard ratios (HRs), and 95% confidence intervals (CIs) for all-cause mortality according to exposure categories. Unless otherwise noted, all multivariable models included baseline age (years), examination year, current smoker (yes/no), alcohol intake (≥5 drinks per week or not), hypertension (yes/no), hypercholesterolemia (yes/no), and family history of DM (present or not). Tests of linear trends across exposure categories were computed by using ordinal scoring. To assess the shape of the CRF-mortality curve or the BMI-mortality curve, we examined the risk of overall death across increments of METs or BMI. The proportional hazards assumption was examined by comparing the cumulative hazard plots grouped on exposure; no appreciable violations were noted. All statistical analyses were performed with SAS software (version 9.1; SAS Institute, Cary, NC). P values are 2-sided; statistical significance was assigned at the level of P<.05.
RESULTS
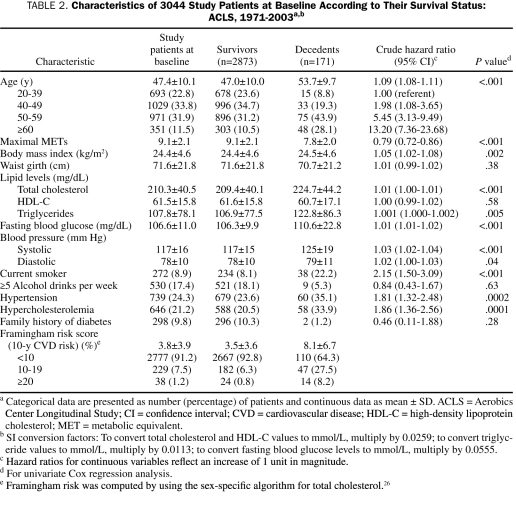
During a mean follow-up period of 15.6 years, 171 deaths (48 due to CVD) occurred. On the basis of the Framingham risk scores, more than 90% of women in the study were members of the low-risk group. Of the entire group of women, 66.4% were of normal weight, 22.2% were overweight, and 11.4% were obese. Compared with survivors, decedents tended to be older, had a lower level of CRF, and were more likely to be current smokers or to have hypertension or hypercholesterolemia (Table 2). Univariate Cox regression analyses showed that both CRF and BMI were associated with a higher risk of mortality (Table 2). The risk of dying was more than 10 times higher for women aged 60 years or older than for women aged 20 to 39 years (Table 2).
TABLE 2.
Characteristics of 3044 Study Patients at Baseline According to Their Survival Status: ACLS, 1971-2003a,b
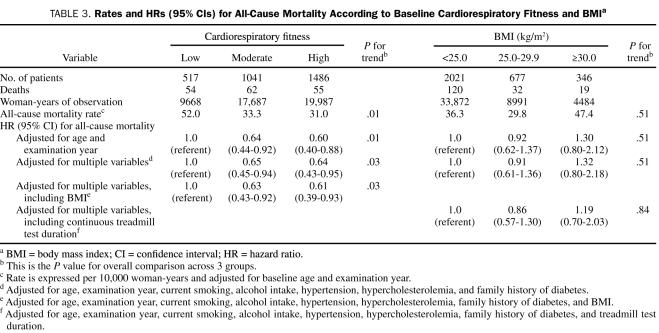
Table 3 shows the independent association between CRF, BMI, and all-cause mortality. The all-cause death rate (adjusted for age and examination year) per 10,000 woman-years was 52.0% for women in the low CRF group, 33.3% for those in the moderate CRF group, and 31.0% for those in the high CRF group (Ptrend=.01). After the analyses were adjusted for covariables (age, examination year, current smoking, alcohol intake, hypertension, hypercholesterolemia, and family history of DM), the risk of death was 35% lower for the moderate CRF group and 36% lower for the high CRF group than for the low CRF group (Ptrend=.03). The inverse association was slightly attenuated but remained statistically significant after additional adjustments for BMI (Ptrend=.03). The multivariable-adjusted HR (adjusted for age, examination year, current smoking, alcohol intake, hypertension, hypercholesterolemia, and family history of DM) for all-cause mortality was 1.00 for women with a BMI lower than 25.0 kg/m2, 0.91 for women with a BMI of 25.0 to 29.9 kg/m2, and 1.32 for women with a BMI of 30.0 kg/m2 or higher (Ptrend=.51), respectively. After an additional adjustment for baseline treadmill test duration, the association between BMI and mortality risk remained nonsignificant (Ptrend=.84).
TABLE 3.
Rates and HRs (95% CIs) for All-Cause Mortality According to Baseline Cardiorespiratory Fitness and BMIa
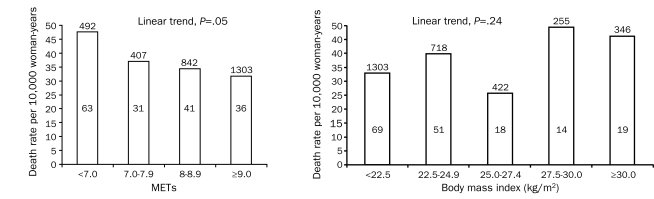
To further examine the dose-response characteristics of the association among CRF, BMI, and risk of all-cause mortality, we computed the age-adjusted death rates (per 10,000 woman-years) for CRF categories defined by 1-MET increments (Figure, left) and for BMI categories (2.5 units; Figure, right). An exercise capacity lower than 7 METs was associated with a 1.5-fold higher death risk than an exercise capacity of 9 METs or higher (Ptrend=.05). The HR across the incremental METs group is 1.00; the HRs (95% CIs) for the categories of exercise capacities are as follows: 0.78 (95% CI, 0.51-1.20) for the group with an exercise capacity of 7.0 to 7.9 METs, 0.72 (95% CI, 0.49-1.08) for the group with an exercise capacity of 8.0 to 8.9 METs, and 0.67 (95% CI, 0.43-1.03) for the group with an exercise capacity of 9.0 METs or higher. No association trend was observed between BMI and death (Ptrend=.24).
FIGURE.
Age-adjusted all-cause mortality rates (per 10,000 woman-years) by cardiorespiratory fitness levels quantified in increments of 1 metabolic equivalent (MET) as determined by a maximal treadmill exercise test (left) and by body mass index levels in 2.5 kg/m2 increments (right) in women. Number of patients at risk is located above each associated bar, and number of deaths is located within each associated bar.
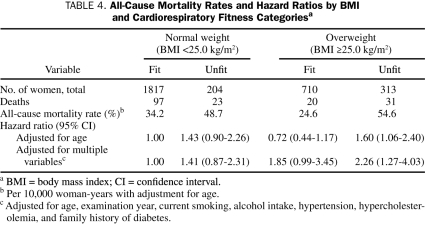
Finally, we examined the combined effect of CRF and BMI (Table 4) and found statistical evidence of an interaction between CRF and BMI in predicting all-cause mortality (χ2=23.86; P<.001). The death rates among unfit women with higher BMI (≥25 kg/m2) were more than twice those among fit women with higher BMI. When fit women with normal BMI were used as the comparator group, the highest mortality risk was found for unfit women with higher BMI (HR, 2.26; 95% CI, 1.27-4.03).
TABLE 4.
All-Cause Mortality Rates and Hazard Ratios by BMI and Cardiorespiratory Fitness Categoriesa
DISCUSSION
We addressed the single and joint associations among CRF, BMI, and the risk of mortality among women with IFG and undiagnosed DM. Low CRF was associated with a significantly higher risk of all-cause mortality, and this inverse association remained significant after the analyses were adjusted for age, year of examination, smoking status, alcohol intake, prevalence of hypertension, hypercholesterolemia, family history of DM, and BMI at baseline. No association was observed between being overweight or obese and overall deaths. The protective effect of CRF held true for overweight or obese women, whereas the death rate among unfit women with higher BMI (≥25 kg/m2) was more than twice that among fit women with higher BMI.
We have found no previous reports of an association between CRF and risk of all-cause mortality among women with IFG or undiagnosed DM. Most of the few published studies focused on patients with abnormal glucose metabolism or on men only or a combination of women and men. Wei et al8 examined this association among 1263 middle-aged men and found that the adjusted risk of all-cause mortality among men in the low CRF group was 2.1-fold higher (95% CI, 1.5- to 2.9-fold) than that of physically active men and that the adjusted risk of all-cause mortality among men classifying themselves as physically inactive was 1.7-fold higher (95% CI, 1.2-fold to 2.3-fold) than that among men who were physically active. They also found that both low CRF and physical inactivity were independent predictors of all-cause mortality among men with DM. A study by Church et al9 involving 2196 middle-aged men with DM found that the mortality risk was 4.5 (95% CI, 2.6-7.6), 2.8 (95% CI, 1.6-4.7), and 1.6 (95% CI, 0.93-2.76) across the first 3 fitness quartiles; the fourth quartile served as the reference group (P for trend <.0001). This steep inverse association between CRF and all-cause mortality was found to be independent of BMI.9 Kavanagh et al28,29 also found an inverse association between CRF and risk of all-cause mortality among men who either had undergone a heart transplant or were candidates for cardiovascular rehabilitation. Most studies focusing on the association between CRF and mortality have focused on sedentary but apparently healthy persons.12 Our results provide evidence that supports the formal assessment of CRF among women with IFG and the use of this information in physical activity counseling aimed at reducing the risk of premature death.
Although it is well recognized that IFG is a risk factor for the development of DM, Barr et al1 demonstrated that IFG is an independent risk factor for increased mortality, not simply an antecedent of DM. These findings suggest that strategies aimed at preventing premature mortality should focus on persons at earlier stages of metabolic dysfunction, such as IFG, and not only on those with frank DM. Thompson et al30 found that young, urban Native American women with lower CRF levels were at a higher risk of IFG, but not of metabolic syndrome, when the statistical analyses were adjusted for BMI. This finding further supports the suggestion by Barr et al1 that IFG is an important risk factor for premature mortality. The findings of these 2 studies also further support our recommendation that the CRF of women with IFG be assessed by formal treadmill testing for a determination of which groups are at higher risk of mortality. These women should be counseled to intensify their physical activity as a part of primary prevention efforts.
Obesity is an independent risk factor for mortality among women,13-17 but little is known about the mortality risk of women with IFG and DM. The current study found no association between obesity and mortality, a finding that agrees with the observations of Johnson et al.31 Some studies have also found an inverse association between BMI and mortality, a finding termed the obesity paradox.32,33 The mechanism responsible for this paradox is currently unclear, but the finding is more common among patients with CVD.32,33 This paradox may be explained by nonpurposeful weight loss before study participation or by dyspnea due to deconditioning (caused by factors other than CVD) among obese patients.33
Another explanation for the paradox may be the limitations associated with using BMI to define at-risk obesity. However, Lavie et al found that a higher percentage of body fat predicts a better prognosis for patients with heart failure33 and coronary heart disease.34 Further research is warranted in this area, but these findings are in direct contrast to those of most other studies, which have found either a J-shaped or a U-shaped association between BMI and mortality risk.13-17 In the current study, low fitness was associated with a higher risk of mortality in 51% of obese patients, 20% of overweight patients, and 10% of patients of normal weight; these rates were higher than those of women with all other combinations of CRF and BMI (Table 4). This association between low fitness and higher risk of mortality was also found when the overweight and obese groups were combined. Higher fitness was associated with a lower risk of overall mortality for overweight or obese women but not for normal-weight women, although there was a nonsignificant trend in this direction.
Although CRF has a genetic component (25%-40% of cases),12,35,36 the primary determinant of fitness is the physical activity routine. Recently, Church et al37 reported that women with activity levels as low as 4 kcal/kg/wk (approximately 72 min/wk of moderate-intensity walking) experienced significant improvements in CRF when they were compared with women in a control group who did not exercise. Engaging in activities such as brisk walking, bicycling, or jogging for 30 minutes or more on most days of the week30 would move most of these women out of the low fitness category.
This study has several strengths, including the extensive baseline examination aimed at detecting subclinical disease, the use of measured risk factors, the relatively long follow-up period (average, 15 years), and the broad age range of the study population (20-79 years).
One limitation of this study is homogeneity of the patient population: patients were predominantly white, well-educated, middle- to upper-class, and female. This homogeneity limits our ability to generalize our findings to a broader population but should not affect the internal validity of the study. There is no strong reason to assume that CRF assessment would have fewer benefits for men or other ethnic groups. Our previous studies, in which the number of deaths that occurred was sufficient to allow parallel analyses of women and men, showed that the inverse gradient of mortality across CRF groups is similar for men and women.9,38-40 In terms of exposure assessment, we classified women according to CRF at the time of study enrollment, but in the current analysis we were unable to evaluate the effect on outcome of changes in fitness or BMI over time. During the follow-up interval, many women in the low fitness category may have increased their fitness levels, and many obese women may have decreased their BMI. Therefore, we cannot determine whether changes in fitness, obesity status, or both occurred during follow-up or if there were any other exposures. However, such misclassification of exposure would probably lead to an underestimation of the magnitude of the associations observed in the current study.
CONCLUSION
Cardiorespiratory fitness, but not BMI, is a significant predictor of all-cause mortality among women with IFG or undiagnosed DM, independently of traditional risk factors. Determining CRF levels with exercise stress testing, which has already been shown to be an effective tool for risk stratification of men with diabetes, may also be an effective tool for risk stratification of women with IFG. We encourage health care professionals to consider the potential preventive and diagnostic value of assessing CRF levels in this high-risk group of women.
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and we thank the staff at the Cooper Institute for data entry and data management.
Footnotes
The Aerobics Center Longitudinal Study was supported by National Institutes of Health grants AG06945 and HL62508.
REFERENCES
- 1.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116(2):151-157 Epub 2007 Jun 18 [DOI] [PubMed] [Google Scholar]
- 2.Wen CP, Cheng TY, Tsai SP, Hsu HL, Wang SL. Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care 2005;28(11):2756-2761 [DOI] [PubMed] [Google Scholar]
- 3.Genuth S. Lowering the criterion for impaired fasting glucose is in order. Diabetes Care 2003;26(12):3331-3332 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005 Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2005. Centers for Disease Control and Prevention; 2005 http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf Accessed April 7, 2009 [Google Scholar]
- 5.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes 1987;36(4):523-534 [DOI] [PubMed] [Google Scholar]
- 6.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114-2120 [DOI] [PubMed] [Google Scholar]
- 7.Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes 2002;51(8):2637-2641 [DOI] [PubMed] [Google Scholar]
- 8.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605-611 [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27(1):83-88 [DOI] [PubMed] [Google Scholar]
- 10.McAuley PA, Myers JN, Abella JP, Tan SY, Froelicher VF. Exercise capacity and body mass as predictors of mortality among male veterans with type 2 diabetes. Diabetes Care 2007;30(6):1539-1543 Epub 2007 Mar 10 http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=17351282&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25(2):59-66 [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary coronary prevention. Mayo Clin Proc. 2009;84(4):373-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30(5):822-829 [DOI] [PubMed] [Google Scholar]
- 14.Matsuo T, Sairenchi T, Iso H, et al. Age- and gender-specific BMI in terms of the lowest mortality in Japanese general population. Obesity (Silver Spring) 2008;16(10):2348-2355 Epub 2008 Jul 24 [DOI] [PubMed] [Google Scholar]
- 15.Song YM, Ha M, Sung J. Body mass index and mortality in middle-aged Korean women. Ann Epidemiol. 2007;17(7):556-563 Epub 2007 Mar 29 [DOI] [PubMed] [Google Scholar]
- 16.Sui X, LaMonte MJ, Laditka JN, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 2007;298(21):2507-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:(21)1925-1932 [DOI] [PubMed] [Google Scholar]
- 18.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183-1197 [DOI] [PubMed] [Google Scholar]
- 19.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men [published correction appears in Ann Intern Med. 1999;131(5):394] Ann Intern Med. 1999;130(2):89-96 [DOI] [PubMed] [Google Scholar]
- 20.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1, blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005;45(1):142-161 Epub 2004 Dec 20 [DOI] [PubMed] [Google Scholar]
- 21.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 1989;262(17):2395-2401 [DOI] [PubMed] [Google Scholar]
- 22.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413-1423 Epub 2007 Apr 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. U S Armed Forces Med J. 1959;10:675-688 [PubMed] [Google Scholar]
- 24.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103(3):363-373 [DOI] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine ACSM's Guidelines for Exercise Testing and Prescription 6th ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 26.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837-1847 [DOI] [PubMed] [Google Scholar]
- 27.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 2002;106(25):3143-3421 [PubMed] [Google Scholar]
- 28.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12,169 men referred for cardiac rehabilitation. Circulation 2002;106(6):666-671 [DOI] [PubMed] [Google Scholar]
- 29.Kavanagh T, Mertens DJ, Shephard RJ, et al. Long-term cardiorespiratory results of exercise training following cardiac transplantation. Am J Cardiol. 2003;91(2):190-194 [DOI] [PubMed] [Google Scholar]
- 30.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity). Arterioscler Thromb Vasc Biol. 2003;107(24):3109-3116 [DOI] [PubMed] [Google Scholar]
- 31.Johnson NP, Wu E, Bonow RO, Holly TA. Relation of exercise capacity and body mass index to mortality in patients with intermediate to high risk of coronary artery disease. Am J Cardiol. 2008;102(8):1028-1033 Epub 2008 Jul 31 [DOI] [PubMed] [Google Scholar]
- 32.Artham SM, Lavie CJ, Patel HM, Ventura HO. Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr. 2008;3(3):155-161 [DOI] [PubMed] [Google Scholar]
- 33.Lavie CJ, Ventura HO, Milani RV. The “obesity paradox”: is smoking/lung disease the explanation? [editorial]. Chest 2008;134(5):896-898 [DOI] [PubMed] [Google Scholar]
- 34.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891-894 [DOI] [PubMed] [Google Scholar]
- 35.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: The HERITAGE Family Study. Med Sci Sports Exerc. 1998;30(2):252-258 [DOI] [PubMed] [Google Scholar]
- 36.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2 max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87(3):1003-1008 [DOI] [PubMed] [Google Scholar]
- 37.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 2007;297(19):2081-2091 [DOI] [PubMed] [Google Scholar]
- 38.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996;276(3):205-210 [PubMed] [Google Scholar]
- 39.Kampert JB, Blair SN, Barlow CE, Kohl HW., III Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6(5):452-457 [DOI] [PubMed] [Google Scholar]
- 40.Marquis P, Fayol C, Joire JE, Leplège A. Psychometric properties of a specific quality of life questionnaire in angina pectoris patients. Qual Life Res. 1995;4(6):540-546 [DOI] [PubMed] [Google Scholar]