Abstract
OBJECTIVE: To assess the role of uvulopalatopharyngoplasty (UPPP) in the treatment of obstructive sleep apnea (OSA) using polysomnography (PSG) data within 6 months before and after surgery.
PATIENTS AND METHODS: We analyzed PSG and body mass index (BMI) data from patients with OSA who were 18 years or older and who underwent UPPP between January 1, 1988, and August 31, 2006.
RESULTS: Sixty-three patients (51 men [81.0%]; mean ± SD age, 42.1±13.9 years; mean ± SD BMI, 34.9±7.2) underwent PSG a mean ± SD of 50±47 days before and 88.5±34.0 days after UPPP. Surgical cure was defined as a postoperative apnea-hypopnea index (AHI) of 5 or less. Fifteen patients (24%) achieved a surgical cure. Twenty-one patients (33%) had a postoperative AHI of 10 or less, whereas 32 (51%) achieved a 50% or greater reduction in AHI and/or an AHI of 20 or less. No significant changes were noted in BMI before and 6 months after UPPP. Patients who attained an AHI of 5 or less were younger (mean ± SD age, 35.9±13.1 vs 44±13.7 years; P=.05), had lower BMIs (mean ± SD, 30.8±6.5 vs 34.6±6.6; P=.05), and had less severe OSA (mean ± SD AHI, 38.1±33.6 vs 69.6±32.8; P=.004). Of the 48 patients (76%) with a post-UPPP AHI greater than 5, 35 (56%) received continuous positive airway pressure, with a mean reduction in pressure of 1.4 cm H2O (95% confidence interval, -0.4 to -2.4 cm H2O).
CONCLUSION: Independent of changes in BMI, in our retrospective analysis, UPPP achieved an AHI of 5 or less in 24% and an AHI of 10 or less in 33% of patients with OSA who underwent PSG 6 months before and after surgery. In those with residual OSA who received continuous positive airway pressure, the required pressure setting decreased by 1.4 cm H2O.
Independent of changes in body mass index, uvulopalatopharyngoplasty achieved an apnea-hypopnea index of 5 or less in 15 patients and of 10 or less in 21 patients with obstructive sleep apnea (OSA) who underwent polysomnography 6 months before and after surgery. In those with residual OSA who received continuous positive airway pressure, the required pressure setting decreased by 1.4 cm H2O.
AHI = apnea-hypopnea index; BMI = body mass index; CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea; PSG = polysomnography; UPPP = uvulopalatopharyngoplasty
Obstructive sleep apnea (OSA) is highly prevalent, affecting 4% of men and 2% of women who meet a disease-defining threshold of at least 5 episodes of apnea or hypopnea per hour of sleep (apnea-hypopnea index [AHI] ≥5) and excessive daytime sleepiness.1 Continuous positive airway pressure (CPAP), a technique that pneumatically supports the upper airway, is a therapeutic mainstay for OSA. It has been shown to reduce the AHI, improve sleepiness and quality of life, and reduce cardiovascular risk.2,3 Despite demonstrable benefits and technological equipment advances, compliance with CPAP therapy varies, with 29% to 83% of patients using CPAP for less than 4 hours a night in various studies.4 Accordingly, physicians may recommend other options for their patients with OSA, including risk factor modification such as weight loss, oral appliances that advance the mandible or tongue during sleep,5 or a variety of surgical procedures to bypass or expand the upper airway.6
The most common surgical procedure performed for OSA is uvulopalatopharyngoplasty (UPPP).7,8 Introduced by Fujita et al9 in 1981, UPPP involves tonsillectomy (if not previously performed), trimming and reorientation of the posterior and anterior tonsillar pillars, and excision of the uvula and posterior palate. Often, UPPP is combined with other nasopharyngeal or oropharyngeal procedures. The reported success of UPPP as a treatment of OSA is between 16% and 83%, depending on the definition of a positive outcome.7,10 Some authors have defined surgical success or cure after UPPP as a 50% reduction in the AHI, whereas others combine this criterion with an absolute AHI of 20 or less.11-14 Unfortunately, use of these criteria means that successfully treated patients may still have mild to moderate residual OSA. Increasing evidence shows that, when treating OSA, reducing the AHI to less than 5 is necessary to improve health care-related outcome measures, such as hypertension.15 Accordingly, there have been calls for caution about UPPP as first-line therapy for OSA and for all future studies of UPPP to base surgical success on AHI outcomes of 5 or less or 10 or less, targets typically expected from CPAP therapy.7,16 Therefore, to better define response to UPPP, we reviewed the UPPP experience at Mayo Clinic's site in Rochester, MN, using these more stringent and contemporary criteria.
PATIENTS AND METHODS
We retrospectively analyzed the records of all patients 18 years or older who had been diagnosed as having OSA by PSG and had undergone UPPP between January 1, 1988, and August 31, 2006, after obtaining approval from the Mayo Clinic Institutional Review Board. Patients who had undergone PSG within 6 months before and after UPPP were included in this analysis. Board-certified sleep specialists at the Mayo Clinic Center for Sleep Medicine evaluated all patients before and after PSG, reviewed the PSG data using standard criteria,17,18 and discussed the results and treatment options with all patients.
All PSG studies were technologist-attended, in-laboratory examinations using a digital polygraph (NCI-LAMONT Medical Inc, Madison, WI, or Bio-logic Systems Corp, Mundelein, IL). The following parameters were recorded: electroencephalography, electrooculography, submental and anterior tibialis electromyography, snoring by laryngeal microphone, oxygen saturation (finger or ear oximeter), and respiratory effort (thoracic, abdominal, and summated inductive plethysmography). From January 1, 1988, through September 30, 2001, airflow was analyzed by an in-house manufactured oronasal thermocouple array. From October 1, 2001, through August 31, 2006, a nasal pressure transducer (Pro-Tech Services Inc, Mukilteo, WA) was used to assess airflow.
Until April 30, 2002, hypopnea was defined as at least a 30% decrease in airflow for at least 10 seconds despite respiratory effort and accompanied by at least a 2% decrease in oxyhemoglobin saturation. For the remaining period, the desaturation criterion for hypopnea was 4% or less to comply with revised CPAP coverage requirements issued by the Centers for Medicare and Medicaid Services. Obstructive apnea was defined throughout as cessation of airflow for at least 10 seconds despite respiratory effort. Both pre- and post-UPPP PSG were performed either as a diagnostic study or in a split-night manner with CPAP titration during the second half if the AHI was 5 or greater.
Data were analyzed using JMP software (SAS Institute, Cary, NC). Paired-sample t tests were used to compare presurgery and postsurgery data and independent-sample (unpaired) t tests to compare groups that did and did not achieve an AHI of 5 or less. Paired data were also analyzed using the Wilcoxon signed rank test and unpaired data using the Wilcoxon rank sum test. No differences were noted between parametric and nonparametric measures; therefore, only the results of parametric testing are reported. P<.05 was considered statistically significant. Data are summarized as mean ± SD or median (interquartile range). In addition, 95% confidence intervals are reported around the point estimate.
RESULTS
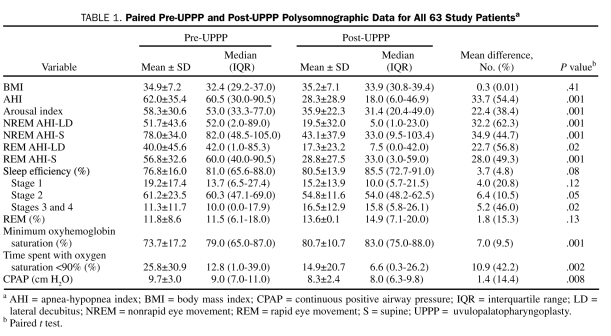
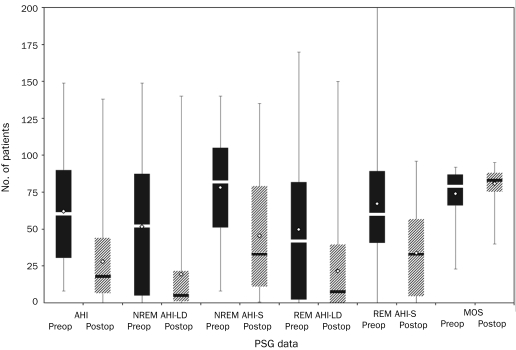
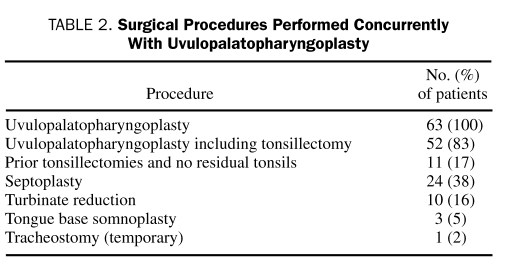
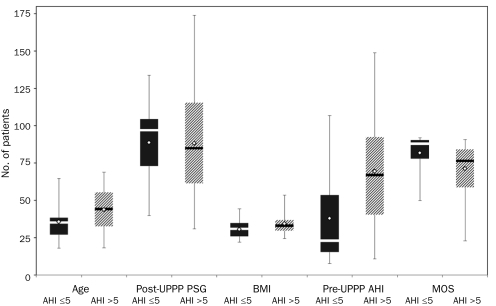
Sixty-three patients were identified who met the study inclusion criteria. The mean age was 42.1±13.9 years, and the group consisted primarily of men (51 [81.0%]). Polysomnography was performed 50±47 days before and 88.5±34 days after UPPP. The mean preoperative AHI was 62±35.4. Preoperative and postoperative PSG data are provided in Table 1 and Figure 1. Procedures performed concurrently with UPPP are listed in Table 2. Although tonsillectomy is usually performed as part of UPPP at our institution, for the purposes of this analysis, it was recorded as a second procedure.
TABLE 1.
Paired Pre-UPPP and Post-UPPP Polysomnographic Data for All 63 Study Patientsa
FIGURE 1.
Box and whisker plot. Mean (open diamonds) and median (horizontal lines) preoperative (preop) (dark bars) and postoperative (postop) (cross-hatched bars) polysomnography (PSG) data for 63 patients who underwent uvulopalatopharyngoplasty (UPPP). AHI = apnea-hypopnea index; LD = lateral decubitus; MOS = minimum oxygen saturation; NREM = nonrapid eye movement; REM = rapid eye movement; S = supine. Whiskers indicate minimum and maximum values.
TABLE 2.
Surgical Procedures Performed Concurrently With Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty with adjunctive surgical procedures resulted in a 54.4% reduction in the mean AHI to 28.3±28.9 postoperatively (P=.001). There was a 38.4% decrease in the mean arousal index from 58.3±30.6 to 35.9±22.3 (P=.001), with improvements in the mean nadir oxygen saturation and percentage of time spent with oxygen saturation of less than 90% (Table 1).
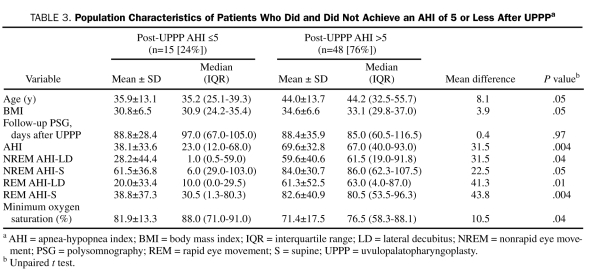
Use of the traditional outcome definition of achieving a 50% or greater reduction in the AHI and/or an AHI of 20 or less resulted in successful UPPP in 32 patients (51%). Use of more stringent response criteria resulted in 21 patients (33%) achieving a postoperative AHI of 10 or less and 15 patients (23.8%) achieving a postoperative AHI of 5 or less. Data on patients who achieved an AHI of 5 or less (surgical success) compared with those who did not are shown in Table 3 and Figure 2. No difference was found in the duration between surgery and follow-up PSG in patients with surgical success and failure (88.8±28.4 vs 88.4±35.9 days; P=.97). Patients who attained an AHI of 5 or less were younger (35.9±13.1 vs 44±13.7 years; P=.05), had a lower body mass index (BMI) (30.8±6.5 vs 34.6±6.6; P=.05), had less severe OSA (AHI, 38.1±33.6 vs 69.6±32.8; P=.004), and had higher preoperative minimum oxygen saturation (81.9%±13.3% vs 71.4%±17.0%; P=.04) (Table 3 and Figure 2).
TABLE 3.
Population Characteristics of Patients Who Did and Did Not Achieve an AHI of 5 or Less After UPPPa
FIGURE 2.
Box and whisker plot. Mean (open diamonds) and median (horizontal lines) population characteristics of patients with a postoperative apnea-hypopnea index (AHI) of 5 or less (n=15) (dark bars) and a postoperative AHI greater than 5 (n=48) (cross-hatched bars) after uvulopalatopharyngoplasty (UPPP). BMI = body mass index; MOS = minimum oxygen saturation; PSG = polysomnography. Whiskers indicate minimum and maximum values.
The mean BMI remained stable before and 6 months after UPPP (34.9±7.2 vs 35.2±7.1; P=.41). Four (80%) of the 5 patients with a BMI of 25 or less achieved a postoperative AHI of 5 or less compared with 11 (19%) of 58 with a BMI greater than 25. The difference was statistically significant (P=.005). Ten of 17 patients with a pre-UPPP AHI of 30 or less had a post-UPPP AHI of 5 or less compared with 5 of 46 with a pre-UPPP AHI greater than 30, resulting in an odds ratio of 11.7 (95% confidence interval, 3.1-44.7) for surgical success for those with a pre-UPPP AHI of 30 or less.
Of the 48 patients with a post-UPPP residual AHI greater than 5, 13 (21%) refused CPAP therapy and opted for weight reduction and positional therapy, whereas 35 (56%) accepted CPAP therapy. Paired CPAP data were available in 27 of those patients, with a mean reduction in CPAP of 9.7±3.0 cm H2O preoperatively to 8.3±2.4 cm H2O postoperatively for a point estimate of 1.4 cm H2O (95% confidence interval, -0.4 to -2.4 cm H2O).
DISCUSSION
The role of upper airway surgery in general and UPPP in particular in the management of OSA remains unclear because most studies are limited by small sample size, lack of consensus on a clear definition of surgical success, reliance on subjective end points, and an inability to compare UPPP in a blinded manner with CPAP.7,14 In a recent review by Megwalu and Piccirillo14 of 30 UPPP trials from January 1996 to August 2005, 7 different definitions of OSA and 17 definitions of surgical success were used. Of those articles, 67% evaluated UPPP treatment success without objective postoperative PSG data.14
Traditionally, a successful outcome of UPPP has been defined as achieving a reduction in AHI of at least 50% and/or a residual AHI of 20 or less. The AHI is a continuous measure, and a statistically significant change marked by a 50% reduction in the index and/or an index of 20 or less may not represent a satisfactory clinical outcome other than in patients struggling to comply with any form of OSA treatment.19 If a disease-defining threshold for OSA is an AHI of 5 or greater, achieving an AHI of less than 5 might be optimal for controlling disease-related consequences. Indeed, a 50% reduction in AHI was insufficient to lower blood pressure in the control arm of a CPAP treatment trial.15 Most patients diagnosed as having OSA are offered a CPAP treatment trial after a laboratory-based pressure titration. An optimal CPAP titration is one that reduces the frequency of obstructive sleep-disordered breathing events to an AHI of 5 or less, whereas a good titration is defined as an AHI of less than 10.20 Some investigators have suggested that outcomes of UPPP be subjected to similar criteria.7,16
Only 24% of our study patients achieved an AHI of 5 or less after UPPP as judged by PSG within 6 months of surgery, whereas 33% achieved an AHI of 10 or less. If a more traditional outcome definition were applied (≥50% reduction in the AHI and/or an AHI of ≤20), UPPP was successful in one-half of our patients. These data are similar to the findings of Elshaug et al7, in which the success rates as defined by an AHI of 5 or less, an AHI of 10 or less, or an AHI equal to a 50% reduction in AHI and/or an AHI of 20 or less were 16.1%, 34.1%, and 51.5%, respectively. Our results reiterate how widely the success rates of UPPP will vary depending on the definition used and the importance of objective follow-up of the patient who has undergone UPPP, because a significant proportion of patients undergoing the surgery are likely to have residual OSA.
We performed a univariate analysis to determine which patients were more likely to achieve an AHI of 5 or less after surgery and found that those who were younger, had lower BMIs, had lower preoperative AHIs, and had higher minimum oxygen desaturation during initial diagnostic PSG were more likely to achieve an AHI of 5 or less with UPPP. The site of anatomic narrowing in the upper airway10 or use of an anatomic grading system21 may also help improve the predictions of successful UPPP. Unfortunately, we had no uniform reporting of upper airway anatomic grading in most of our patients and thus could not assess this in our retrospective analysis.
In our study, BMI was a predictor of UPPP success because a greater percentage of patients with a BMI of 25 or less had a postoperative AHI of 5 or less. Obesity is one of the most important risk factors for OSA.22 Disposition of adipose tissue in the lateral parapharyngeal fat pads, intraluminal structures (eg, tongue), and neck heightens the propensity for upper airway collapse with sleep by compressing upper airway size, changing upper airway geometry, and/or altering soft tissue properties.23,24 Accumulation of abdominal viscera fat may also be a risk for OSA, perhaps by decreasing “tracheal tug,” a caudally directed, pharyngeal-stabilizing, lung volume-dependent traction force directed via the trachea.25 Uvulopalatopharyngoplasty cannot directly address either parapharyngeal or central excess adipose tissue, so it is biologically plausible that individuals with higher BMIs would be more likely to have persistently increased AHIs after surgery.
Continuous positive airway pressure was recommended in patients with a residual AHI greater than 5 after UPPP. Among the 35 patients who accepted CPAP therapy, paired data were available for CPAPs before and after surgery in 27 patients, and their CPAP requirement decreased by a point estimate of 1.4 cm H2O after UPPP. Whether this reduction improved compliance with CPAP therapy because of reduced pressure adverse effects and improved comfort requires further study.
One of the strengths of the current study is the assessment of pre-UPPP and post-UPPP BMI because weight changes may confound interpretation of the surgical results. No significant changes were noted in BMI after surgery. Other strengths of our study include the comprehensive data on evolution of AHI relative to sleep stage and position and the number of analyzed patients, which makes this one of the larger UPPP series published since 2002.
Our study has several important limitations. Although 978 patients underwent UPPP at our institution between 1988 and 2006, only 63 met our inclusion criteria for analysis (ie, availability of PSG data within 6 months before and after UPPP). The low number of patients who underwent follow-up PSG undoubtedly introduces a selection bias because it is possible that less satisfied patients were more likely to be studied again within our chosen postoperative window of 6 months. Some patients likely did not undergo follow-up PSG because of lack of perceived need and/or insurance limitations, issues difficult to sort out by retrospective analysis. Patients with more severe OSA may have been more likely to be studied because of concerns that they were less likely to be cured with UPPP. Indeed, our study patients typically had severe OSA with a mean preoperative AHI of 62.6±35.4. Our patients with preoperative AHIs of less than 30 had a higher likelihood of a successful surgical outcome (odds ratio, 11.7), a finding similar to that in other studies.21,26 Determining the full spectrum of postoperative complications was not possible via retrospective review. Although many of the sleep architecture parameters changed in a statistically significant positive manner after UPPP, the clinical importance of these changes is unclear, and we cannot rule out some confounding from resolution of first-night effect.27 Another limitation, pervasive in the UPPP literature, is that UPPP is infrequently performed in isolation. Thirty-four of our patients had undergone procedures that addressed the nasal airway, and 3 had single-session radiofrequency ablation treatments that addressed the level of the base of the tongue. Although single-session radiofrequency ablation combined with UPPP has been shown to produce better outcomes, with 50% of patients achieving a postoperative AHI of less than 5,26 excluding patients who underwent radiofrequency ablation from our analysis did not change our results.
We elected to analyze PSG data within an arbitrarily chosen 6-month window after UPPP. We hoped that this would allow for a more uniform interval between surgery and PSG assessment. We realize a limited time window prevents detection of delayed complications that might jeopardize UPPP response over time,28,29 such as cicatricial narrowing at the velopharyngeal-oropharyngeal junction and potential alterations in pharyngeal sensitivity,30,31 because the effectiveness of UPPP has been shown to decrease in the long term.32
CONCLUSION
The possibility of achieving the contemporary treatment goal of a postoperative AHI of 5 or less or 10 or less with UPPP is low for unselected patients who have varying differences in age, BMI, and severity of OSA defined by AHI and minimal oxygen saturation. However, this does not mean that UPPP does not have a role in the treatment of OSA. Modifications in clinical criteria for selection for UPPP have been occurring; however, most patients considered for UPPP at our institution in the period up to 2006 did not achieve an AHI of 5 or less. Our study suggests a greater likelihood of OSA cure with milder baseline OSA and normal BMI. This is important information for physicians whose patients may want to eschew CPAP therapy for an opportunity of surgical cure of OSA with UPPP.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-1235 [DOI] [PubMed] [Google Scholar]
- 2.Gay P, Weaver T, Loube D, Iber C, Positive Airway Pressure Task Force. Standards of Practice Committee. American Academy of Sleep Medicine Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006;29(3):381-401 [DOI] [PubMed] [Google Scholar]
- 3.Khan A, Latif F, Hawkins B, Tawk M, Sivaram CA, Kinasewitz G. Effects of obstructive sleep apnea treatment on left atrial volume and left atrial volume index. Sleep Breath 2008;12(2):141-147 [DOI] [PubMed] [Google Scholar]
- 4.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 2006;29(2):244-262 [DOI] [PubMed] [Google Scholar]
- 6.Sher AE. Upper airway surgery for obstructive sleep apnea. Sleep Med Rev. 2002;6(3):195-212 [DOI] [PubMed] [Google Scholar]
- 7.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 2007;30(4):461-467 [DOI] [PubMed] [Google Scholar]
- 8.Kezirian EJ, Weaver EM, Yueh B, et al. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope 2004;114(3):450-453 [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89(6):923-934 [DOI] [PubMed] [Google Scholar]
- 10.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19(2):156-177 [DOI] [PubMed] [Google Scholar]
- 11.Dattilo DJ, Drooger SA. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: comparison of subjective and objective results. J Oral Maxillofac Surg. 2004;62(2):164-168 [DOI] [PubMed] [Google Scholar]
- 12.Kao YH, Shnayder Y, Lee KC. The efficacy of anatomically based multilevel surgery for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2003;129(4):327-335 [DOI] [PubMed] [Google Scholar]
- 13.Miller FR, Watson D, Boseley M. The role of the Genial Bone Advancement Trephine system in conjunction with uvulopalatopharyngoplasty in the multilevel management of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2004;130(1):73-79 [DOI] [PubMed] [Google Scholar]
- 14.Megwalu UC, Piccirillo JF. Methodological and statistical problems in uvulopalatopharyngoplasty research: a follow-up study. Arch Otolaryngol Head Neck Surg. 2008;138(8):805-809 [DOI] [PubMed] [Google Scholar]
- 15.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003;107(1):68-73 [DOI] [PubMed] [Google Scholar]
- 16.Elshaug AG, Moss JR, Hiller JE, Maddern GJ. Upper airway surgery should not be first line treatment for obstructive sleep apnoea in adults. BMJ 2008;336(7634):44-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet M, Carley D, Carskadon M, et al. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15(2):173-184 [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects Bethesda, MD: US Dept of Health, Education, and Welfare; 1968. [Google Scholar]
- 19.Elshaug AG, Moss JR, Southcott AM, Hiller JE. An analysis of the evidence-practice continuum: is surgery for obstructive sleep apnoea contraindicated? J Eval Clin Pract. 2007;13(1):3-9 [DOI] [PubMed] [Google Scholar]
- 20.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171 [PMC free article] [PubMed] [Google Scholar]
- 21.Li HY, Wang PC, Lee LA, Chen NH, Fang TJ. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep 2006;29(12):1537-1541 [DOI] [PubMed] [Google Scholar]
- 22.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592-1599 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep 1996;19(2):104-115 [DOI] [PubMed] [Google Scholar]
- 25.Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75(5):2084-2090 [DOI] [PubMed] [Google Scholar]
- 26.Eun YG, Kim SW, Kwon KH, Byun JY, Lee KH. Single-session radiofrequency tongue base reduction combined with uvulopalatopharyngoplasty for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2008December;265(12):1495-1500 Epub 2008 Apr 29 [DOI] [PubMed] [Google Scholar]
- 27.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology 1966;2(3):263-266 [DOI] [PubMed] [Google Scholar]
- 28.Larsson H, Carlsson-Nordlander B, Svanborg E. Long-time follow-up after UPPP for obstructive sleep apnea syndrome: results of sleep apnea recordings and subjective evaluation 6 months and 2 years after surgery. Acta Otolaryngol. 1991;111(3):582-590 [DOI] [PubMed] [Google Scholar]
- 29.Larsson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994;104(11, pt 1):1362-1368 [DOI] [PubMed] [Google Scholar]
- 30.Dematteis M, Lévy P, Pépin JL. A simple procedure for measuring pharyngeal sensitivity: a contribution to the diagnosis of sleep apnoea. Thorax 2005;60(5):418-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimoff RJ. Upperairway myopathy is important in the pathophysiology of obstructive sleep apnea. J Clin Sleep Med. 2007;3(6):567-569 [PMC free article] [PubMed] [Google Scholar]
- 32.Janson C, Gislason T, Bengtsson H, et al. Long-term follow-up of patients with obstructive sleep apnea treated with uvulopalatopharyngoplasty. Arch Otolaryngol Head Neck Surg. 1997;123(3):257-262 [DOI] [PubMed] [Google Scholar]