Abstract
BACKGROUND
The review presents the 2005–2006 peer-reviewed marine pharmacology literature, and follows a similar format to the authors’ 1998–2004 reviews. The preclinical pharmacology of chemically characterized marine compounds isolated from marine animals, algae, fungi and bacteria is systematically presented.
RESULTS
Anthelminthic, antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis and antiviral activities were reported for 78 marine chemicals. Additionally 47 marine compounds were reported to affect the cardiovascular, immune and nervous system as well as possess anti-inflammatory effects. Finally, 58 marine compounds were shown to bind to a variety of molecular targets, and thus could potentially contribute to several pharmacological classes.
CONCLUSIONS
Marine pharmacology research during 2005–2006 was truly global in nature, involving investigators from 32 countries, and the United States, and contributed 183 marine chemical leads to the research pipeline aimed at the discovery of novel therapeutic agents.
SIGNIFICANCE
Continued preclinical and clinical research with marine natural products demonstrating a broad spectrum of pharmacological activity and will probably result in novel therapeutic agents for the treatment of multiple disease categories.
Keywords: drugs, marine, metabolites, natural products, pharmacology, review, toxicology
1. Introduction
The current article reviews the 2005–6 preclinical pharmacology of marine natural products using a similar format to the previous reviews on pharmacological research [1–5]. The review of the literature on the pharmacology of antitumor and cytotoxic marine compounds has been reported elsewhere [6–11]. Only those articles reporting on the bioactivity or pharmacology of marine chemicals that were structurally characterized are included in the current article. As in our previous reviews, we used a modification of Schmitz’s chemical classification [12] to assign structures to four major chemical classes, namely, polyketides, terpenes, nitrogen-containing compounds or polysaccharides. Those articles that reported anthelminthic, antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis and antiviral properties of marine chemicals have been presented in Table 1 with the corresponding structures shown in Fig. 1. The publications describing marine compounds affecting the cardiovascular, immune and nervous systems, as well as those with anti-inflammatory effects are grouped in Table 2, and their structures shown in Fig. 2. Finally, marine compounds with activity towards a series of cellular and molecular targets are exhibited in Table 3, and their structures depicted in Fig. 3. Publications regarding the bioactivity of marine extracts or as yet structurally uncharacterized marine compounds have been excluded from the present review, although several promising reports were published during 2005–6: anti-inflammatory and analgesic effects of Egyptian Red Sea sponge extracts [13]; proangiogenic effects of 15–20 kDa fucoidans on endothelial cells [14]; antioxidative and anti-inflammatory effects of phlorotannin-containing extracts with potential for osteoarthritis from the brown alga Ecklonia cava [15]; immunostimulating activity in vivo of a novel sulfated exopolysaccharide derived from a red-tide microalga Gyrodinium impudicum [16]; antiherpetic activity in vitro of sulfated fucans from the marine brown alga Stoechospermum marginatum [17]; in vitro bioactivity of Brazilian marine sponge extracts against herpes, adenovirus and rotaviruses [18]; antifungal activity of glycolipid fractions from the red alga Chondria armata [19]; antiviral and immunoregulatory activity of an exopolysaccharide from the marine Bacillus licheniformis [20]; potent anticoagulant activity of a sulfated polysaccharide from the brown alga Ecklonia cava [21]; antimicrobial activity of Red Sea coral extracts [22]; a novel broad-spectrum antibacterial protein produced by the bacterium Marinomonas mediterranea [23]; antiviral activity of polysaccharide fractions isolated from the cyanobacterium Arthrospira platensis (formerly Spirulina platensis) [24]; antiangiogenic and antimicrobial activity of sponge-associated bacterial extracts [25], and a β-galactose-specific lectin with anti-HIV-1 activity isolated from the marine worm Chaetopterus variopedatus [26].
Table 1.
Marine Pharmacology in 2005–6: Marine Compounds with Anthelmintic, Antibacterial, Anticoagulant, Antifungal, Antimalarial, Antiprotozoal, Antituberculosis, and Antiviral Activities
| Drug Class | Compound/Organisma | Chemistry | Pharmacologic Activity | IC50b | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|---|
| Anthelmintic | (−)-echinobetaine A & (+)-B (1,2)/sponge | Alkaloidf | Activity against nematode Haemonchus contortus | 8.3–83 μg/mL++ | Undetermined | AUS | [27,28] |
| Anthelmintic | Laurencia scoparia terpene (3)/alga | Sesquiterpenee | Activity against nematode Nippostrongylus brasiliensis | 0.11 mM | Undetermined | URY, BRA | [29] |
| Antibacterial | caminosides B & D (4,5)/sponge | Polysaccharideg | Methicillin-resistant S. aureus & vancomicin- resistant Enterococcus inhibition | 3.1–6.3 μg/disk+ | E. coli Type III secretion inhibition | CAN, NLD, USA | [30] |
| Antibacterial | Spongosorites sp. alkaloids (6,7)/sponge | Alkaloidf | S. aureus inhibition | 3.12–6.25 | Sortase A inhibition | S.KOR | [31] |
| Antibacterial | aurelin (8)/jellyfish | Peptidef | E. Coli inhibition | 7.7 μg/mL+ | Undetermined | RUS | [32] |
| Antibacterial | batzellaside A (9)/sponge | Alkaloidf | S. epidermidis inhibition | ≤ 6.3 μg/mL+ | Undetermined | USA | [33] |
| Antibacterial | dendridine A (10)/sponge | Alkaloidf | B. subtilis & M. luteus inhibition | 4.2–8.3 μg/mL+ | Undetermined | AUS, JPN | [34] |
| Antibacterial | 6-oxo-de-O-methyllasiodiplodi n (11)/fungus | Polyketided | B. subtilis, S. aureus & S. enteritidis inhibition | 6.25–12.5 μg/mL+ | Undetermined | CHN | [35] |
| Antibacterial | grammistins (12)/fish | Peptidef | B. subtilis, S. aureus & E. coli inhibition | 3.13–12.5 μg/mL+ | Undetermined | JPN | [36] |
| Antibacterial | halichonadin C (13)/sponge | Sesquiterpenee | M. luteus inhibition | 0.52 μg/mL+ | Undetermined | JPN | [37] |
| Antibacterial | lajollamycin (14)/bacterium | Polyketided | S. aureus & S. pneumoniae inhibition | 1.5–4 μg/mL+ | Undetermined | USA | [38] |
| Antibacterial | marinomycins A–D (15–18)/bacterium | Polyketided | S. aureus & E. faceium inhibition | 0.1–0.6 μM | Undetermined | USA | [39] |
| Antibacterial | resistoflavin methyl ether(19)/bacteria | Polyketided | B. subtilis inhibition | 3.1 μg/mL+ | Undetermined | DEU | [40] |
| Antibacterial | Streptomyces anthraquinones (20,21)/bacterium | Polyketided | Methicillin-resistant S. aureus inhibition | 0.15–0.36 | Undetermined | USA | [41] |
| Antibacterial | Streptomycetaceae quinone (22)/bacterium | Polyketided | Methicillin-resistant S. aureus & vancomicin-resistant Enterococcus inhibition | 1.95–3.90 μg/mL+ | Undetermined | USA | [42] |
| Antibacterial | xeniolide I (23)/soft coral | Terpenee | E. coli & B. subtilis inhibition | 1.2 μg/mL+ | Undetermined | ISR | [43] |
| Anticoagulant | Limandra aspera protein/fish | Peptidef | Factor XIIa and platelet integrins inhibition | < 1 μM | Formation of inactive complex with XIIa | KOR | [57] |
| Anticoagulant | fucoidans (24)/alga | Polysaccharideg | Thrombin and factor Xa inhibition in vitro and in vivo | ND | RUS | [58] | |
| Anticoagulant | heparin (25)/clam | Polysaccharideg | Activated partial thromboplastin time & Xa inhibition in vitro | 52–97 IU/mg | Lower activity than bovine mucosal heparin | ITA | [59] |
| Anticoagulant | sulfated galactans (26,27)/alga | Polysaccharideg | Thrombin and factor Xa inhibition in vitro | ND | 2,3-disulfated a- galactose units critical motif | BRA | [60] |
| Anticoagulant | sulfated galactofucan (28)/alga | Polysaccharideg | Endothelial cell heparan sulfate synthesis stimulation | ND | Factor Xa inhibition in vitro | BRA | [61] |
| Antifungal | capisterones A & B (29,30)/alga | Steroide | Enhancement of fluconazole activity | ND | CDR1 & MDR1 efflux pump reversal activity | USA | [62] |
| Antifungal | Dysidea herbacea phenol (31)/sponge | Polyketided | C. albicans & A. niger inhibition | 1.95–7.8 μg/mL+ | Leakage of K+ from fungal cells | ISR | [63] |
| Antifungal | spongistatin (32)/sponge | Polyketided | Broad panel of yeasts and filamentous fungi | 1–32 μg/mL+ | Disruption of microtubule network | USA | [64] |
| Antifungal | halocidin (33)/ascidian | Peptidef | C. albicans inhibition | 1–4 μg/mL+ | Membrane pore formation | KOR | [66] |
| Antifungal | hassallidin A (34)/bacterium | Lipopeptidef | C. albicans & A. fumigatus inhibition | 4.8 μM+ | Undetermined | DEU | [67] |
| Antifungal | latrunculins (35–42)/sponge | Polyketided | C. albicans inhibition comparable to clotrimazole | 2.5–19 μM+ | Undetermined | EGY, USA | [49] |
| Antifungal | majusculoic acid (43)/bacterium | Polyketided | C. albicans inhibition, less potent than fluconazole | 8 μM+ | Undetermined | USA | [68] |
| Antimalarial | pycnidione (44)/fungus | Polyketided | P. falciparum W2 & D6 strain inhibition | 0.2–0.4 ng/mL | Undetermined | AUS, USA | [77] |
| Antimalarial | plakortide Q (45)/sponge | Polyketided | P. falciparum D10 & W2 strain inhibition | 0.5–1 μM | Undetermined | ITA | [78] |
| Antimalarial | Xestospongia sp. xestoquinone (46)/sponge | Polyketided | FCB1 P. falciparum inhibition | 3 μM | Pfnek-1 kinase inhibition | FRA | [79] |
| Antimalarial | manzamine Y (47)/sponge | Alkaloidf | P. falciparum D6 & W2 strain inhibition | 0.4–0.85 μg/mL | Undetermined | IDN, ESP, USA | [80] |
| Antimalarial | caucanolides A & D (48,49)/soft coral | Diterpenee | P. falciparum W2 inhibition | 17 μg/mL | Undetermined | COL, PAN, USA | [81] |
| Antimalarial | Eunicea sp. sesquiterpenoids (50–54)/coral | Sesquiterpenee | P. falciparum W2 strain inhibition | 10–18 μg/mL | Undetermined | COL, PAN, USA | [82] |
| Antimalarial | kallolide D (55)/sea whip | Diterpenee | P. falciparum inhibition | 30.6 μM | Undetermined | PAN, USA | [83] |
| Antimalarial | leptolide & deoxypseudopter olide (56,57)/coral | Diterpenee | P. falciparum W2 strain inhibition | 50 & 74 μM | Undetermined | ESP, PAN | [84] |
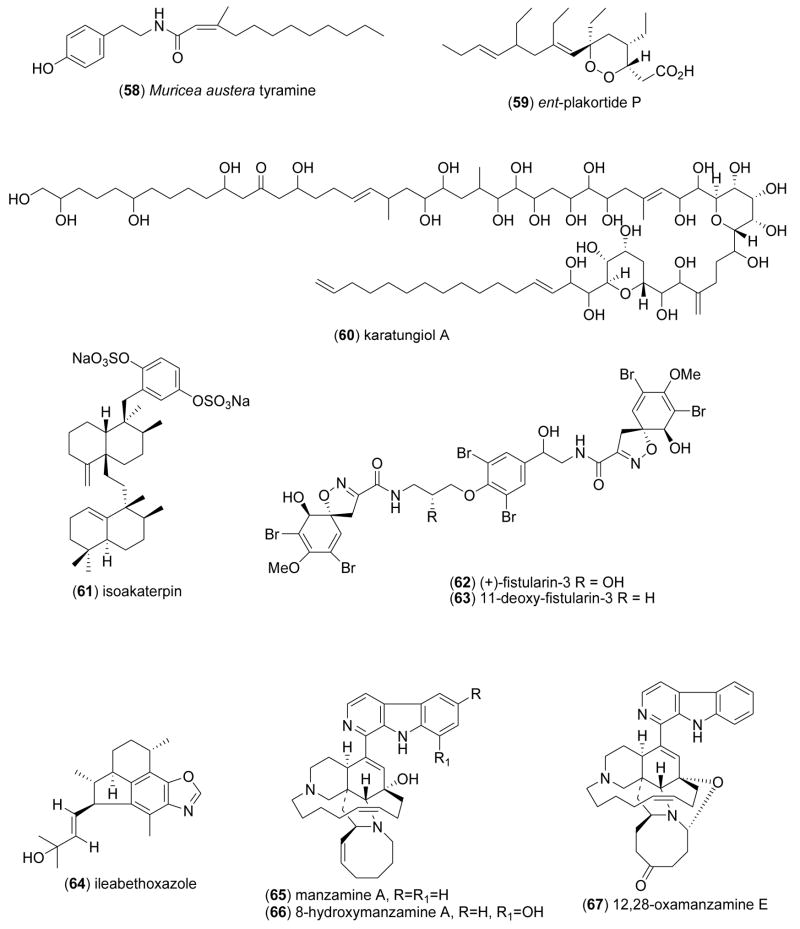
| Antimalarial | Muricea austera tyramine (58)/coral | Tyramine | P. falciparum W2 strain inhibition | 36 μM | Undetermined | ESP, PAN | [85] |
| Antiprotozoal | ent-plakortide P (59)/sponge | Polyketided | Leishmania mexicana inhibition | 1 μg/mL | Undetermined | KOR | [86] |
| Antiprotozoal | karatungiol A (60)/alga | Polyketided | Trichomonas foetus inhibition | 1 μg/mL+ | Undetermined | JPN | [87] |
| Antiprotozoal | isoakaterpin (61)/sponge | Meroterpenoide | Leishmania spp. adenosine phosphoribosyl transferase inhibition | 1.05 μM | Undetermined | CAN, BRA | [88] |
| Antituberculosi s | fistularin-3 & 11- deoxyfistularin-3 (62,63)/sponge | Tyrosine | M. tuberculosis inhibition | 7.1–7.3 μM+ | Undetermined | BRA | [89] |
| Antituberculosi s | ileabethoxazole (64)/soft coral | Diterpenee | M. tuberculosis inhibition | 61 μg/mL+ | Undetermined | USA | [91] |
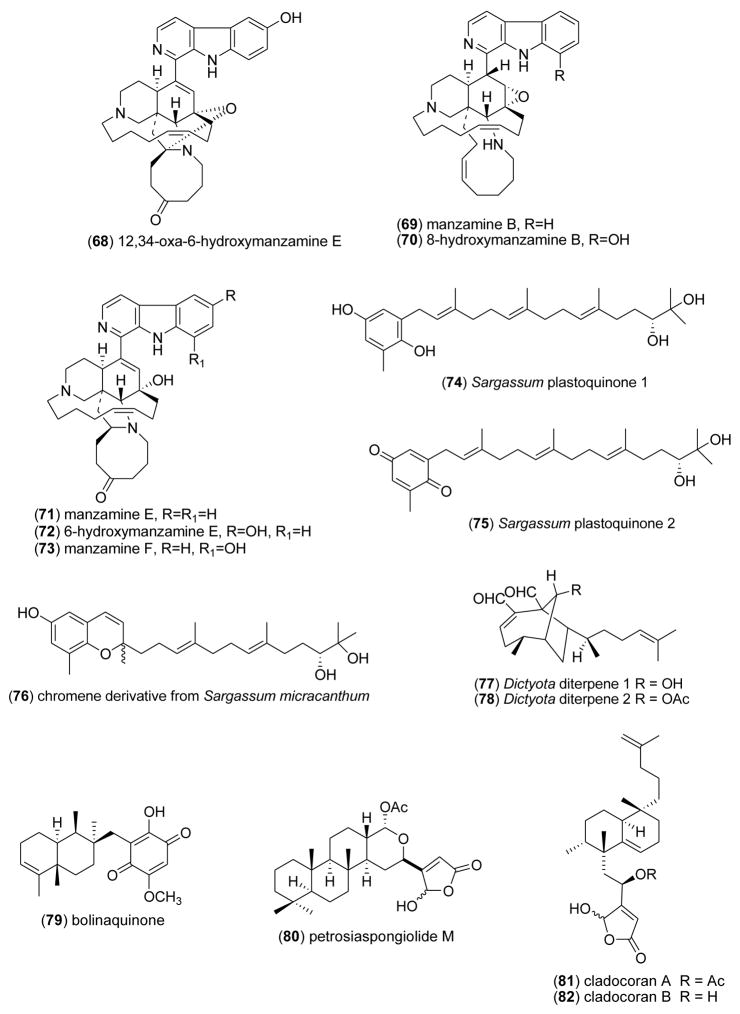
| Antituberculosi s | manzamine alkaloid (65- 73)/sponge | Alkaloidsf | M. tuberculosis inhibition | 0.4–5.2 μg/mL+ | Undetermined | IDN, ESP, USA | [80] |
| Antiviral | Callophylis variegata galactans/alga | Polysaccharideg | Herpes simplex & dengue type 2 inhibition | 0.1–2.2 μg/mL | Undetermined | ARG | [92] |
| Antiviral | naviculan/diatom | Polysaccharideg | Herpes simplex 1 & 2 inhibition | 7.4–14 μM | Undetermined | JPN | [93] |
| Antiviral | Schizymenia binderi sulfated galactan/alga | Polysaccharideg | Herpes simplex 1 & 2 inhibition | 0.18–0.76 μg/mL | Interference with HSV-heparan sulfate cellular residues | ARG, CHL | [94] |
| Antiviral | Sargassum plastoquinones (74–76)/alga | Terpenoide | Measles & cytomegalovirus inhibition | 0.49–3.1 μM | Lipid peroxidation observed | JPN | [95] |
| Antiviral | Dictyota diterpenes (77,78)/alga | Diterpenee | Inhibition of HIV-1 reverse transcriptase | 10–35 μM** | RNA-dependent DNA-polymerase activity inhibition | BRA | [96] |
| Antiviral | griffithsin/alga | Proteinf | T- & M-tropic HIV-1 inhibition | 0.043–0.63 nM | Inhibition of CD4- dependent gp120 binding | USA | [97] |
Organism, Kingdom Animalia: fish and ascidian (Phylum Chordata); sea star (Phylum Echinodermata), clam (Phylum Mollusca), sponges (Phylum Porifera); corals, sea whips and jellyfish (Phylum Cnidaria); Kingdom Monera: bacteria (Phylum Cyanobacteria); Kingdom Fungi: fungus; Kingdom Plantae: diatom, alga;
IC50: concentration of a compound required for 50% inhibition in vitro,
estimated IC50,
Ki: inhibition constant for a drug,
Kd: concentration at which 50% of ligand binding sites are occupied, ND: not determined;
MIC: minimum inhibitory concentration,
LD99: dose required to kill 99% of test population;
MMOA: molecular mechanism of action
Country: ARG: Argentina; AUS: Australia; BRA: Brazil; CAN: Canada; CHN: China; CHL: Chile; COL: Colombia; DEU: Germany; EGY: Egypt; ESP: Spain; FRA: France; IDN: Indonesia; IND: India; ISR: Israel; ITA: Italy; JPN: Japan; NLD: The Netherlands; NZL: New Zealand; PAN: Panama; PRT: Portugal; RUS: Russia; SVN: Slovenia; URY: Uruguay;
Polyketide;
Terpene;
Nitrogen-containing compound;
Polysaccharide.
Figure 1.
Table 2.
Marine Pharmacology in 2005–6: Marine Compounds with Anti-inflammatory activity, and affecting the Cardiovascular, Immune and Nervous System
| Drug Class | Compound/organisma+ | Chemistry | Pharmacological activity | IC50b | MMOAc | Countryd | References |
|---|---|---|---|---|---|---|---|
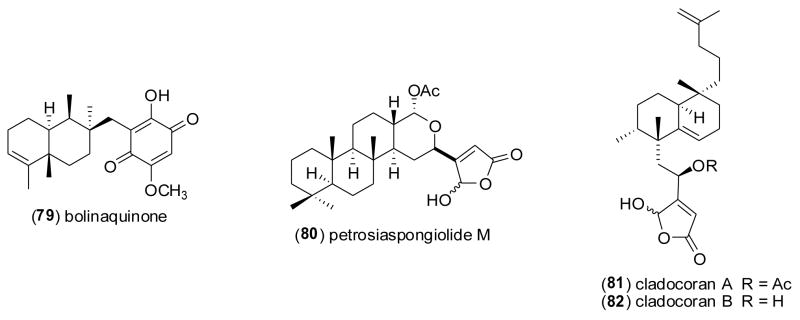
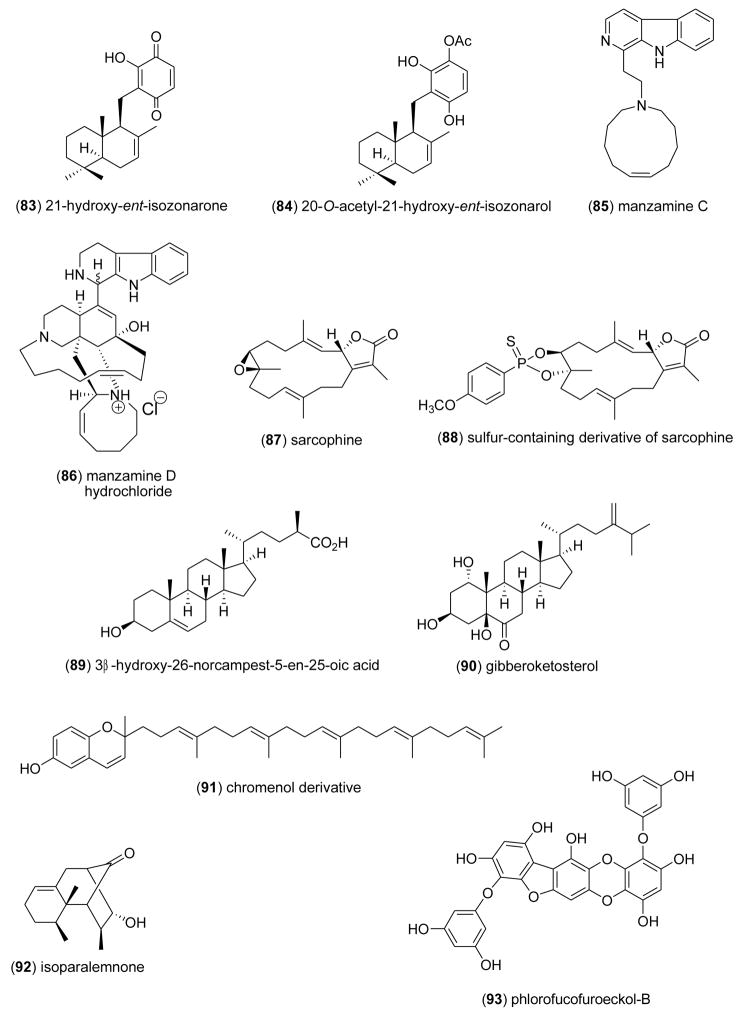
| Anti-inflammatory | bolinaquinone (79) & petrosias pongiolid e M (80)/sponge | Merosesquiterpenef & Sesterterpenef | Inhibition of colonic inflammation in vivo | ND | iNOS, NO, IL-1β & PGE2 inhibition | ESP, ITA | [98] |
| Anti-inflammatory | cladocorans A & B (81,82)/coral | Sesterterpenef | Secretory phospholipase A2 inhibition | 0.8–1.95 μM | Undetermined | JPN | [99] |
| Anti-inflammatory | Dysidea quinones (83,84)/sponge | Sesquiterpene-quinonef | Human neutrophil free radical release inhibition in vitro | 3–11 μM | Superoxide anion inhibition | NZL | [100] |
| Anti-inflammatory | manzamines A–F (65,69,71,73, 85,86)/sponge | Indole-derived alkaloidg | Modulation of LPS-activated brain microglia in vitro | 0.016–10 μM | TXB2 and superoxide anion inhibition | USA | [101] |
| Anti-inflammatory | sarcophines (87,88)/soft coral | Diterpenef | Modulation of LPS-activated brain microglia in vitro | 1 μM | TXB2 and superoxide anion inhibition | EGY, USA | [102] |
| Anti-inflammatory | Euryspongia n. sp. sterol (89)/sponge | Steroidf | HU keratinocyte 6-keto-PGF1α inhibition | 10 μg/mL* | Undetermined | FRA | [104] |
| Anti-inflammatory | gibberoketosterol (90)/soft coral | Steroidf | iNOS and COX-2 protein inhibition | 10 μM* | Undetermined | EGY, TAIW | [105] |
| Anti-inflammatory | Ircinia spinosula chromenol (91)/sponge | Triterpene-polyketidee | Porcine leukocyte LTB4 inhibition | 1.9 μM | Undetermined | GRC, DEU | [106] |
| Anti-inflammatory | isoparalemnone (92)/soft coral | Sesquiterpenef | Inhibition of iNOS protein | 10 μM* | Undetermined | EGY, TAIW | [107] |
| Anti-inflammatory | PFF-B (93)/alga | Shikimate-derivativee | Inhibition of histamine release in vitro | 7.8 μM | Undetermined | JPN | [108] |
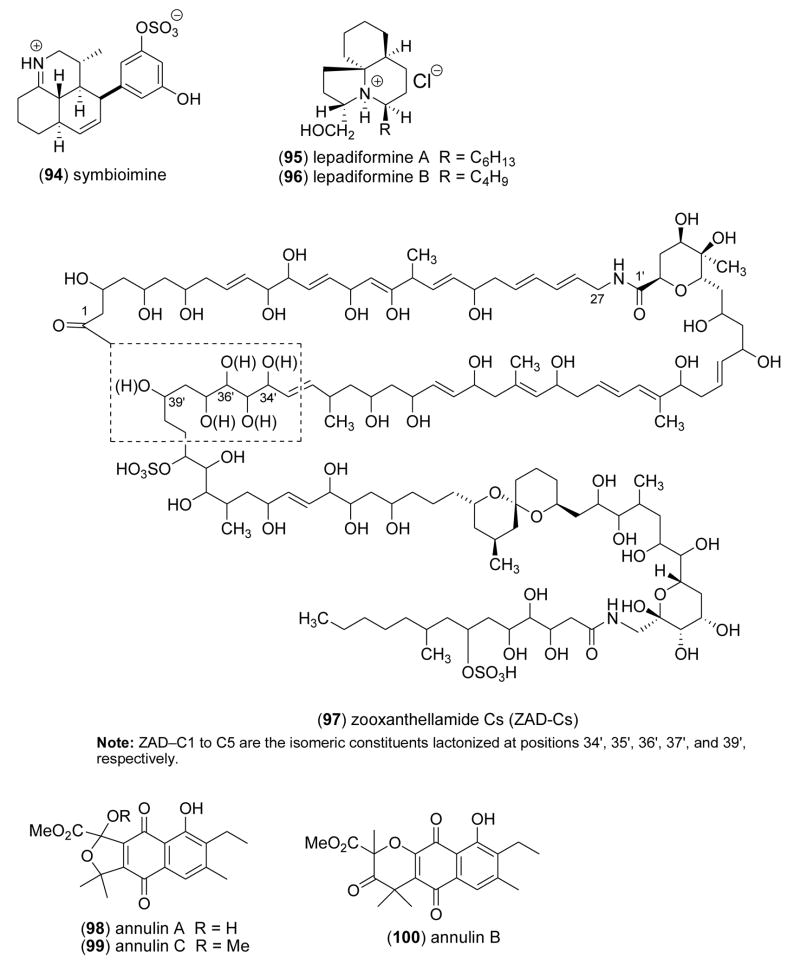
| Anti-inflammatory | symbioimine (94)/dinoflagellate | Alkaloidg | COX-2 protein inhibition | > 10 μM* | Undetermined | JPN | [109] |
| Cardiovascular | lepadiformines A & B (95,96)/ascidian | Alkaloidg | Cardiac inward rectifying K+ current inhibition | 1.4–1.6 μM*** | Voltage-dependent block | FRA | [110] |
| Cardiovascular | zooxanthellamide Cs (97)/alga | Polyketidee | Vasoconstriction of rat blood vessels | 0.39 μM | Undetermined | JPN | [111] |
| Immune system | annulins A–C (98–100)/hydroid | Polyketidee | Indoleamine 2,3- dioxygenase inhibition | 0.1–1.1μM** | Undetermined | CAN | [112] |
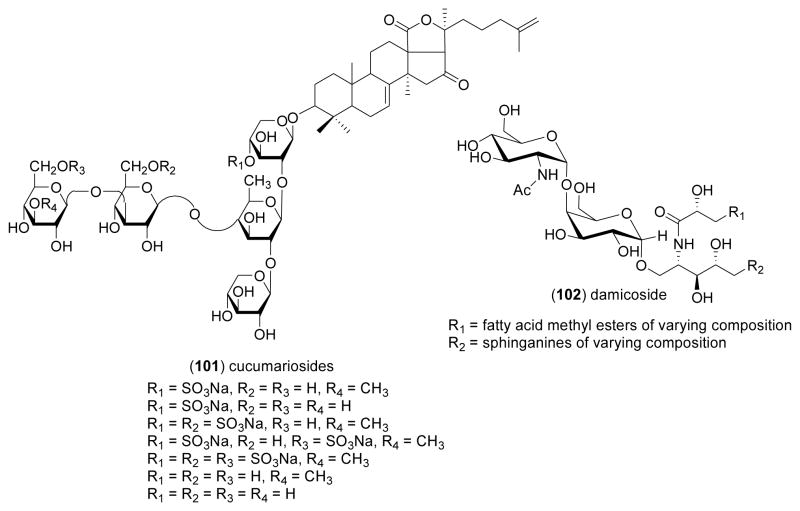
| Immune system | cucumariosides (101)/sea cucumber | Triterpene- oligoglycosidef | Stimulation of lymphocytes & neutrophils | ND | IL-6 & TNF-α increase | RUS | [113] |
| Immune system | damicoside (102)/sponge | Glycosphingolipid | Stimulation of spleen cell proliferation | 0.001 μg/m L* | Free galactose group required for activity | ITA | [114] |
| Immune system | laminarin/alga | Polysaccharideh | Inhibition of lymphocyte apoptosis | 1–4 mg/mL | Induction of 33 immune response genes | S.KOR | [115] |
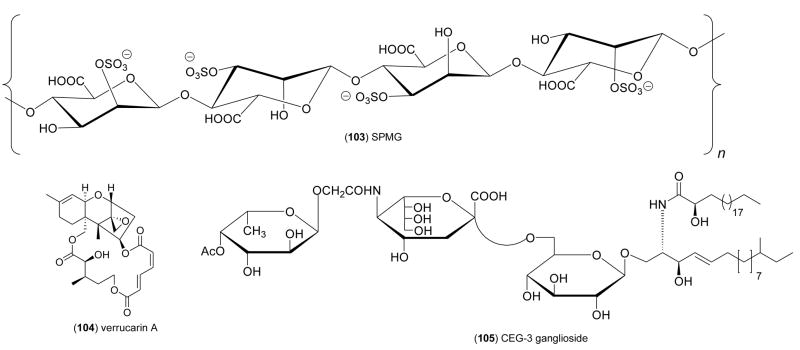
| Immune system | sulfated SPMG (103)/alga | Polysaccharideh | In vivo activation of T cells | 10 mg/kg | IL-2, IFN-γincrease; TNF-α decrease | CHN | [116] |
| Immune system | verrucarin A (104)/fungus | Polyketidee | Interleukin-8 inhibition | > 10 ng/mL* | p38 & JNK MAP kinase inhibition | JPN | [117] |
| Nervous system | CEG-3 ganglioside (105)/sea cucumber | Glycolipid | Induction of neurite outgrowth | 10 μM* | Undetermined | JPN | [120] |
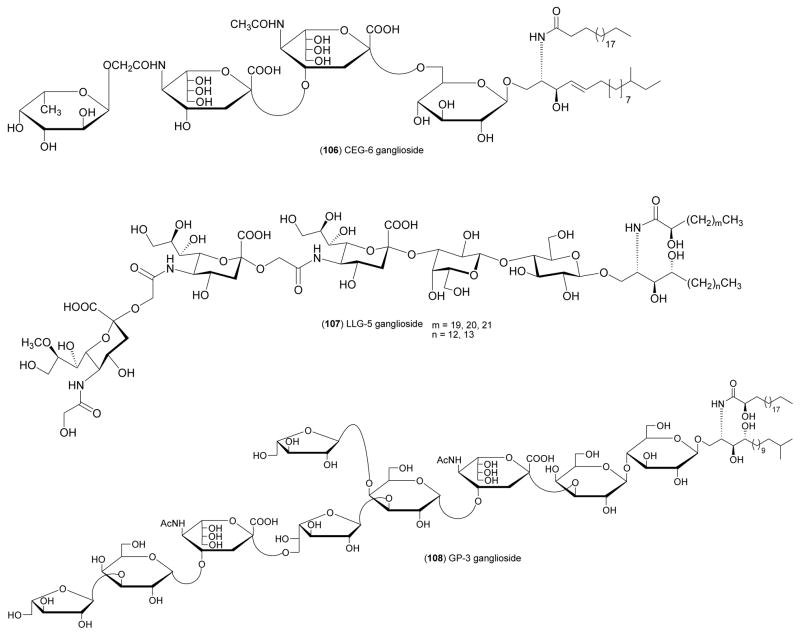
| Nervous system | CEG-6 ganglioside (106)/sea cucumber | Glycolipid | Induction of neurite outgrowth | <10 μM* | Undetermined | JPN | [121] |
| Nervous system | LLG-5 ganglioside (107)/sea star | Ganglioside | Induction of neurite outgrowth | < 10 μM* | Undetermined | JPN | [122] |
| Nervous system | GP-3 ganglioside (108)/sea star | Ganglioside | Induction of neurite outgrowth | > 10 μM* | Undetermined | JPN | [123] |
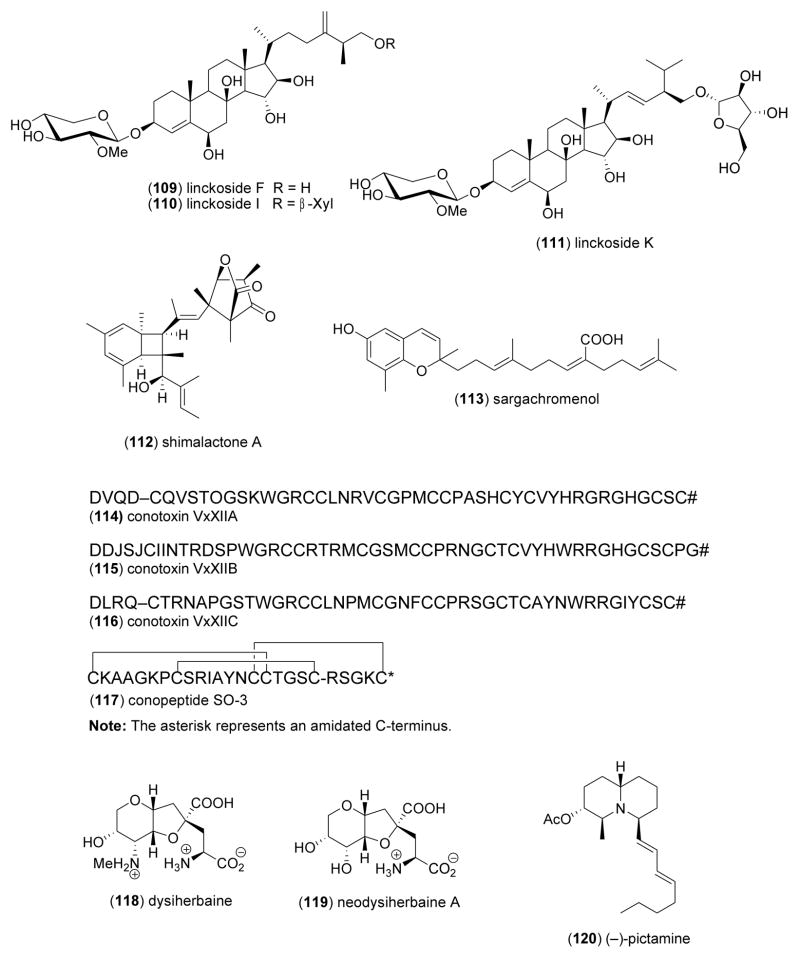
| Nervous system | linckosides F, I, K (109–111)/sea star | Steroidf | Induction of neurite outgrowth | ND | Dependent on pentose modified C branch | JPN | [124] |
| Nervous system | shimalactone A (112)/fungus | Polyketidee | Induction of neuritogenesis | 10 μg/mL* | Undetermined | JPN | [125] |
| Nervous system | sargachromenol (113)/alga | Diterpene-polyketidee | Promotion of NGF-stimulated neurite outgrowth | 9 μM | cAMP & MAP kinase pathways required | JPN | [118] |
| Nervous system | Conus vexillum conotoxins (114–116)/snail | Peptideg | Non-competitive nicotinic receptor antagonists | 0.4–8.4 nM | Slow block of agr;7 & α3β2 nicotinic receptor | AUS, DEU | [126] |
| Nervous system | SO-3 conopeptide (117)/snail | Peptideg | N-type neuronal Ca2+ current inhibition | 0.16 μM | Selective N-type voltage-sensitive Ca channel blocker | CHN | [127] |
| Nervous system | dysiherbaines (118,119)/sponge | Aminoacidg | Ionotropic glutamate receptor binding | 0.5–4.3 nM** | GluR5, GluR6 & KA2 receptor binding | FIN, JPN, GBR, USA | [128,129] |
| Nervous system | (−)-pictamine (120)/ascidian | Quinolizidine alkaloidg | Nicotinic acetylcholine receptor block | 1.5 μM | α4β2 receptor irreversible inhibition | JPN, USA | [130] |
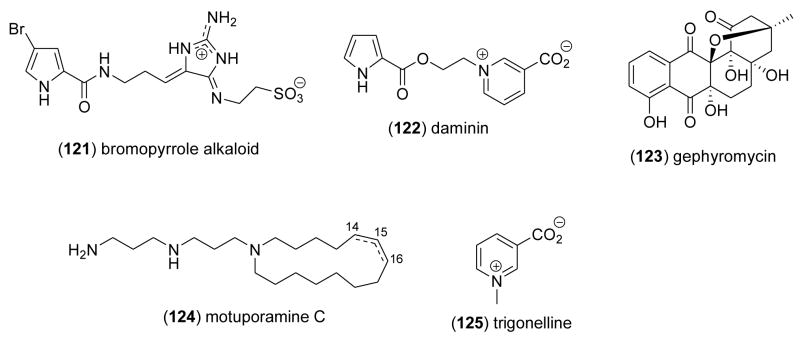
| Nervous system | bromopyrrole alkaloid (121)/sponge | Bromopyrrole alkaloidg | Glutamate and serotonin antagonist | 10 μg/mL* | Inhibition of neuronal Ca2+ entry | ITA, DEU | [131] |
| Nervous system | daminin (122)/sponge | Pyrrole alkaloidg | Inhibition of neuronal Ca2+ levels | 1 μg/mL* | Undetermined | ITA, DEU | [132] |
| Nervous system | gephyromycin (123)/bacterium | Polyketidee | Increase of neuronal Ca2+ levels | ND | Undetermined | GBR, DEU | [133] |
| Nervous system | motuporamine C (124)/sponge | Alkaloidg | Neuronal growth collapse | 5 μM* | Upregulation of Rho pathway | CAN | [134] |
| Nervous system | trigonelline (125)/soft coral | Pyridinium alkaloidg | Voltage-activated K+ current inhibition | > 0.1 mM* | Enhanced Ca2+ influx | EGY, GBR | [135] |
Organism: Kingdom Animalia: hydroid, corals (Phylum Cnidaria) ; ascidian, blue shark (Phylum Chordata), sea star, cucumber ( Phylum Echinodermata); snail (Phylum Mollusca); sponge (Phylum Porifera ); Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium (Phylum Cyanobacteria);
IC50: concentration of a compound required for 50% inhibition in vitro,
estimated IC50,
Ki: inhibition constant for a drug,
Kd: concentration at which 50% of ligand binding sites are occupied, ND: not determined;
MMOA: molecular mechanism of action, NO: nitric oxide;
Country: AUS: Australia; CHN: China; DEU: Germany; EGY: Egypt; FIN: Finland; FRA: France; GBR: United Kingdom; GRC: Greece; ITA: Italy; JPN: Japan; NZL: New Zealand; S.KOR: South Korea; TAIW: Taiwan ;
Polyketide;
Terpene;
Nitrogen-containing compound;
Polysaccharide.
Figure 2.
Table 3.
Marine Pharmacology in 2005–6: Marine Compounds with Miscellaneous Mechanisms of Action.
| Compound/Organisma | Chemistry | Pharmacological Activity | IC50b | MMOAc | Countryd | References |
|---|---|---|---|---|---|---|
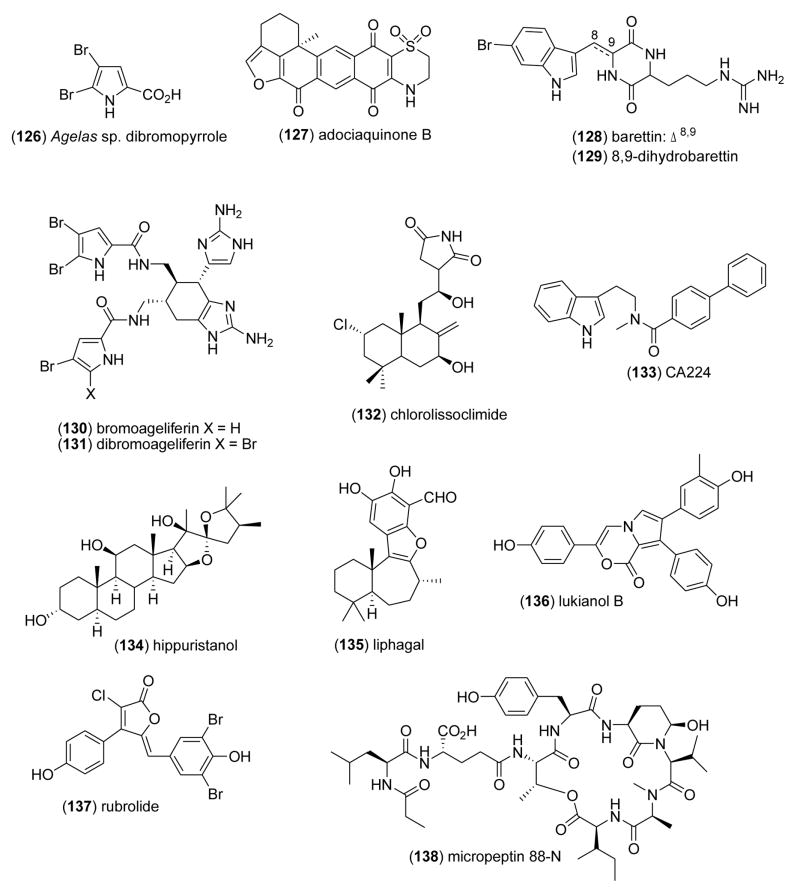
| Agelas sp. dibromopyrrole (126)/sponge | Alkaloidg | Reduction in Ca2+ elevation induced by K+ depolarization | < 0.3 mM | Voltage-gated calcium channel inhibition | DEU | [169] |
| adociaquinone B (127)/sponge | Alkaloidg | Cdc25B phosphatase inhibition | 0.07 μM | Selective oxidation of catalytic cysteine | USA | [170] |
| barettin (128) & 8,9-dihydrobarettin (129)/sponge | Diketopiperazineg | Serotonin uptake inhibition | 0.34–4.63 μM | Binding to 5-HT2A, 5-HT2C, 5-HT4 & 5-HT2C | SWE | [171] |
| bromoageliferin (130) & dibromoageliferin (131)/sponge | Alkaloidg | Inhibition of Ca2+ entry | 4–6.6 μM | Reduction of voltage-dependent calcium entry | DEU | [172] |
| chlorolissoclimide (132)/marine slug | Alkaloidal diterpenef | Reversible protein synthesis inhibition | 0.7 μM | Blocked elongation & ribosome release from polysomes | CAN, | [173] |
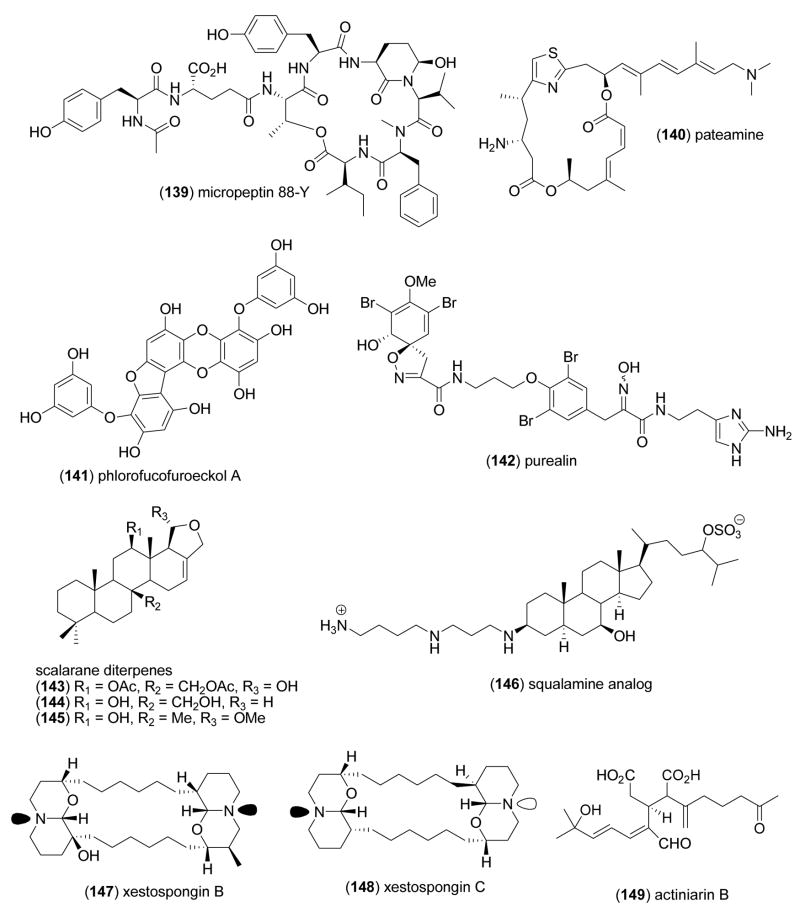
| fascaplysin analogue CA224 (133)/synthetic | Alkaloid | Cyclin-dependent kinase 4 inhibition | 5.5 μM | No Cdk2-cyclin A inhibition; no DNA intercalation | GBR | [174] |
| hippuristanol (134)/coral | Steroidf | Translation inhibition in vitro & in vivo | 0.4–2 μM | Translation initiation factor eIF4A RNA-binding inhibition | JPN, | [175] |
| liphagal (135)/sponge | Meroterpenef | Phosphatidylinositol-3-kinase inhibition | 0.1 μM | More selectivity to PI3Kα than PI3Kγ | CAN, NLD, USA | [176] |
| lukianol B (136) & rubrolide (137)/ascidian | Tyrosine derivativeg | Antidiabetic activity | 0.6–0.8 μM | Aldose reductase inhibition | ESP | [177] |
| micropeptin 88N (138) & 88-Y (139)/bacterium | Depsipeptideg | Chemotrypsin inhibition | 1.3–15 μM | Attachment to active site of enzyme, no hydrolysis | JPN | [178] |
| pateamine (140)/sponge | Polyketidee | Protein synthesis inhibition | 5 nM | Translation initiation factor eIF4A I/II & III inhibition NZL, USA | CAN, | [179] |
| phlorofucofuroeckol A(141)/alga | Shikimate-derivative | Angiotensin-converting enzyme 1 inhibition | 12.7 μM | Reactive oxygen species/peroxynitrite scavenger | S.KOR | [180] |
| purealin (142)/sponge | Dibromotyrosine derivativeg | Cytosplamatic dynein heavy chain inhibitor | 35 μM | Uncompetitive inhibition, no binding to ATP site | USA | [181] |
| Spongia sesterterpenoid (143–145)/sponge | Sesterterpenef | Hypercholesterolemia antagonist | 8.1–64.5 μM | Farnesoid X-activated receptor inhibition | S. KOR | [182] |
| squalamine analog (146)/shark | Sterol derivativef | Activation of bidirectional Cl− transport | Undetermined | Cl− transport dependent on IP3-insensitive stores & unidentified receptor | USA | [183] |
| xestospongin B (147)/sponge | Alkaloidg | IP3-induced Ca2+ signalling inhibition | 27–44 μM | Competitive to IP3 receptor binding | CHL, FRA, NCL | [184] |
| xestospongin C (148)/sponge | Alkaloidg | IP3-induced Ca2+ release inhibition | 458 nM | Enhanced rayanodyne receptor activity | USA | [185] |
| actiniarin B (149)/anemone | Polyketidee | Cdc25B phosphatase inhibition | 1.6 μg/mL | Undetermined | USA | [186] |
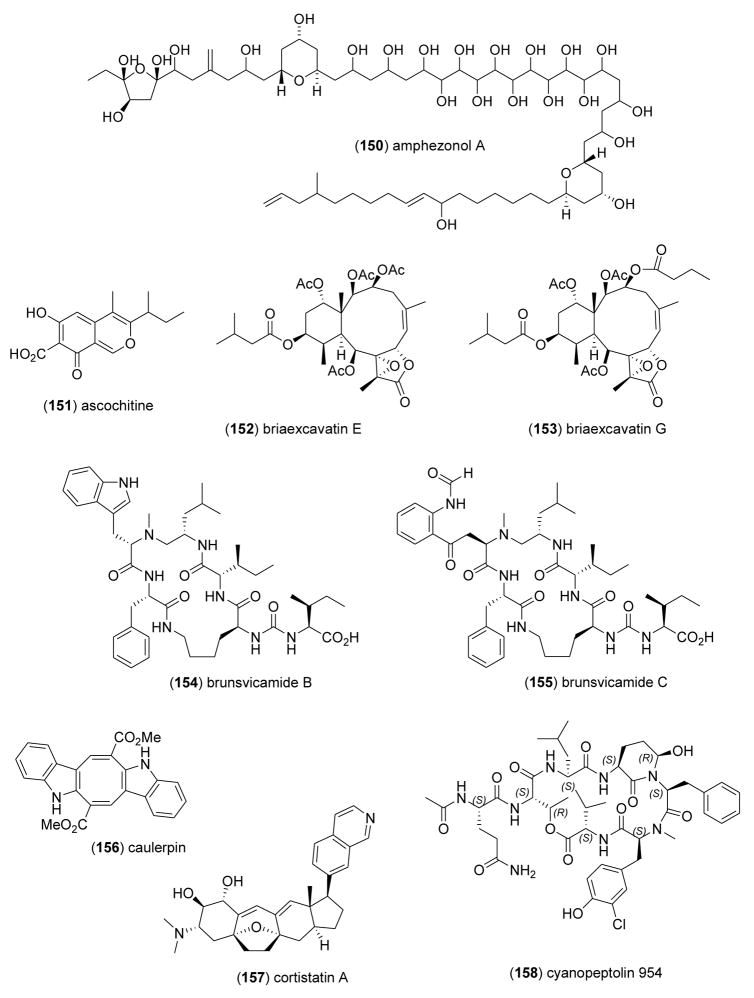
| amphezonol A (150)/alga | Polyketidee | DNA polymerase α inhibition | 15 μM | Undetermined | JPN | [187] |
| ascochitine (151)/fungus | Polyketidee | M. tuberculosis tyrosine phosphatase inhibition | 11.5 μM | Undetermined | DEU | [188] |
| briaexcavatin E (152)/coral | Diterpenef | Neutrophil elastase inhibition | 5–10 μM | Undetermined | TAIW | [189] |
| briaexcavatin G (153)/coral | Diterpenef | Neutrophil elastase inhibition | ND | Undetermined | TAIW | [190] |
| brunsvicamides B & C (154, 155)/bacterium | Peptidesg | M. tuberculosis tyrosine phosphatase inhibition | 7.3–8 μM | Undetermined | GBR, | [191] |
| caulerpin (156)/alga | Alkaloidg | HU protein tyrosine phosphatase 1 B inhibition | 3.77 μM | Undetermined | CHN | [192] |
| cortistatin A (157)/sponge | Alkaloidg | Antiangiogenic | 2 nM | Undetermined | IDN, JPN | [193] |
| cyanopeptolin 954 (158)/bacterium | Depsipeptideg | A-chymotrypsin inhibition | 54 nM | Undetermined | DEU, | [194] |
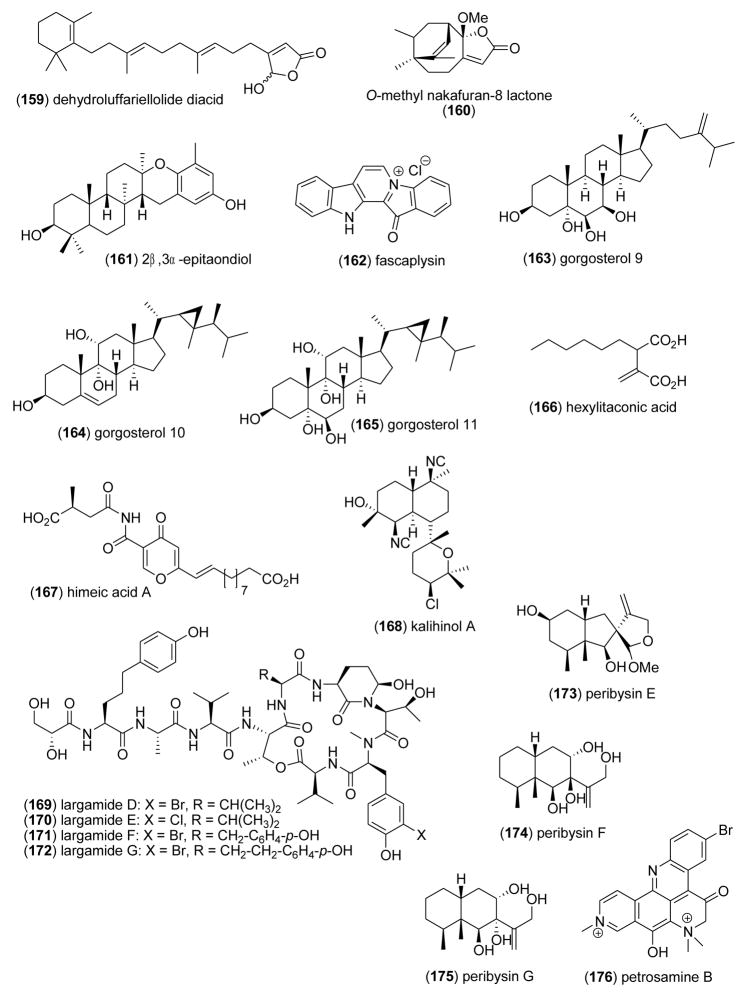
| dehydroluffariellolide diacid (159)/sponge | Sesterterpenef | Cdc25B phosphatase inhibition | 1.6 μg/mL | Undetermined | USA | [195] |
| O-methyl nakafuran-8-lactone (160)/sponge | Sesquiterpenef | Protein tyrosine phosphatase 1B inhibition | 1.58 μM | Undetermined | CHN, S. | [196] |
| 2β,3α-epitaondiol (161)/alga | Meroterpenef | Sodium channel inhibition | 0.7 μM | Undetermined | USA | [197] |
| fascaplysin (162)/sponge | Alkaloidg | Cdc25B phosphatase inhibition | 1.0 μg/mL | Undetermined | USA | [195] |
| gorgosterols (163–165)/coral | Sterolf | Binding to liver X receptor α | 0.07–1.3 μM | Undetermined | CRI, | [198] |
| hexylitaconic acid (166)/fungus | Polyketidee | Inhibition of p53-HDM2 ubiquitin-protein ligase | 50 μg/mL | Undetermined | JPN | [199] |
| himeic acid A (167)/fungus | Polyketidee/Peptide | Ubiquitin-activating enzyme inhibition | < 50 μM | Undetermined | JPN | [200] |
| kalihinol A (168)/sponge | Diterpenef | Cyclooxygenase 2 inhibition | 1.07 μM | Undetermined | CHN, | [201] |
| largamides D–G(169–172)/bacterium | Depsipeptideg | α-chymotrypsin type II inhibition | 4.0–25.0 μM | Undetermined | USA | [202] |
| peribysins E–G (173–175)/fungus | Sesquiterpenef | Cell adhesion inhibition | 15–20.1 μM | Undetermined | JPN | [203,204] |
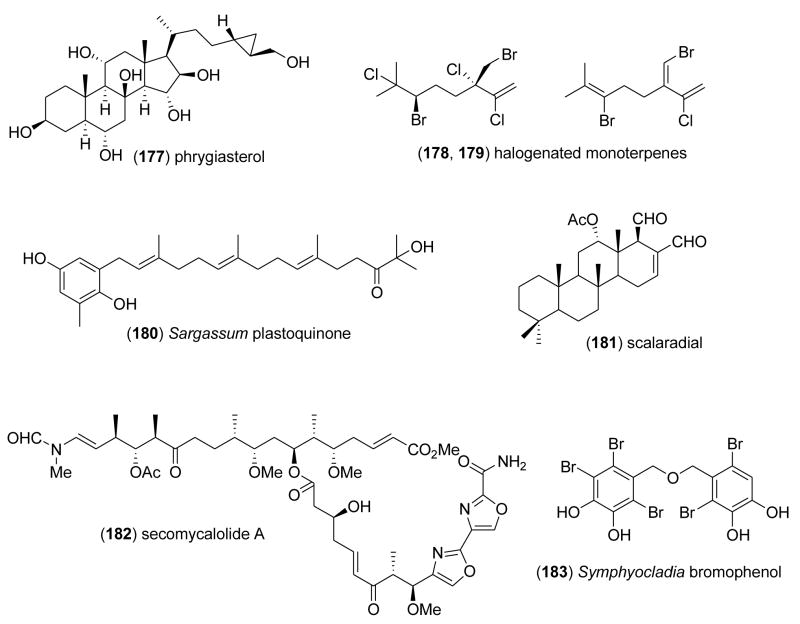
| petrosamine B (176)/sponge | Alkaloidg | Aspartyl semialdehyde dehydrogenase inhibition | 306 μM | Undetermined | AUS | [205] |
| phrygiasterol (177)/starfish | Sterolf | Inhibition of Ca2+ influx | 20 μg/mL | Undetermined | RUS | [206] |
| Portieria hornemannii monoterpenes (178,179)/alga | Monoterpenef | DNA methyl transferase-1 inhibition | 1.25–1.65 μM | Undetermined | USA | [207] |
| Sargassum micracanthum plastoquinone (180)/alga | Meroterpenef | Lipid peroxidation inhibition | 0.95 μg/m L | Undetermined | JPN | [208] |
| scalaradial (181)/sponge | Sesterterpenef | PI3K/Akt signaling inhibition | 2.9 μM | Undetermined, but independent of sPLA2 | CHN | [209] |
| secomycalolide A(182)/sponge | Polyketidee/Peptide | Rat proteasome activity inhibition | 11 μg/mL | Undetermined | JPN | [210] |
| Symphyocladia latiuscula bromophenol (183)/alga | Polyketide | Aldose reductase inhibition | 0.11–1.15 μg/mL | Undetermined | CHN | [211] |
Organism, Kingdom Animalia: ascidians, shark (Phylum Chordata), anemone, corals (Phylum Cnidaria), starfish ( Phylum Echinodermata), sea slug (Phylum Mollusca), sponge (Phylum Porifera); Kingdom Fungi: fungus; Kingdom Plantae: alga;
IC50: concentration of a compound required for 50% inhibition in vitro;
MMOA: molecular mechanism of action;
Country: CAN: Canada; CHE: Switzerland; CHL: Chile; CHN: China; CRI: Costa Rica; DEU: Germany; ESP: Spain; FRA: France;GBR: United Kingdom; IDN: Indonesia; ITA: Italy; JPN: Japan; NCL: New Caledonia; NZL: New Zealand; RUS: Russia; S. KOR: South Korea; SWE: Sweden; TAIW: Taiwan;
Polyketide;
Terpene;
Nitrogen-containing compound;
polysaccharide.
Figure 3.
2. Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral activities
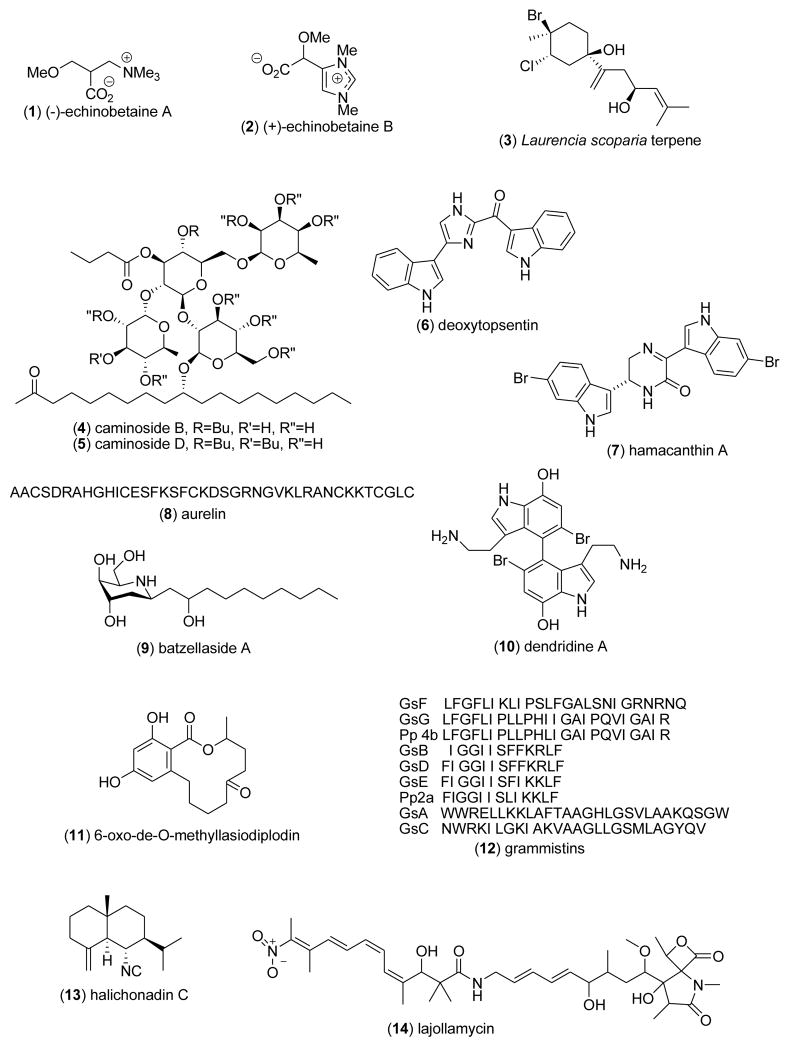
Table 1 presents new pharmacological findings reported during 2005–6 on the anthelmintic, antibacterial, anticoagulant, antifungal, antimalarial, antiprotozoal, antituberculosis, and antiviral pharmacology of the 78 marine natural products shown in Fig. 1.
2.1 Anthelmintic and antibacterial activity
Three studies contributed to the search of novel anthelmintic marine natural products during 2005–6. Capon and colleagues [27,28] described two novel betaines (−)-echinobetaine A (1) and (+)-echinobetaine B (2), from the Australian sponge Echinodictyum sp. which were nematocidal (LD99=83 and 8.3μg/mL, respectively) to the commercial livestock parasite Haemonchus contortus. Although the mechanism of action of these compounds remains undetermined, (+)-echinobetaine B’s nematocidal activity was comparable to that of “two commercially available synthetic antihelmintics, closantel and levamisole”. Davyt and colleagues [29] reported a novel halogenated β-bisabolene sesquiterpenoid (3) from the red alga Laurencia scoparia that showed anthelmintic activity (EC50=0.11 mM) against the parasitant stage (L4) of Nippostrongilus brasiliensis, a rat gastrointestinal parasite that has a similar lifestyle and morphology to human hookworms.
As part of an ongoing global effort to discover novel antimicrobials to treat infections caused by resistant pathogenic bacteria, during 2005–6, 27 studies contributed novel antibacterial marine natural products isolated from marine fungi, bacteria, sponges, soft corals, jellyfish and fish, a considerable increase from our previous reviews [1–5]. Only two reports provided detailed mechanism of action studies. Linington and colleagues [30] discovered that the novel caminosides B (4) and D (5) glycolipids, isolated from the Caribbean marine sponge Caminus sphaeoroconia, were inhibitors of pathogenic E.coli type III secretion system. Both caminosides were observed to “possess a number of structural features not found in sponge glycolipids” and were also noted to be effective against Gram-positive methicillin-resistant S. aureus and vancomycin–resistant Enteroccocus (MIC=3.1–6.3 μg/disk). Oh and colleagues [31] reported that the bis(indole) alkaloids deoxytopsentin (6) and hamacanthin A (7) isolated from the marine sponge Spongosorites sp. exhibited potent antibacterial activity against S. aureus (MIC=3.12–6.35 μg/mL). Interestingly, both alkaloids inhibited the enzyme sortase A (IC50=15.7 & 86.3 μg/mL, respectively), a membrane-associated transpeptidases that plays a key role in Gram-positive pathogenic bacterial invasion of host cells.
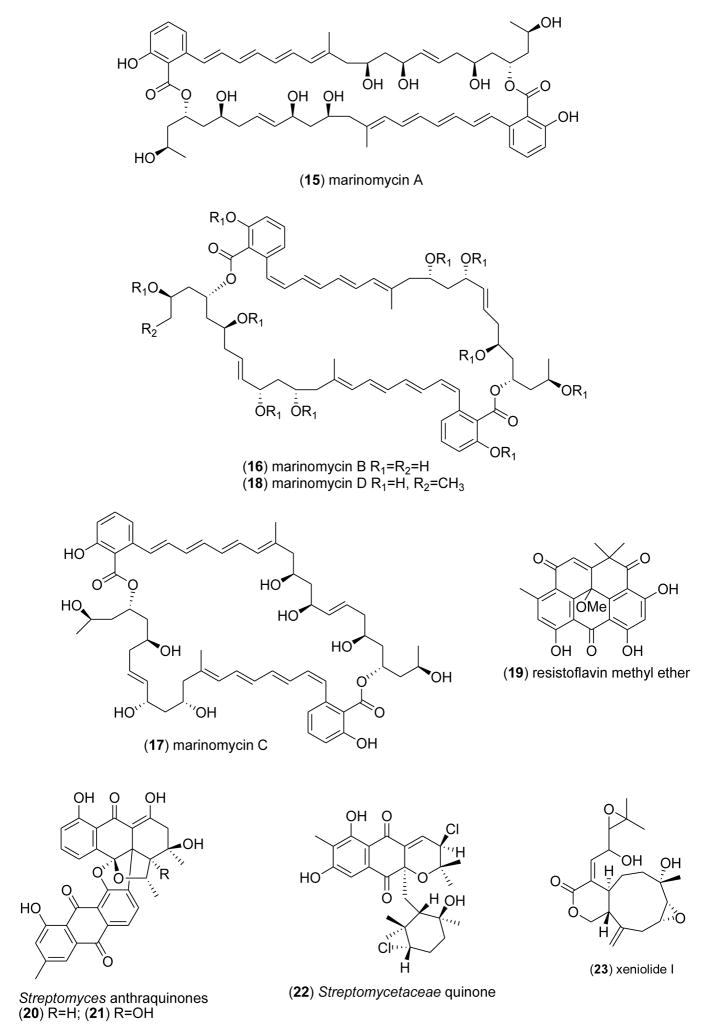
As shown in Table 1, several potent marine antibacterials were also reported in 2005–6 (Fig 1), with MICs less than 10 μg/mL against several antibiotic-resistant bacterial strains, but unfortunately the articles did not include data on putative mechanisms of action: aurelin (8) [32]; batzellaside A (9) [33]; dendridine A (10) [34]; 6-oxo-de-O-methyllasiodiplodin (11) [35]; grammistins (12) [36]; halichonadin C (13) [37]; lajollamycin (14) [38]; marinomycins A (15), B (16), C (17) and D (18) [39]; resistoflavin methyl ether (19) [40]; Streptomyces anthraquinones (20–21) [41]; Streptomycetaceae quinone (22) [42] and, xeniolide I (23) [43].
Furthermore, novel structurally characterized marine molecules with MICs greater than 10 μg/mL were also isolated during this period, but are not included in Table 1 or Fig. 1 because of their weaker antibacterial activity: agelasidine A, (MIC=50 μg/mL) [44], alkylpyridinium(MIC<25 μg/mL) [45]; diaporthelactone (MIC=50 μg/mL) [46]; Geniculosporium sp. botryanes [47]; guangomide A & B (MIC=100 μg/mL) [48]; latrunculins (MIC=14.7–17.8 μg/mL) [49]; norresistomycin (MIC=16 μg/mL) [50]; perinadine A (MIC=33–66.7 μg/mL) [51]; Pseudomonas aeruginosa quinoline (MIC=50–100 μg/mL) [52]; rifamycin B & SV [53]; sarasinoside A1 and J [54]; scalusamide A (MIC=33 μg/mL) [55], and Thorectandra sp. alkaloid (MIC=12.5 μg/mL) [56]. Although these marine compounds demonstrated weaker antimicrobial activity, they highlight the fact that novel antimicrobial leads may result from further research into the chemical biodiversity present in marine bacteria, fungi and sponges.
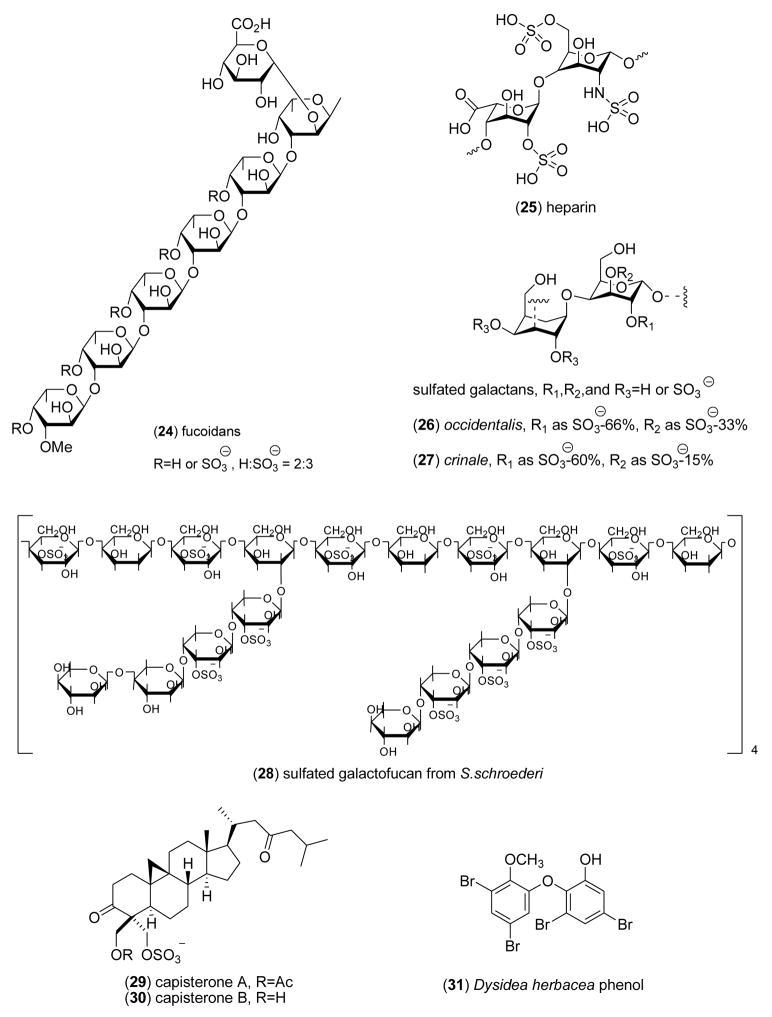
2.2 Anticoagulant activity
As shown in Table 1, during 2005–6, 5 articles reported anticoagulant marine natural products isolated from algae, fish and clams, an increase from our previous reviews [1–5]. Rajapakse and colleagues [57] characterized a 12.01 kDa single-chain monomeric protein from the marine yellowfin sole (Limanda aspera) which inhibited the blood coagulation serine endopeptidase factor XII (IC50<1 μM) by forming an inactive complex, and also triggered platelet aggregation by binding to a membrane glycoprotein integrin. Drozd and colleagues [58] extended the pharmacology of the fucoidans (24) from the marine algae Fucus evanescens and Laminaria cichorioides, showing that these sulfated polysaccharides inhibited both thrombin and factor Xa with potency comparable to non-fractioned and low-molecular weight heparins, although with considerable variability attributed to the “degree of sulfation and various types of glycoside bonds”. Luppi and colleagues [59] reported the purification and structural characterization of an unusual low-sulfated heparin (25) from the marine Italian bivalve mollusk Callista chione that decreased anti-factor Xa and activated partial thromboplastin time activity (IC50=52–97 IU/mg), probably as the result of a specific decrease in sulfation at position 2 of the uronic acid units. Pereira and colleagues [60] using an approach that combined structural analysis with specific biological assays, investigated the anticoagulant pharmacology of sulfated galactans (26,27) isolated from the red marine alga Gelidium crinale. Their detailed mechanistic studies demonstrated that 2,3-disulfated a-galactose units along the galactan chain were of major significance for the sulfated galactans’s anticoagulant activity, because the chains modulated interactions of the polysaccharides with “target proteases and coagulation inhibitors”. Rocha and colleagues [61] described a novel sulfated galactofucan (28) isolated from the marine brown alga Spatoglossum schroederi with a unique structure composed of a central core of 4-linked, partially 3-sulfated β-galactose units. Remarkably, the polysaccharide had no anticoagulant activity, yet showed potent antithrombotic activity resulting from the synthesis of heparan sulfate by vascular endothelial cells.
2.3 Antifungal activity
As shown in Table 1, sixteen studies during 2005–6 reported on the antifungal activity of several novel marine natural products isolated from marine algae, fungi, bacteria, sponges and sea stars, a substantial increase from our 1998–2004 reviews [1–5].
Four reports extended the molecular pharmacology of novel antifungal marine chemicals. Li and colleagues [62] discovered that the capisterones A and B (29,30) from the green alga Penicillus capitatus reversed drug resistance to clinically relevant azole-resistant fungal strains. Interestingly, although both compounds had no inherent antifungal activity, they enhanced fluconazole activity in efflux pump-overexpressing Candida albicans strains, suggesting their utility in protocols for resistant fungal infections. Sionov and colleagues [63] observed that a phenol compound (31) from the marine sponge Dysidea herbacea had significant activity against the human fungal pathogens C. albicans and Aspergillus fumigatus (MIC=1.95–7.8 μg/mL) which compared well with the clinically used antifungal amphotericin B (MIC=1–2 μg/mL). The phenol compound caused significant concentration-dependent changes in fungal cell morphology and cell membrane, resulting in K+ ion leakage. Pettit and colleagues [64] extended the in vitro and in vivo pharmacology of the marine spongistatin 1 (32) isolated from the marine sponge Hyrios erecta, a previously described anticancer agent [65]. The macrocyclic lactone polyether was shown to be fungicidal to 74 reference strains and clinical isolates (MIC=1–32 μg/mL), including several fungal strains resistant to the clinically used drugs flucytosine, ketoconazole and fluconazole. Furthermore, mechanism of action studies revealed that spongistatin 1 disrupted cytoplasmatic and spindle microtubules in Cryptococcus neoformans in a time- and concentration-dependent manner, preventing nuclear migration, and both nuclear and cellular cell division. Jang and colleagues [66] found that a synthetic analogue of halocidin (33), a previously reported antimicrobial peptide isolated from the hemocytes of a marine ascidian, had potent antifungal activity (MIC=1–4 μg/mL). The synthetic Di-K19Hc peptide derivative of 33 was shown to bind to C. albicans very rapidly (30 seconds) via an interaction with β-1,3-glucan, a component of the fungal cell wall, and concomitantly inducing ion channel formation, K+ efflux, and death of the fungal cell.
Additionally, and as shown in Table 1, several marine chemicals showed significant antifungal activity (i.e. MICs that were less than 10 μg/mL (Fig 1; 34–43), but unfortunately mechanism of action studies were lacking at the time of publication: the lipopeptide hassallidin A (34), (MIC=4.8 μM) [67], the polyketide latrunculins (35–42), (MIC=2.5–19 μM) [49], and the fatty acid majusculoic acid (43), (MIC=8 μM) [68]. Further investigation of the molecular pharmacology of these compounds will be required to determine their mechanism of action.
Finally, additional novel structurally-characterized marine molecules demonstrated MICs greater than 10 μg/mL, and therefore because of the weaker antifungal activity they have been excluded from Table 1 and Fig. 1: amphidinols (IC50=10–58 μM) [69,70], callipeltins F–I (IC50=100 μM) [71], Lamellodysidea herbacea sterols [72], minutosides A and B [73], oceanalin A (IC50=30 μM) [74], sokodoside A and B [75], and sterigmatocyn [76]. Although these marine chemicals showed weaker antifungal activity, they represent potential pharmacological leads perhaps possessing novel and uncharacterized mechanisms of action that might ultimately benefit the ongoing global search for clinically useful antifungal agents.
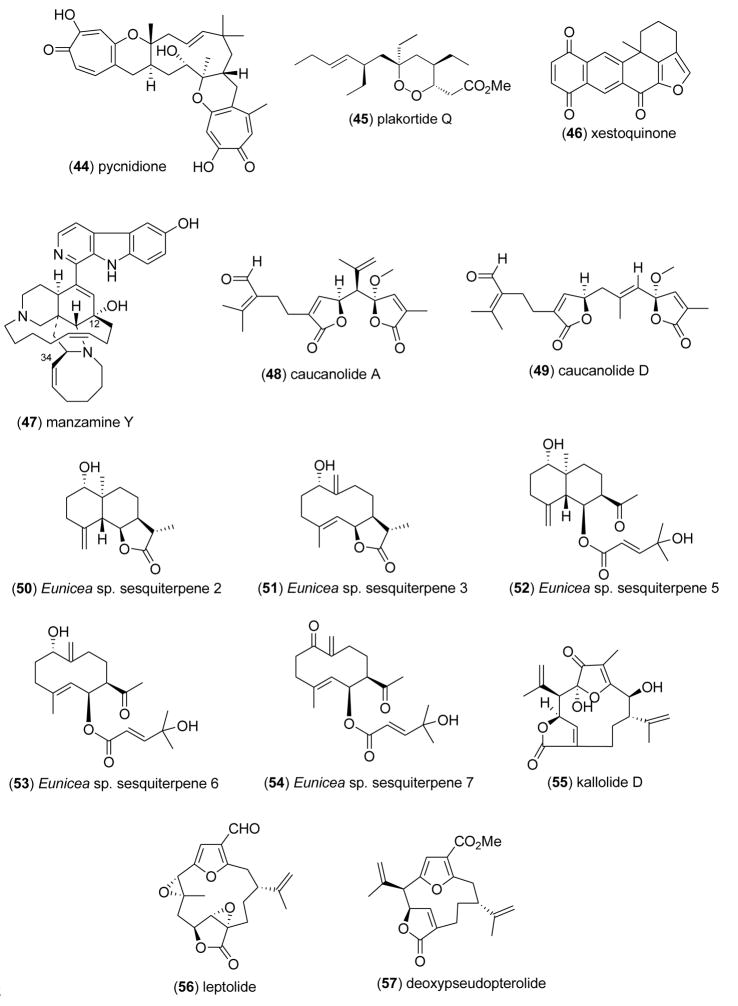
2.4 Antimalarial, antiprotozoal, and antituberculosis activity
As shown in Table 1, in 2005–6 nine studies were reported in the area of antimalarial, antiprotozoal and antituberculosis pharmacology of structurally characterized marine natural products, a significant increase from our previous 1998–2004 reviews [1–5].
Wright and Lan-Unnasch [77] reported that pycnidione (44) isolated from the marine fungus Phoma sp., had significant antiplasmodial activity against three strains of Plasmodium falciparum (IC50=0.15–0.4 μM). Because of structural similarities between pycnidione and atovaquone, an ingredient of the antimalarial medication Malarone®, the investigators proposed that the antiplasmodial activity of pycnidione was “significant in terms of lead structure development”. Campagnuolo and colleagues [78] identified antimalarial activity in novel polyketide cycloperoxides isolated from the marine sponge Plakortis simplex. The known plakortide Q (45) demonstrated the highest inhibition of P. falciparum chloroquine-sensitive and chloroquine-resistant strains (IC50=0.52–1 μM), suggesting that the configuration at C-3 exerted a significant effect on antimalarial activity of these compounds. Laurent and colleagues [79] proved that the known xestoquinone (46) isolated from the Pacific Ocean sponge Xestospongia sp. had significant in vitro antiplasmodial activity (IC50=3μM), and inhibited Pfnek-1(IC50=1 μM), a protein kinase of P. falciparum that plays a yet undetermined role in its biochemistry. Rao and colleagues [80] highlighted the bioactivity of four new manzamine-type alkaloids, as well as that of 13 known manzamine alkaloids isolated from Indonesian sponges of the genus Acanthostrongylophora against the chloroquine-sensitive and chloroquine-resistant strains of P. falciparum. Although less potent than artemisinin, used as a control in these studies (IC50=10 & 6.3 ng/mL, respectively), the higher bioactivity of manzamine Y (47) against P. falciparum (IC50=0.42–0.85 μg/mL) demonstrated the importance of hydroxy and the 8-membered ring in the aliphatic region of this molecule for the antimalarial activity.
Several additional marine chemicals were reported in 2005–6 to possess antimalarial activity, but their bioactivity appeared to be less significant, i.e. MIC >10μM: The diterpenes caucanolides A and D (48,49) from the Colombian gorgonian coral Pseudopterogorgia bipinnata, (IC50=17 μg/mL) [81], sesquiterpenoid metabolites (50–54) from a Caribbean gorgonian coral Eunicea sp., (IC50=10–18 μg/mL) [82], the diterpene kallolide D (55) from a Colombian Pseudopterogorgia species, (IC50=30.6 μM) [83], the furanocembranolide diterpenes leptolide (56) and deoxypseudopterolide (57) from the Panamanianoctocorals Leptogorgia alba and Leptogorgia rigida, (IC50= 50 & 74 μM, respectively)[84], and a tyramine derivative (58) from the Panamanian octocoral Muricea austera (IC50=36 μM) [85].
Three marine compounds were reported to possess antiprotozoal activity. Lim and colleagues [86] found that ent-plakortide P (59), a new natural product from the sponge Plakortis sp., inhibited Leishmania mexicana proliferation (IC50=1 μg/mL), although it appeared to be less potent than ketoconazole (IC50=0.06 μg/mL). Washida and colleagues [87] examined a novel polyol compound karatungiol A (60) isolated from the symbiotic Indonesian marine dinoflagellate Amphidinium sp., and observed antiprotozoal activity against Trichomonas foetus (IC50=1 μg/mL). This constitutes an important observation in view of the fact that this flagellated protozoan parasite of both the bovine and feline reproductive tract appears to show increasing resistance to the anthelmintics fenbendazole and metronidazole. Gray and colleagues [88] discovered a new disulfated meroterpenoid, isoakaterpin (61), from extracts of the Brazilian marine sponge Callyspongia sp. that inhibited Leishmania spp. adenine phosphoribosyl transferase (IC50=1.05 μM), an enzyme that is part of the purine salvage pathway in the parasite, and “should compromise parasite but not mammal metabolism”.
Three novel marine compounds were contributed to the global search for novel antituberculosis agents. De Oliveira and colleagues [89] reported that (+)-fistularin -3 (62) and 11-deoxy-fistularin-3 (63) isolated from the Brazilian sponge Aplysina cauliformis inhibited growth of Mycobacterium tuberculosis H37Rv (MIC=7.1–7.3 μM, respectively), thus extending previous observations on the antituberculosis activity of fistularin-3 (62)[90]. Because these compounds evidenced very low toxicity to macrophages (IC50=200 and 630 μM, respectively), there is definite potential for these compounds to become leads for antituberculosis drug development. As part of the investigation of the extensive chemodiversity of the Caribbean sea whip Pseudopterogorgia elisabethae, Rodriguez and colleagues [91] noted that at the concentration range of 128-64 mg/mL the novel benzoxazole alkaloid ileabethoxazole (64) inhibited M. tuberculosis (H37Rv, MIC=61 μg/mL), with a potency that “lies within the same range as that of the very active rifampin”. As a result of an ongoing investigation to identify new manzamines from the Indo-Pacific sponge, Acanthostrongylophora sp., Rao and colleagues [80] identified two of the alkaloids, namely (+)-8-hydroxymanzamine A (66) and manzamine F (73), that inhibited M. tuberculosis (H37Rv, MIC=0.9 & 0.4 μg/mL, respectively), results which compared very favorably with rifampicin (MIC=0.5 μg/mL), a first-line antituberculosis drug.
2.5 Antiviral activity
As shown in Table 1, interest in the antiviral pharmacology of novel marine natural products remained high during 2005–6. Four studies reported novel marine chemicals with antiviral activity against herpes simplex, measles and cytomegalovirus. Rodriguez and colleagues [92] isolated three galactan polysaccharide fractions from the Argentinian marine algae Callophyllis variegata which showed potent antiviral activity against herpes simplex types 1 (HSV-1) and 2 (HSV-2) (IC50=0.16–2.19 μg/mL) and dengue type 2 (IC50=0.1–0.41 μg/mL), together with low cytotoxicity, suggesting that these compounds might become “promising antiviral agents”. Lee and colleagues [93] described a sulfated polysaccharide naviculan from Navicula directa, a diatom collected from deep-sea water in Toyama Bay, Japan, which inhibited HSV-1 and HSV-2 (IC50=7–14 μg/mL) by interferring with early stages of viral replication, probably affecting viral binding, adsorption and penetration into host cells. Matsuhiro and colleagues [94] reported the structural analysis and antiviral activity of a sulfated galactan isolated from the marine red seaweed Schizymenia binderi. The sulfated galactan exhibited highly selective antiviral activity against HSV-1 and HSV-2 (IC50=0.18–0.76 μg/mL), very low cytotoxicity, appeared to inhibit viral adsorption to host cells and was thus considered to be superior to “other previously reported sulfated galactans of algal origin”. Iwashima and colleagues [95] discovered that three plastoquinones (74–76) isolated from the marine alga Sargassum micracanthum inhibited cytomegalovirus (IC50=0.49–2.6 μM) and measles virus (IC50=2.7–3.1 μM), suggesting that the compounds could become “lead compounds in an anti-human cytomegalovirus drug” development.
Two reports contributed additional pharmacology against human immunodeficiency virus type-1 (HIV-1), the causative agent of the acquired immunodeficiency disease syndrome (AIDS), a disease that infects more than 40 milion people worldwide. In a detailed mechanistic study De Souza and colleagues [96] described the biochemical pharmacology of two diterpenes (77–78) isolated from a Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase enzyme. Both diterpenes were shown to behave as classical non-competitive reversible inhibitors of the RNA-dependent DNA polymerase activity of HIV-1 reverse transcriptase (Ki=10 and 35 μM, respectively). Mori and colleagues [97] contributed the characterization of a novel and potent HIV-inactivating protein griffithsin from the red alga Griffithsia sp. Griffithsin, a new type of lectin, displayed potent antiviral activity against laboratory strains and primary isolates of HIV-1 (IC50=0.043–0.63 nM), by a mechanism that required binding to viral glycoproteins (eg. gp120, gp41 and gp160) in a monosaccharide-dependent manner. Furthermore, the authors noted griffithsin was a potential “candidate microbicide to prevent the sexual transmission of HIV and AIDS”.
3. Marine compounds with anti-inflammatory effects and affecting the cardiovascular, immune and nervous system
Table 2 summarizes the preclinical pharmacological research completed during 2005–2006 with the 47 marine secondary metabolites shown in Fig. 2.
3.1 Anti-inflammatory compounds
The anti-inflammatory pharmacology of marine compounds reported during 2005–6 showed a considerable increase from our previous reviews [1–5].
Busserolles and colleagues [98] tested the hypothesis that oral administration of bolinaquinone (79) and petrosiaspongiolide M (80), two marine terpenes isolated from the sponges Dysidea sp. and Petrosaspongia nigra, could inhibit inflammation and oxidative stress in an in vivo murine model of inflammatory bowel disease in humans. The observation that both compounds inhibited neutrophilic infiltration, interleukin-1β, prostaglandin E2 levels and cyclooxygenase 2 protein expression in vivo, supports further development of these compounds for “protective strategies” against intestinal inflammatory diseases. Miyaoka and colleagues [99] contributed to the pharmacology of phospholipase A2 inhibitors by investigating two sesterterpenoids, cladocorans A (81) and B (82) from the coral Cladocora cespitosa, which possess a -hydroxy-butenolide moiety. Cladocorans A and B were observed to potently inhibit secretory phospholipase A2 (IC50=0.8–1.9 μM), with a potency similar to manoalide (IC50=0.6 μM). McNamara and colleagues [100] reported the isolation of a novel isozonarone derivative (83) and of isozonarol (84) from the New Zealand sponge Dysidea cf. cristagalli. In vitro studies with human neutrophils demonstrated a concentration-dependent reduction of superoxide anion release (IC50=3–11 μM) by a mechanism hypothesized to involve the accumulation of the lipophilic sesquiterpene moiety in cell membranes, where it could interfere with superoxide production. Mayer and colleagues [101] conducted a structure-activity relationship (SAR) study to investigate the anti-neuroinflammatory properties of the indole-derived alkaloids manzamines A (65), B (69), C (85), D (86), E (71) and F (73), isolated from the marine sponges Haliclona sp., Amphimedon sp., and Xestospongia sp. Manzamine A’s potent inhibition of both superoxide anion (IC50=0.1 μM) and thromboxane B2 (IC50=0.016 μM) release by activated brain microglia cells, suggested that the “solubility or ionic forms of manzamine A as well as changes such as saturation or oxidation of the β carboline or 8-membered amine ring” played a critical role in the observed SAR results. Sawant and colleagues [102] investigated both the marine cembranoid diterpene sarcophine (87) and a semisynthetic sulfur-containing derivative (88) in an in vitro anti-neuroinflammatory assay [103]. Only compound (87) significantly inhibited both generation of superoxide anion and thromboxane B2 (IC50=1 μM) from activated rat brain macrophages, demonstrating that “targeting the epoxide ring of sarcophine” enhanced sarcophine’s anti-inflammatory activity. Mandeau and colleagues [104] showed that a new steroid, 3β-hydroxy-26-norcampest-5-en-25-oic acid (89) from the sponge Euryspongia n. sp. reduced 6KPGF1α production by human keratinocytes by 41% at 10 μg/mL. Interestingly, Ahmed and colleagues [105] reported that the known steroid gibberoketosterol (90), isolated from the Formosan soft coral Sinularia gibberosa, significantly reduced proinflammatory iNOS and COX-2 proteins in lipopolysaccharide-stimulated murine macrophages at a concentration of 10 μM to 44.5 % and 68.3 % of control values, respectively. Tziveleka and colleagues [106] submitted anti-inflammatory studies with the known chromenol (91) isolated from the marine Greek sponge Ircinia spinosula. The authors noted that the compound’s potent inhibition of leukotriene B4 generation by stimulated porcine leukocytes (IC50=1.9 μM), was related to the “absence of a side chain OH group as well as the reduced number of prenyl moieties” on the sponge metabolite. Huang and colleagues [107] described a novel sesquiterpenoid isoparalemnone (92) from the Formosan soft coral Paralemnalia thyrsoides that significantly inhibited inflammatory iNOS protein expression (70% at 10 μM) in activated RAW 264.7 cells. Sugiura and colleagues [108] reported that a phlorofucofuroeckol-B (93) from an edible Japanese marine brown alga, Eisenia arborea, inhibited histamine release (IC50=7.8 μM) from a rat basophilic leukemia in a concentration-dependent manner, an observation which compared favorably with a clinically used antihistamine Tranilast (IC50=46.6 μM). Kita and colleagues [109] discovered a novel amphoteric iminium metabolite, symbioimine (94) in a dinoflagellate Symbiodinium sp. isolated from the marine flatworm Amphiscolops sp., and showed that it inhibited the cyclooxygenase 2 enzyme by 32% at 10 μM. The authors suggested that symbioimine might become a useful lead to develop new nonsteroidal anti-inflammatory drugs.
3.2 Cardiovascular compounds
Sauviate and colleagues [110] reported novel studies on the mechanism of action of lepadiformines A and B (95,96), previously described marine alkaloids from the tunicate Clavelina moluccensis. Lepadiformines A and B dose-dependently inhibited the background inward rectifying K+ current (IC50=1.42 μM) by blocking the cardiac muscle Kir channel, and putatively interacting with “one of the negatively charged aminoacids located in the inner vicinity of the narrow K+ selectivity filter, candidates being residues D172, E224 or E229. Onodera and colleagues [111] isolated zooxanthellamide Cs (97) from cultures of the marine dinoflagellate Symbiodinium sp., and showed they were vasoconstrictive to rat blood vessels (EC50= 0.39 μM). The structure-activity relationship study suggested that the “huge macrolactone structure” played an as yet undetermined but critical role in the vasoconstrictive activity.
3.3 Compounds affecting the immune system
As a significant contribution to the discovery of novel indoleamine 2,3-dioxygenase (INDO) inhibitors, agents shown to prevent immunological rejection of tumors, Pereira and colleagues [112], reported that the polyketides annulins A, B, and C (98–100) purified from the marine Northeastern Pacific hydroid Garveia annulata, potently inhibited INDO in vitro (Ki= 0.12–0.68 μM). Interestingly, the annulins were more potent than 1-methyltryptophan (Ki=6.6 μM), one of the most potent agents currently available. Aminin and colleagues [113] investigated the immunomodulatory properties of a “medical lead” named cumaside, which consisted of a complex of cholesterol with monosulfated cucumariosides (101), triterpene oligoglycosides from the Far-Eastern edible sea cucumber Cucumaria japonica. The investigators observed that cumaside, while lowering the membranolytic activity of the cucumariosides, appeared to significantly enhance their immunomodulatory properties on both human and murine macrophages and lymphocytes. Costantino and colleagues [114] contributed a new α-galactoglycosphingolipid, damicoside (102), isolated from the marine sponge Axinella damicornis. Damicoside exhibited concentration-dependent stimulatory activity in a murine spleen proliferation assay, showing that a free galactose 2-OH and 3-OH are critical for activity, while in contrast, a free galactose 4-OH is not required for the immunostimulatory activity of these bioactive glycosphingolipids compounds. Kim and colleagues [115] investigated the antiapoptotic activity of laminarin polysaccharides isolated from the alga Laminaria japonica. A detailed pharmacological investigation revealed that the laminarin polysaccharides suppressed mouse thymocyte apoptosis, while also significantly inducing the upregulation of 33 immunomodulatory genes from a total of 7,410 genes which were examined using a cDNA microarray. Xia and colleagues [116] extended the pharmacology of a sulfated polymannuroguluronate (SPMG) (103), a polysaccharide with an average molecular weight of 8.0 kDa isolated from the brown alga Laminaria japonica, which recently entered Phase II clinical trials in China as an anti-AIDS drug candidate. Although SPMG appeared to exert immunopotentiation by direct activation of T cell proliferation, and the concomitant modulation of cytokines, namely enhancement of interleukin-2 and interferon- generation and inhibition of tumor necrosis factor-α release, the authors concluded that “much remains, however, unknown about the immunomodulation mechanism of SPMG”. Oda and colleagues [117] described the pharmacology of verrucarin A (104), a compound isolated from the culture broth of the Palauan marine fungus Myrothecium roridum. Verrucarin A significantly inhibited interleukin-8 production from human promyelocytic leukemia cells, by a mechanism that involved inhibition of the activation of the mitogen activated kinases c-JUN and p38.
3.4 Compounds affecting the nervous system
Pharmacological studies with marine compounds affecting the nervous system during 2005–6 focused on three main areas of neuropharmacology: the stimulation of neurogenesis, the targeting of receptors, and other miscellaneous activities on the nervous system.
Biologically active molecules which stimulate neurogenesis and rescue damaged neuronal cells are potentially promising therapeutic strategies to treat neurodegenerative diseases [118]. As shown in Table 2, the enhancement of the neuritogenic properties of nerve growth factor (NGF), a chemical that has a critical role in differentiation, survival and neuronal regeneration, was reported for several marine natural compounds isolated from sea cucumbers, sea stars, brown algae and a fungus, respectively.
Nandini and colleagues [119] isolated a novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chain from the skin of the blue shark Prionace glauca which exhibited neuritogenic activity of both an axonic and a dendritic nature, as well as binding activities for various growth factors and two neurotrophic factors. The unique structure and biological activity of the proteoglycans demonstrated that shark skin has “immense potential to be exploited for pharmaceutical purposes”. Although it is clear that the harvest of sharks for either food or pharmaceutical purposes is highly questionable, from a sustainability point of view the characterization of biological metabolites from these animals is extremely interesting and significant. Kisa and colleagues [120,121] contributed two new monosialo- and disialo- gangliosides CEG-3 (105) and CEG-6 (106) from the Japanese sea cucumber Cucumaria echinata. Although the molecular mechanism of action remains undetermined, both gangliosides induced neurite outgrowth in 42–50% of rat pheochromocytoma PC12 cells at 10 μM in the presence of NGF, suggesting the “isolation and characterization of such neuritogenically active ganglosides” will require considerable further study. Inagaki and colleagues [122] contributed the first isolation and characterization of a trisialo-ganglioside LLG-5 (107) from the sea star Linckia laevigata. LLG-5 proved to be more neuritogenic (59.3 % at 10 μM) to rat pheochromocytoma PC12 cells than CEG-3 and CEG-6. Higuchi and colleagues [123] isolated a biologically active glycoside GP-3 (108) from the starfish Asterina pectinifera which proved to be slightly less neuritogenic (38.2 % at 10 μM) to rat pheochromocytoma PC12 cells than CEG-3, CEG-6 and LLG-5. Han and colleagues [124] reported a structure-activity relationship with new steroid glycosides, namely linckosides (109–111) isolated from the Okinawan sea star Linckia laevigata. All linckosides enhanced the neuritogenic activity of NGF by 40–98%, with a SAR study revealing the “importance of the carbon branch modified by a pentose at the side chain” in the neuritogenic activity. Wei and colleagues [125] investigated a novel polyketide shimalactone A (112) isolated from the cultured marine-derived fungus Emericella variecolor GF10. Shimalactone A induced neuritogenesis in a neuroblastoma Neuro 2A cell line at 10 μg/mL by an as yet undetermined mechanism. Tsang and colleagues [118] described sargachromenol (113) from the marine brown alga Sargassum macrocarpum. Sargachromenol was shown to “markedly” promote NGF-dependent neurogenesis in PC12D cells (ED50=9 μM). Interestingly, mechanistic studies demonstrated that both the cyclic AMP-mediated protein kinase and mitogen-activated protein kinase 1/2 signal transduction pathways were required for neurite growth stimulated by sargachromenol. Tsang’s detailed molecular studies clearly suggests that additional mechanism of action investigations with the gangliosides, linckosides and shimalactones might possibly help develop these chemicals as potentially new medicines for the treatment of neurodegenerative diseases.
As shown in Table 2, the conotoxins αD-VxXIIA, αD-VxXIIB, and αD-VxXIIC, conopeptide SO-3 and dysiherbaine, were shown to target receptors present in the nervous system.
Loughnan and colleagues [126] reported three novel conotoxins αD-VxXIIA, αD-VxXIIB, and αD-VxXIIC (114–116), purified from the venom of the marine snail Conus vexillum. A detailed series of mechanistic studies revealed that the three post-translationally modified conotoxins were non-competitive inhibitors of nicotinic acetylcholine receptors with selectivity towards α7 and β-containing neuronal receptor subtypes, and with αD-VxXIIB conotoxin being the most potent (IC50=0.4 nM for α7). Wen and colleagues [127] described a new O-superfamily conopeptide SO-3 (117), derived from the marine snail Conus striatus. Because the new conopeptide was shown to selectively target N-type voltage-sensitive calcium currents in cultured hippocampal neurons (IC50=0.16 μM), the authors suggested that it may have “therapeutic potential as a novel analgesic agent”. Sanders and colleagues [128,129] extended the pharmacology of dysiherbaines (118,119), potent kainate receptor agonists derived from the marine sponge Dysidea herbacea. Detailed molecular studies revealed the site residues responsible for subunit selectivity of the two compounds on kainate receptors, observations which could aid in the rational design of “selective ligands with distinct pharmacological properties”. Tsuneki and colleagues [130] investigated the preclinical pharmacology of the marine quinolizidine alkaloid (−) pictamine (120), isolated from the ascidian Clavelina picta. Pictamine irreversibly blocked α4β2 and α7 nicotinic acetylcholine receptors (IC50= 1.5 μM), and thus could become a valuable tool to study neuronal activity mediated by these two major types of nicotinic receptors.
As shown in Table 2, during 2005–6, additional marine compounds were reported to exhibit pharmacological effects on the nervous system. Aiello and colleagues [131] established the molecular pharmacology of a novel bromopyrrole alkaloid (121), isolated from the Mediterranean sponge Axinella verrucosa. In a series of in vitro studies, the alkaloid was observed to display potent neuroprotective activity against the agonists serotonin and glutamate. Aiello and colleagues [132] also reported another marine natural product, namely the alkaloid daminin (122) isolated from the Mediterranean sponge Axinella damicornis that was observed to reduce Ca2+ levels in neuronal cells in vitro stimulated with either glutamic acid or n-methyl-D-aspartate, agents that cause a strong rise in Ca2+ in these cells. Bringmann and colleagues [133] isolated a novel angucyclinone gephyromycin (123) from the bacterium Streptomyces griseus. Gephyromcyin appeared to “represent a new potent glutamate agonist” towards neuronal cells, and at 3 μg/mL caused significant increase in intracellular Ca2+ concentration, a response comparable to the potent glutamate agonist DCG-IV. To and colleagues [134] while studying the mechanisms involved in neuronal outgrowth observed that the alkaloid motuporamine C (124), isolated from the Papua New Guinea marine sponge Xestospongia exigua, stimulated concentration-dependent neuronal growth cone collapse. The intracellular signaling mechanisms involved significant upregulation of the Rho-Rho- kinase collapse pathway, suggesting this compound might be useful to examine mechanisms “utilized by neurons for outgrowth”. Temraz and colleagues [135] noted that Red Sea soft corals Sarcophyton glaucum and Lobophyton crassum contained natural products which include trigonelline (125), that increased the electrophysiological excitability of rat cultured dorsal root ganglion neurons. The increased excitability was associated with enhanced KCl-evoked Ca2+ influx consistent with an increase in action potential firing, perhaps contributing to “chemical defenses”.
4. Marine Compounds with Miscellaneous Mechanisms of Action
Table 3 lists 58 marine compounds with miscellaneous pharmacological mechanisms of action, and with their respective structures presented in Fig. 3. Because during 2005–2006 additional pharmacological data were unavailable, it was not possible to assign these compounds to a particular drug class as was the case for the compounds included in Tables 1 and 2.
As shown in Table 3, the pharmacological activity, respective IC50s, and a molecular mechanism of action have been reported for 23 marine natural products: Agelas sp. dibromopyrrole (126), adociaquinone B (127), barrettins (128 and 129), bromoageliferins (130 and 131), chlorolissoclimide (132), fascaplysin analogue CA224 (133), hippuristanol (134), liphagal ( 135), lukianol B (136), rubrolide (137), micropeptins (138 and 139), pateamine (140), phlorofucofuroeckol A (141), purealin (142), Spongia sesterterpenoids (143–145), squalamine analog (146), and xestospongin B (147) and C (148).
In contrast, although a pharmacological activity was described, and an IC50 for inhibition of an enzyme or receptor determined, detailed molecular mechanism of action studies were unavailable for the following 35 marine compounds included in Table 3: actiniarin B (149), amphezonol A (150), ascochitine (151), briaexcavatin E and G (152 and 153), brunsvicamides B and C (154 and 155), caulerpin (156), cortistatin A (157), cyanopeptolin 954 (158), dehydroluffariellolide diacid (159), O-methyl nakafuran-8-lactone (160), 2β,3α-epitaondiol (161), fascaplysin (162), gorgosterols (163–165), hexylitaconic acid (166), himeic acid A (167), kalihinol A (168), largamides D–G 169–172, peribysins E–G (173–175), petrosamine B (176), phrygiasterol (177), Portieria hornemannii monoterpenes (178 and 179), Sargassum micracanthum plastoquinone (180), scalaradial (181), secomycalolide A (182), and Symphyocladia latiuscula bromophenol (183).
5. Reviews on marine pharmacology
Several reviews covering both general and specific subject areas of marine pharmacology were published during 2005–6: (a) general marine pharmacology: biodiversity as a continuing source of novel drug leads [136]; international collaboration in drug discovery and development [137]; indole alkaloid marine natural products as a promising source of new drug leads for multiple disease categories [138]; the biopotential of marine actinomycete diversity and natural product discovery [139]; the renaissance of natural products as drug candidates [140]; bioactive compounds from cyanobacteria and microalgae [141]; drug discovery from natural sources [142]; a new resource for drug discovery: marine actinomycete bacteria [143]; bioactive compounds from marine processing byproducts [144]; implications of marine biotechnology on drug discovery [145]; (b) antimicrobial marine pharmacology: advances in antimicrobial and antiangiogenic pharmacology of squalamine [146]; marine natural products as anti-infective agents [147]; chemotyping/metabolomics use for metabolite profiling in microbial drug discovery [148]; the status of natural products from fungi and their potential as anti-infective agents [149]; (c) cardiovascular pharmacology: dietary long-chain omega-3 fatty acids of marine origin and their protective cardiovascular effects [150]; (d) antituberculosis, antimalarial and antifungal marine pharmacology: compounds for infectious diseases [151]; marine natural products against tuberculosis [152]; (e) antiviral marine pharmacology: antiviral activities of polysaccharides from natural sources [153]; antiplasmodial marine natural products in the perspective of current chemotherapy and prevention of malaria [154]; (f) anti-inflammatory marine pharmacology: therapeutic potential of the antioxidative properties of coelenterazine, a marine bioluminescent substrate [155]; chemistry and biology of anti-inflammatory marine phospholipase A2 inhibitors [156]; the structures, biosynthesis and pharmacology of the marine natural products of Pseudopterogoria elisabethae [157]; chemistry and biology of anti-inflammatory marine natural products [158]; marine sponge metabolites for the control of inflammatory diseases [159]; antioxidant metabolites from marine derived fungi [160]; (g) nervous system marine pharmacology: marine compounds for the treatment of neurological disorders [161]; potential candidates for Alzheimer’s disease [151]; novel pain relief via marine snails [162]; bryostatin-1: pharmacology and therapeutic potential as a CNS drug [163], and (h) miscellaneous molecular targets: V-ATPases as drug targets [164]; topoisomerase inhibitors of marine origin [165]; enzyme inhibitors from marine actinomycetes [166]; marine compounds as a new source for glycogen kinase 3 inhibitors [167].
6. Conclusion
Four years after the approval of the marine compound ziconotide (Prialt®) by the U.S. Food and Drug Administration [168], global research focused on the therapeutic potential of marine natural products remains very active and sustained. The latest update on the clinical pipeline of marine-derived agents is available at http://marinepharmacology.midwestern.edu/clinDev.htm.
The current contribution to the marine pharmacology reviews series which was begun in 1998 [1–5], demonstrates that marine pharmacology research continued to proceed at a sustained pace in 2005–2006, as a result of the active participation of natural product chemists and pharmacologists from Argentina, Australia, Brazil, Canada, Chile, China, Colombia, Costa Rica, Egypt, Finland, France, Germany, Greece, India, Indonesia, Israel, Italy, Japan, the Netherlands, New Caledonia, New Zealand, Panama, Portugal, Russia, Slovenia, South Korea, Spain, Sweden, Switzerland, Taiwan, United Kingdom, Uruguay, and the United States. Thus, if the rate of preclinical and clinical pharmacological research continues, we anticipate that more marine natural products will probably become potential leads for clinical development as novel therapeutic agents for the treatment of multiple disease categories.
Acknowledgments
This review was made possible with financial support from Midwestern University to AMSM; grant number 1R01A136596, from the National Institute of Allergy and Infectious Diseases, NIH, and the Medicines for Malaria Venture to MTH; NIH-SCORE Program (Grant S06GM08102) of the University of Puerto Rico to ADR; and FAPESP grant 05/60175-2 (São Paulo, Brazil) to RGSB. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Assistance with extensive searches of the 2005-2006 marine pharmacology literature in PubMed, Marinlit, Current Contents® and Chemical Abstracts®, as well as article retrieval by library staff members, medical and pharmacy students of Midwestern University, is most gratefully acknowledged. The authors especially wish to thank Mr. Bing Wang for assistance with the preparation of figures, and Ms. Mary Hall for carefully reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities;with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. The Pharmacologist. 2000;42:62–69. [Google Scholar]
- 2.Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities;affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol. 2002;132:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 3.Mayer AMS, Hamann MT. Marine pharmacology in 2001–2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol. 2005;140:265–286. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer AMS, Rodriguez AD, Berlinck RG, Hamann MT. Marine pharmacology in 2003–4: marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem Physiol C: Pharmacol. 2007;145:553–581. doi: 10.1016/j.cbpc.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer AMS, Hamann MT. Marine pharmacology in 2000: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer AMS. Marine Pharmacology in 1998 Antitumor and Cytotoxic Compounds. The Pharmacologist. 1999;41:159–164. [Google Scholar]
- 7.Mayer AMS, Lehmann VKB. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res. 2001;21:2489–2500. [PubMed] [Google Scholar]
- 8.Mayer AMS, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- 9.Mayer AMS, Gustafson KR. Marine pharmacology in 2001–2: antitumour and cytotoxic compounds. Eur J Cancer. 2004;40:2676–2704. doi: 10.1016/j.ejca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Mayer AMS, Gustafson KR. Marine pharmacology in 2003–2004: anti-tumour and cytotoxic compounds. Eur J Cancer. 2006;42:2241–2270. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Mayer AMS, Gustafson KR. Marine pharmacology in 2005–2006: antitumour and cytotoxic compounds. Eur J Cancer. 2008;44:2357–2387. doi: 10.1016/j.ejca.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz FJ, Bowden BF, Toth SI. Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR, editors. Marine Biotechnology, Pharmaceutical and Bioactive Natural Products. Vol. 1. Plenum Press; New York: 1993. pp. 197–308. [Google Scholar]
- 13.Fakhr I, Hamdy N, Radwan M, El-Batran S, El Shabrawy O. Studies on the anti-inflammatory and analgesic effects of extracts from marine sponges. Nat Prod Sci. 2006;12:74–78. [Google Scholar]
- 14.Matsubara K, Xue C, Zhao X, Mori M, Sugawara T, Hirata T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int J Mol Med. 2005;15:695–699. [PubMed] [Google Scholar]
- 15.Shin HC, Hwang HJ, Kang KJ, Lee BH. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharmacol Res. 2006;29:165–171. doi: 10.1007/BF02974279. [DOI] [PubMed] [Google Scholar]
- 16.Yim JH, Son E, Pyo S, Lee HK. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar Biotechnol (NY) 2005;7:331–338. doi: 10.1007/s10126-004-0404-6. [DOI] [PubMed] [Google Scholar]
- 17.Adhikari U, Mateu CG, Chattopadhyay K, Pujol CA, Damonte EB, Ray B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry. 2006;67:2474–2482. doi: 10.1016/j.phytochem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 18.da Silva AC, Kratz JM, Farias FM, Henriques AT, Dos SJ, Leonel RM, Lerner C, Mothes B, Barardi CR, Simoes CM. In vitro antiviral activity of marine sponges collected off Brazilian coast. Biol Pharm Bull. 2006;29:135–140. doi: 10.1248/bpb.29.135. [DOI] [PubMed] [Google Scholar]
- 19.Al-Fadhli A, Wahidulla S, D’Souza L. Glycolipids from the red alga Chondria armata (Kutz.) Okamura. Glycobiology. 2006;16:902–915. doi: 10.1093/glycob/cwl018. [DOI] [PubMed] [Google Scholar]
- 20.Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int Immunopharmacol. 2006;6:8–13. doi: 10.1016/j.intimp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Athukorala Y, Jung WK, Vasanthan T, Jeon YJ. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym. 2006;66:184–191. [Google Scholar]
- 22.Kelman D, Kashman Y, Rosenberg E, Kushmaro A, Loya Y. Antimicrobial activity of red sea corals. Mar Biol. 2006;149:357–363. [Google Scholar]
- 23.Lucas-Elio P, Hernandez P, Sanchez-Amat A, Solano F. Purification and partial characterization of marinocine, a new broad-spectrum antibacterial protein produced by Marinomonas mediterranea. Biochim Biophys Acta. 2005;1721:193–203. doi: 10.1016/j.bbagen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Rechter S, Konig T, Auerochs S, Thulke S, Walter H, Dornenburg H, Walter C, Marschall M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral Res. 2006;72:197–206. doi: 10.1016/j.antiviral.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Thakur AN, Thakur NL, Indap MM, Pandit RA, Datar VV, Muller WE. Antiangiogenic, antimicrobial, and cytotoxic potential of sponge-associated bacteria. Mar Biotechnol (NY) 2005;7:245–252. doi: 10.1007/s10126-004-4085-y. [DOI] [PubMed] [Google Scholar]
- 26.Wang JH, Kong J, Li W, Molchanova V, Chikalovets I, Belogortseva N, Luk’yanov P, Zheng YT. A beta-galactose-specific lectin isolated from the marine worm Chaetopterus variopedatus possesses anti-HIV-1 activity. Comp Biochem Physiol C: Pharmacol. 2006;142:111–117. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Capon RJ, Vuong D, Lacey E, Gill JH. (−)-Echinobetaine A: isolation, structure elucidation, synthesis, and SAR studies on a new nematocide from a southern Australian marine sponge, Echinodictyum sp. J Nat Prod. 2005;68:179–182. doi: 10.1021/np049687h. [DOI] [PubMed] [Google Scholar]
- 28.Capon RJ, Vuong D, McNally M, Peterle T, Trotter N, Lacey E, Gill JH. (+)-Echinobetaine B: isolation, structure elucidation, synthesis and preliminary SAR studies on a new nematocidal betaine from a southern Australian marine sponge, Echinodictyum sp. Org Biomol Chem. 2005;3:118–122. doi: 10.1039/b414839h. [DOI] [PubMed] [Google Scholar]
- 29.Davyt D, Fernandez R, Suescun L, Mombru AW, Saldana J, Dominguez L, Fujii MT, Manta E. Bisabolanes from the red alga Laurencia scoparia. J Nat Prod. 2006;69:1113–1116. doi: 10.1021/np060235+. [DOI] [PubMed] [Google Scholar]
- 30.Linington RG, Robertson M, Gauthier A, Finlay BB, MacMillan JB, Molinski TF, Van SR, Andersen RJ. Caminosides B–D, antimicrobial glycolipids isolated from the marine sponge Caminus sphaeroconia. J Nat Prod. 2006;69:173–177. doi: 10.1021/np050192h. [DOI] [PubMed] [Google Scholar]
- 31.Oh KB, Mar W, Kim S, Kim JY, Oh MN, Kim JG, Shin D, Sim CJ, Shin J. Bis(indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg Med Chem Lett. 2005;15:4927–4931. doi: 10.1016/j.bmcl.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Ovchinnikova TV, Balandin SV, Aleshina GM, Tagaev AA, Leonova YF, Krasnodembsky ED, Men’shenin AV, Kokryakov VN. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem Biophys Res Commun. 2006;348:514–523. doi: 10.1016/j.bbrc.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 33.Segraves NL, Crews P. A Madagascar Sponge Batzella sp. as a source of alkylated iminosugars. J Nat Prod. 2005;68:118–121. doi: 10.1021/np049763g. [DOI] [PubMed] [Google Scholar]
- 34.Tsuda M, Takahashi Y, Fromont J, Mikami Y, Kobayashi J. Dendridine A, a bis-indole alkaloid from a marine sponge Dictyodendrilla Species. J Nat Prod. 2005;68:1277–1278. doi: 10.1021/np050076e. [DOI] [PubMed] [Google Scholar]
- 35.Yang RY, Li CY, Lin YC, Peng GT, She ZG, Zhou SN. Lactones from a brown alga endophytic fungus (No. ZZF36) from the South China Sea and their antimicrobial activities. Bioorg Med Chem Lett. 2006;16:4205–4208. doi: 10.1016/j.bmcl.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama N, Araki M, Ishida M, Nagashima Y, Shiomi K. Further isolation and characterization of grammistins from the skin secretion of the soapfish Grammistes sexlineatus. Toxicon. 2005;45:595–601. doi: 10.1016/j.toxicon.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Ishiyama H, Hashimoto A, Fromont J, Hoshino Y, Mikami Y, Kobayashi J. Halichonadins A–D, new sesquiterpenoids from a sponge Halichondria sp. Tetrahedron. 2005;61:1101–1105. [Google Scholar]
- 38.Manam RR, Teisan S, White DJ, Nicholson B, Grodberg J, Neuteboom ST, Lam KS, Mosca DA, Lloyd GK, Potts BC. Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod. 2005;68:240–243. doi: 10.1021/np049725x. [DOI] [PubMed] [Google Scholar]
- 39.Kwon HC, Kauffman CA, Jensen PR, Fenical W. Marinomycins a–d, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora” [j. Am. Chem. Soc. 2006, 128, 1622–1632] J Am Chem Soc. 2006;128:1622–1632. doi: 10.1021/ja0558948. [DOI] [PubMed] [Google Scholar]
- 40.Kock I, Maskey RP, Biabani MA, Helmke E, Laatsch H. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot (Tokyo) 2005;58:530–534. doi: 10.1038/ja.2005.73. [DOI] [PubMed] [Google Scholar]
- 41.Socha AM, Garcia D, Sheffer R, Rowley DC. Antibiotic bisanthraquinones produced by a streptomycete isolated from a cyanobacterium associated with Ecteinascidia turbinata. J Nat Prod. 2006;69:1070–1073. doi: 10.1021/np050449b. [DOI] [PubMed] [Google Scholar]
- 42.Soria-Mercado IE, Prieto-Davo A, Jensen PR, Fenical W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J Nat Prod. 2005;68:904–910. doi: 10.1021/np058011z. [DOI] [PubMed] [Google Scholar]
- 43.Bishara A, Rudi A, Goldberg I, Benayahu Y, Kashman Y. Novaxenicins A–D and xeniolides I–K, seven new diterpenes from the soft coral Xenia novaebrittanniae. Tetrahedron. 2006;62:12092–12097. [Google Scholar]
- 44.Medeiros MA, Lourenco A, Tavares MR, Curto MJ, Feio SS, Roseiro JC. (−)-Agelasidine A from Agelas clathrodes. Z Naturforsch, C: Biosci. 2006;61:472–476. doi: 10.1515/znc-2006-7-802. [DOI] [PubMed] [Google Scholar]
- 45.Chelossi E, Mancini I, Sepcic K, Turk T, Faimali M. Comparative antibacterial activity of polymeric 3-alkylpyridinium salts isolated from the Mediterranean sponge Reniera sarai and their synthetic analogues. Biomol Eng. 2006;23:317–323. doi: 10.1016/j.bioeng.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Huang Y, Fang M, Wang J, Zheng Z, Su W. Cytotoxic and antimicrobial metabolites from marine lignicolous fungi, Diaporthe sp. FEMS Microbiol Lett. 2005;251:53–58. doi: 10.1016/j.femsle.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 47.Krohn K, Dai J, Florke U, Aust HJ, Drager S, Schulz B. Botryane metabolites from the fungus Geniculosporium sp. isolated from the marine red alga Polysiphonia. J Nat Prod. 2005;68:400–405. doi: 10.1021/np0498206. [DOI] [PubMed] [Google Scholar]
- 48.Amagata T, Morinaka BI, Amagata A, Tenney K, Valeriote FA, Lobkovsky E, Clardy J, Crews P. A chemical study of cyclic depsipeptides produced by a sponge-derived fungus. J Nat Prod. 2006;69:1560–1565. doi: 10.1021/np060178k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Sayed KA, Youssef DT, Marchetti D. Bioactive natural and semisynthetic latrunculins. J Nat Prod. 2006;69:219–223. doi: 10.1021/np050372r. [DOI] [PubMed] [Google Scholar]
- 50.Gorajana A, Kurada BV, Peela S, Jangam P, Vinjamuri S, Poluri E, Zeeck A. 1-Hydroxy-1-norresistomycin, a new cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. J Antibiot (Tokyo) 2005;58:526–529. doi: 10.1038/ja.2005.72. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki M, Tsuda M, Sekiguchi M, Mikami Y, Kobayashi J. Perinadine A, a novel tetracyclic alkaloid from marine-derived fungus Penicillium citrinum. Org Lett. 2005;7:4261–4264. doi: 10.1021/ol051695h. [DOI] [PubMed] [Google Scholar]
- 52.Uzair B, Ahmed N, Ahmad VU, Kousar F. A new antibacterial compound produced by an indigenous marine bacteria--fermentation, isolation, and biological activity. Nat Prod Res. 2006;20:1326–1331. doi: 10.1080/14786410601102017. [DOI] [PubMed] [Google Scholar]
- 53.Kim TK, Hewavitharana AK, Shaw PN, Fuerst JA. Discovery of a new source of rifamycin antibiotics in marine sponge actinobacteria by phylogenetic prediction. Appl Environ Microbiol. 2006;72:2118–2125. doi: 10.1128/AEM.72.3.2118-2125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai HF, Edrada RA, Ebel R, Nimtz M, Wray V, Proksch P. Norlanostane triterpenoidal saponins from the marine sponge melophlussarassinorum. J Nat Prod. 2005;68:1231–1237. doi: 10.1021/np050152d. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda M, Sasaki M, Mugishima T, Komatsu K, Sone T, Tanaka M, Mikami Y, Kobayashi J. Scalusamides A–C, new pyrrolidine alkaloids from the marine-derived fungus Penicillium citrinum. J Nat Prod. 2005;68:273–276. doi: 10.1021/np049661q. [DOI] [PubMed] [Google Scholar]
- 56.Segraves NL, Crews P. Investigation of brominated tryptophan alkaloids from two thorectidae sponges: Thorectandra and Smenospongia. J Nat Prod. 2005;68:1484–1488. doi: 10.1021/np0501334. [DOI] [PubMed] [Google Scholar]
- 57.Rajapakse N, Jung WK, Mendis E, Moon SH, Kim SK. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005;76:2607–2619. doi: 10.1016/j.lfs.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Drozd NN, Tolstenkov AS, Makarov VA, Kuznetsova TA, Besednova NN, Shevchenko NM, Zvyagintseva TN. Pharmacodynamic parameters of anticoagulants based on sulfated polysaccharides from marine algae. Bull Exp Biol Med. 2006;142:591–593. doi: 10.1007/s10517-006-0426-3. [DOI] [PubMed] [Google Scholar]
- 59.Luppi E, Cesaretti M, Volpi N. Purification and characterization of heparin from the Italian clam Callista chione. Biomacromolecules. 2005;6:1672–1678. doi: 10.1021/bm049196b. [DOI] [PubMed] [Google Scholar]
- 60.Pereira MG, Benevides NM, Melo MR, Valente AP, Melo FR, Mourao PA. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr Res. 2005;340:2015–2023. doi: 10.1016/j.carres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Rocha HA, Moraes FA, Trindade ES, Franco CR, Torquato RJ, Veiga SS, Valente AP, Mourao PA, Leite EL, Nader HB, Dietrich CP. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi. An ideal antithrombotic agent? J Biol Chem. 2005;280:41278–41288. doi: 10.1074/jbc.M501124200. [DOI] [PubMed] [Google Scholar]
- 62.Li XC, Jacob MR, Ding Y, Agarwal AK, Smillie TJ, Khan SI, Nagle DG, Ferreira D, Clark AM. Capisterones A and B, which enhance fluconazole activity in Saccharomyces cerevisiae, from the marine green alga Penicillus capitatus. J Nat Prod. 2006;69:542–546. doi: 10.1021/np050396y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sionov E, Roth D, Sandovsky-Losica H, Kashman Y, Rudi A, Chill L, Berdicevsky I, Segal E. Antifungal effect and possible mode of activity of a compound from the marine sponge Dysidea herbacea. J Infect. 2005;50:453–460. doi: 10.1016/j.jinf.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Pettit RK, Woyke T, Pon S, Cichacz ZA, Pettit GR, Herald CL. In vitro and in vivo antifungal activities of the marine sponge constituent spongistatin. Med Mycol. 2005;43:453–463. doi: 10.1080/13693780500050598. [DOI] [PubMed] [Google Scholar]
- 65.Uckun FM, Mao C, Jan ST, Huang H, Vassilev AO, Navara CS, Narla RK. Spongistatins as tubulin targeting agents. Curr Pharm Des. 2001;7:1291–1296. doi: 10.2174/1381612013397492. [DOI] [PubMed] [Google Scholar]
- 66.Jang WS, Kim HK, Lee KY, Kim SA, Han YS, Lee IH. Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Lett. 2006;580:1490–1496. doi: 10.1016/j.febslet.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Neuhof T, Schmieder P, Preussel K, Dieckmann R, Pham H, Bartl F, von Dohren H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J Nat Prod. 2005;68:695–700. doi: 10.1021/np049671r. [DOI] [PubMed] [Google Scholar]
- 68.MacMillan JB, Molinski TF. Majusculoic acid, a brominated cyclopropyl fatty acid from a marine cyanobacterial mat assemblage. J Nat Prod. 2005;68:604–606. doi: 10.1021/np049596k. [DOI] [PubMed] [Google Scholar]
- 69.Echigoya R, Rhodes L, Oshima Y, Satake M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae. 2005;4:383–389. [Google Scholar]
- 70.Morsy N, Matsuoka S, Houdai T, Matsumori N, Adachi S, Murata M, Iwashita T, Fujita T. Isolation and structure elucidation of a new amphidinol with a truncated polyhydroxyl chain from Amphidinium klebsii. Tetrahedron. 2005;61:8606–8610. [Google Scholar]
- 71.Sepe V, D’Orsi R, Borbone N, D’Auria MV, Giuseppe BBA, Monti MC, Catania A, Zampella A. Callipeltins F–I: new antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron. 2006;62:833–840. [Google Scholar]
- 72.Sauleau P, Bourguet-Kondracki ML. Novel polyhydroxysterols from the Red Sea marine sponge Lamellodysidea herbacea. Steroids. 2005;70:954–959. doi: 10.1016/j.steroids.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Chludil HD, Maier MS. Minutosides A and B, antifungal sulfated steroid xylosides from the patagonian starfish Anasterias minuta. J Nat Prod. 2005;68:1279–1283. doi: 10.1021/np050086f. [DOI] [PubMed] [Google Scholar]
- 74.Makarieva TN, Denisenko VA, Dmitrenok PS, Guzii AG, Santalova EA, Stonik VA, MacMillan JB, Molinski TF. Oceanalin A, a hybrid alpha, omega-bifunctionalized sphingoid tetrahydroisoquinoline beta-glycoside from the marine sponge Oceanapia sp. Org Lett. 2005;7:2897–2900. doi: 10.1021/ol050796c. [DOI] [PubMed] [Google Scholar]
- 75.Okada Y, Matsunaga S, van Soest RW, Fusetani N. Sokodosides, steroid glycosides with an isopropyl side chain, from the marine sponge Erylus placenta. J Org Chem. 2006;71:4884–4888. doi: 10.1021/jo060653j. [DOI] [PubMed] [Google Scholar]
- 76.Kralj A, Kehraus S, Krick A, Eguereva E, Kelter G, Maurer M, Wortmann A, Fiebig HH, Konig GM. Arugosins G and H: prenylated polyketides from the marine-derived fungus Emericellanidulans var. acristata. J Nat Prod. 2006;69:995–1000. doi: 10.1021/np050454f. [DOI] [PubMed] [Google Scholar]
- 77.Wright AD, Lang-Unnasch N. Potential antimalarial lead structures from fungi of marine origin. Planta Med. 2005;71:964–966. doi: 10.1055/s-2005-864181. [DOI] [PubMed] [Google Scholar]
- 78.Campagnuolo C, Fattorusso E, Romano A, Taglialatela-Scafati O, Basilico N, Parapini S, Taramelli D. Antimalarial polyketide cycloperoxides from the marine sponge Plakortis simplex. Eur J Org Chem. 2005;23:5077–5083. [Google Scholar]
- 79.Laurent D, Jullian V, Parenty A, Knibiehler M, Dorin D, Schmitt S, Lozach O, Lebouvier N, Frostin M, Alby F, Maurel S, Doerig C, Meijer L, Sauvain M. Antimalarial potential of xestoquinone, a protein kinase inhibitor isolated from a Vanuatu marine sponge Xestospongia sp. Bioorg Med Chem. 2006;14:4477–4482. doi: 10.1016/j.bmc.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 80.Rao KV, Donia MS, Peng J, Garcia-Palomero E, Alonso D, Martinez A, Medina M, Franzblau SG, Tekwani BL, Khan SI, Wahyuono S, Willett KL, Hamann MT. Manzamine B and E and ircinal A related alkaloids from an Indonesian Acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer’s diseases. J Nat Prod. 2006;69:1034–1040. doi: 10.1021/np0601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ospina CA, Rodriguez AD, Sanchez JA, Ortega-Barria E, Capson TL, Mayer AM. Caucanolides A–F, unusual antiplasmodial constituents from a colombian collection of the gorgonian coral Pseudopterogorgia bipinnata. J Nat Prod. 2005;68:1519–1526. doi: 10.1021/np050239z. [DOI] [PubMed] [Google Scholar]
- 82.Garzon SP, Rodriguez AD, Sanchez JA, Ortega-Barria E. Sesquiterpenoid metabolites with antiplasmodial activity from a Caribbean gorgonian coral, Eunicea sp. J Nat Prod. 2005;68:1354–1359. doi: 10.1021/np0501684. [DOI] [PubMed] [Google Scholar]
- 83.Marrero J, Ospina CA, Rodriguez AD, Baran P, Zhao H, Franzblau SG, Ortega-Barria E. New diterpenes of the pseudopterane class from two closely related Pseudopterogorgia species: isolation, structural elucidation, and biological evaluation. Tetrahedron. 2006;62:6998–7008. [Google Scholar]
- 84.Gutierrez M, Capson TL, Guzman HM, Gonzalez J, Ortega-Barria E, Quinoa E, Riguera R. Leptolide, a new furanocembranolide diterpene from Leptogorgia alba. J Nat Prod. 2005;68:614–616. doi: 10.1021/np049745z. [DOI] [PubMed] [Google Scholar]
- 85.Gutierrez M, Capson TL, Guzman HM, Gonzalez J, Ortega-Barria E, Quinoa E, Riguera R. Antiplasmodial metabolites isolated from the marine octocoral Muricea austera. J Nat Prod. 2006;69:1379–1383. doi: 10.1021/np060007f. [DOI] [PubMed] [Google Scholar]
- 86.Lim CW, Kim YK, Youn HD, Park HY. Enantiomeric compounds with antileishimanial activities from a sponge, Plakortis sp. Agric Chem Biotechnol. 2006;49:21–23. [Google Scholar]
- 87.Washida K, Koyama T, Yamada K, Kita M, Uemura D. Karatungiols A and B, two novel antimicrobial polyol compounds, from the symbiotic marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006;47:2521–2525. [Google Scholar]
- 88.Gray CA, de Lira SP, Silva M, Pimenta EF, Thiemann OH, Oliva G, Hajdu E, Andersen RJ, Berlinck RG. Sulfated meroterpenoids from the Brazilian sponge Callyspongia sp. are inhibitors of the antileishmaniasis target adenosine phosphoribosyl transferase. J Org Chem. 2006;71:8685–8690. doi: 10.1021/jo060295k. [DOI] [PubMed] [Google Scholar]
- 89.de Oliveira MF, de Oliveira JH, Galetti FC, de Souza AO, Silva CL, Hajdu E, Peixinho S, Berlinck RG. Antimycobacterial brominated metabolites from two species of marine sponges. Planta Med. 2006;72:437–441. doi: 10.1055/s-2005-916239. [DOI] [PubMed] [Google Scholar]
- 90.Gao H, Kelly M, Hamann MT. Bromotyrosine-derived metabolites from the sponge Aiolochroia crassa. Tetrahedron. 1999;55:9717–9726. [Google Scholar]
- 91.Rodriguez II, Rodriguez AD, Wang YH, Franzblau SG. Ileabethoxazole: a novel benzoxazole alkaloid with antimycobacterial activity. Tetrahedron Lett. 2006;47:3229–3232. [Google Scholar]
- 92.Rodriguez MC, Merino ER, Pujol CA, Damonte EB, Cerezo AS, Matulewicz MC. Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales) Carbohydr Res. 2005;340:2742–2751. doi: 10.1016/j.carres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Lee JB, Hayashi K, Hirata M, Kuroda E, Suzuki E, Kubo Y, Hayashi T. Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol Pharm Bull. 2006;29:2135–2139. doi: 10.1248/bpb.29.2135. [DOI] [PubMed] [Google Scholar]
- 94.Matsuhiro B, Conte AF, Damonte EB, Kolender AA, Matulewicz MC, Mejias EG, Pujol CA, Zuniga EA. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta) Carbohydr Res. 2005;340:2392–2402. doi: 10.1016/j.carres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Iwashima M, Mori J, Ting X, Matsunaga T, Hayashi K, Shinoda D, Saito H, Sankawa U, Hayashi T. Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol Pharm Bull. 2005;28:374–377. doi: 10.1248/bpb.28.374. [DOI] [PubMed] [Google Scholar]
- 96.de Souza PH, Leao-Ferreira LR, Moussatche N, Teixeira VL, Cavalcanti DN, da Costa LJ, Diaz R, Frugulhetti IC. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase. Planta Med. 2005;71:1019–1024. doi: 10.1055/s-2005-873113. [DOI] [PubMed] [Google Scholar]
- 97.Mori T, O’Keefe BR, Sowder RC, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 98.Busserolles J, Paya M, D’Auria MV, Gomez-Paloma L, Alcaraz MJ. Protection against 2,4,6-trinitrobenzenesulphonic acid-induced colonic inflammation in mice by the marine products bolinaquinone and petrosaspongiolide M. Biochem Pharmacol. 2005;69:1433–1440. doi: 10.1016/j.bcp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 99.Miyaoka H, Yamanishi M, Mitome H. PLA2 inhibitory activity of marine sesterterpenoids cladocorans, their diastereomers and analogues. Chem Pharm Bull (Tokyo) 2006;54:268–270. doi: 10.1248/cpb.54.268. [DOI] [PubMed] [Google Scholar]
- 100.McNamara CE, Larsen L, Perry NB, Harper JL, Berridge MV, Chia EW, Kelly M, Webb VL. Anti-inflammatory sesquiterpene-quinones from the New Zealand sponge Dysidea cf. cristagalli. J Nat Prod. 2005;68:1431–1433. doi: 10.1021/np050171n. [DOI] [PubMed] [Google Scholar]
- 101.Mayer AMS, Hall ML, Lynch SM, Gunasekera SP, Sennett SH, Pomponi SA. Differential modulation of microglia superoxide anion and thromboxane B2 generation by the marine manzamines. BMC Pharmacol. 2005;5:6. doi: 10.1186/1471-2210-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawant S, Youssef D, Mayer A, Sylvester P, Wali V, Arant M, El SK. Anticancer and anti-inflammatory sulfur-containing semisynthetic derivatives of sarcophine. Chem Pharm Bull (Tokyo) 2006;54:1119–1123. doi: 10.1248/cpb.54.1119. [DOI] [PubMed] [Google Scholar]
- 103.Mayer AMS, Oh S, Presto E, Glaser KB, Jacobson PB. LPS-primed rat brain microglia: a convenient in vitro model to search for anti-inflammatory marine natural products. SHOCK. 1997;7:49. [Google Scholar]
- 104.Mandeau A, Debitus C, Aries MF, David B. Isolation and absolute configuration of new bioactive marine steroids from Euryspongian. sp. Steroids. 2005;70:873–878. doi: 10.1016/j.steroids.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 105.Ahmed AF, Hsieh YT, Wen ZH, Wu YC, Sheu JH. Polyoxygenated sterols from the Formosan soft coral Sinularia gibberosa. J Nat Prod. 2006;69:1275–1279. doi: 10.1021/np0601509. [DOI] [PubMed] [Google Scholar]
- 106.Tziveleka LA, Abatis D, Paulus K, Bauer R, Vagias C, Roussis V. Marine polyprenylated hydroquinones, quinones, and chromenols with inhibitory effects on leukotriene formation. Chem Biodivers. 2005;2:901–909. doi: 10.1002/cbdv.200590066. [DOI] [PubMed] [Google Scholar]
- 107.Huang HC, Wen ZH, Chao CH, Ahmed AF, Chiang MY, Kuo YH, Hsu CH, Sheu JH. Novel sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2006;47:8751–8755. [Google Scholar]
- 108.Sugiura Y, Matsuda K, Yamada Y, Nishikawa M, Shioya K, Katsuzaki H, Imai K, Amano H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci Biotechnol Biochem. 2006;70:2807–2811. doi: 10.1271/bbb.60417. [DOI] [PubMed] [Google Scholar]
- 109.Kita M, Ohishi N, Washida K, Kondo M, Koyama T, Yamada K, Uemura D. Symbioimine and neosymbioimine, amphoteric iminium metabolites from the symbiotic marine dinoflagellate Symbiodinium sp.*. Bioorg Med Chem. 2005;13:5253–5258. doi: 10.1016/j.bmc.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 110.Sauviat MP, Vercauteren J, Grimaud N, Juge M, Nabil M, Petit JY, Biard JF. Sensitivity of cardiac background inward rectifying K+ outward current (IK1) to the alkaloids lepadiformines A, B, and C. J Nat Prod. 2006;69:558–562. doi: 10.1021/np050215s. [DOI] [PubMed] [Google Scholar]
- 111.Onodera K, Nakamura H, Oba Y, Ohizumi Y, Ojika M. Zooxanthellamide Cs: vasoconstrictive polyhydroxylated macrolides with the largest lactone ring size from a marine dinoflagellate of Symbiodinium sp. J Am Chem Soc. 2005;127:10406–10411. doi: 10.1021/ja050810g. [DOI] [PubMed] [Google Scholar]
- 112.Pereira A, Vottero E, Roberge M, Mauk AG, Andersen RJ. Indoleamine 2,3-dioxygenase inhibitors from the Northeastern Pacific Marine Hydroid Garveia annulata. J Nat Prod. 2006;69:1496–1499. doi: 10.1021/np060111x. [DOI] [PubMed] [Google Scholar]
- 113.Aminin DL, Pinegin BV, Pichugina LV, Zaporozhets TS, Agafonova IG, Boguslavski VM, Silchenko AS, Avilov SA, Stonik VA. Immunomodulatory properties of Cumaside. Int Immunopharmacol. 2006;6:1070–1082. doi: 10.1016/j.intimp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 114.Costantino V, D’Esposito M, Fattorusso E, Mangoni A, Basilico N, Parapini S, Taramelli D. Damicoside from Axinella damicornis: the influence of a glycosylated galactose 4-OH group on the immunostimulatory activity of alpha-galactoglycosphingolipids. J Med Chem. 2005;48:7411–7417. doi: 10.1021/jm050506y. [DOI] [PubMed] [Google Scholar]
- 115.Kim KH, Kim YW, Kim HB, Lee BJ, Lee DS. Anti-apoptotic activity of laminarin polysaccharides and their enzymatically hydrolyzed oligosaccharides from Laminaria japonica. Biotechnol Lett. 2006;28:439–446. doi: 10.1007/s10529-005-6177-9. [DOI] [PubMed] [Google Scholar]
- 116.Xia W, Li J, Geng M, Xin X, Ding J. Potentiation of T cell function by a marine algae-derived sulfated polymannuroguluronate: in vitro analysis of novel mechanisms. J Pharm Sci. 2005;97:107–115. doi: 10.1254/jphs.fpj04026x. [DOI] [PubMed] [Google Scholar]
- 117.Oda T, Namikoshi M, Akano K, Kobayashi H, Honma Y, Kasahara T. Verrucarin a inhibition of map kinase activation in a pma-stimulated promyelocytic leukemia cell line. Marine Drugs. 2005;3:64–73. [Google Scholar]
- 118.Tsang CK, Ina A, Goto T, Kamei Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience. 2005;132:633–643. doi: 10.1016/j.neuroscience.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 119.Nandini CD, Itoh N, Sugahara K. Novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chains with a unique heterogeneous sulfation pattern from shark skin, which exhibit neuritogenic activity and binding activities for growth factors and neurotrophic factors. J Biol Chem. 2005;280:4058–4069. doi: 10.1074/jbc.M412074200. [DOI] [PubMed] [Google Scholar]
- 120.Kisa F, Yamada K, Miyamoto T, Inagaki M, Higuchi R. Constituents of Holothuroidea, 17. Isolation and structure of biologically active monosialo-gangliosides from the sea cucumber Cucumaria echinata. Chem Pharm Bull (Tokyo) 2006;54:982–987. doi: 10.1248/cpb.54.982. [DOI] [PubMed] [Google Scholar]
- 121.Kisa F, Yamada K, Miyamoto T, Inagaki M, Higuchi R. Constituents of Holothuroidea, 18. Isolation and structure of biologically active disialo- and trisialo-gangliosides from the sea cucumber Cucumaria echinata. Chem Pharm Bull (Tokyo) 2006;54:1293–1298. doi: 10.1248/cpb.54.1293. [DOI] [PubMed] [Google Scholar]
- 122.Inagaki M, Miyamoto T, Isobe R, Higuchi R. Biologically active glycosides from asteroidea, 43. Isolation and structure of a new neuritogenic-active ganglioside molecular species from the starfish Linckia laevigata. Chem Pharm Bull (Tokyo) 2005;53:1551–1554. doi: 10.1248/cpb.53.1551. [DOI] [PubMed] [Google Scholar]
- 123.Higuchi R, Inoue S, Inagaki K, Sakai M, Miyamoto T, Komori T, Inagaki M, Isobe R. Biologically active glycosides from asteroidea, 42. Isolation and structure of a new biologically active ganglioside molecular species from the starfish Asterina pectinifera. Chem Pharm Bull (Tokyo) 2006;54:287–291. doi: 10.1248/cpb.54.287. [DOI] [PubMed] [Google Scholar]
- 124.Han C, Qi J, Ojika M. Structure-activity relationships of novel neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg Med Chem. 2006;14:4458–4465. doi: 10.1016/j.bmc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 125.Wei H, Itoh T, Kinoshita M, Kotoku N, Aoki S, Kobayashi M. Shimalactone A, a novel polyketide, from marine-derived fungus Emericella variecolor GF10. Tetrahedron. 2005;61:8054–8058. [Google Scholar]
- 126.Loughnan M, Nicke A, Jones A, Schroeder CI, Nevin ST, Adams DJ, Alewood PF, Lewis RJ. Identification of a novel class of nicotinic receptor antagonists: dimeric conotoxins VxXIIA, VxXIIB, and VxXIIC from Conus vexillum. J Biol Chem. 2006;281:24745–24755. doi: 10.1074/jbc.M603703200. [DOI] [PubMed] [Google Scholar]
- 127.Wen L, Yang S, Qiao H, Liu Z, Zhou W, Zhang Y, Huang P. SO-3, a new O-superfamily conopeptide derived from Conus striatus, selectively inhibits N-type calcium currents in cultured hippocampal neurons. Br J Pharmacol. 2005;145:728–739. doi: 10.1038/sj.bjp.0706223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanders JM, Ito K, Settimo L, Pentikainen OT, Shoji M, Sasaki M, Johnson MS, Sakai R, Swanson GT. Divergent pharmacological activity of novel marine-derived excitatory amino acids on glutamate receptors. J Pharmacol Exp Ther. 2005;314:1068–1078. doi: 10.1124/jpet.105.086389. [DOI] [PubMed] [Google Scholar]
- 129.Sanders JM, Pentikainen OT, Settimo L, Pentikainen U, Shoji M, Sasaki M, Sakai R, Johnson MS, Swanson GT. Determination of binding site residues responsible for the subunit selectivity of novel marine-derived compounds on kainate receptors. Mol Pharmacol. 2006;69:1849–1860. doi: 10.1124/mol.106.022772. [DOI] [PubMed] [Google Scholar]
- 130.Tsuneki H, You Y, Toyooka N, Sasaoka T, Nemoto H, Dani JA, Kimura I. Marine alkaloids (−)-pictamine and (−)-lepadin B block neuronal nicotinic acetylcholine receptors. Biol Pharm Bull. 2005;28:611–614. doi: 10.1248/bpb.28.611. [DOI] [PubMed] [Google Scholar]
- 131.Aiello A, D’Esposito M, Fattorusso E, Menna M, Muller WE, Perovic-Ottstadt S, Schroder HC. Novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella verrucosa. Bioorg Med Chem. 2006;14:17–24. doi: 10.1016/j.bmc.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 132.Aiello A, D’Esposito M, Fattorusso E, Menna M, Muller WEG, Perovic-Ottstadt S, Tsuruta H, Gulder TAM, Bringmann G. Daminin, a bioactive pyrrole alkaloid from the Mediterranean sponge Axinella damicornis. Tetrahedron. 2005;61:7266–7270. [Google Scholar]
- 133.Bringmann G, Lang G, Maksimenka K, Hamm A, Gulder TA, Dieter A, Bull AT, Stach JE, Kocher N, Muller WE, Fiedler HP. Gephyromycin, the first bridged angucyclinone, from Streptomyces griseus strain NTK 14. Phytochemistry. 2005;66:1366–1373. doi: 10.1016/j.phytochem.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 134.To KC, Loh KT, Roskelley CD, Andersen RJ, O’Connor TP. The anti-invasive compound motuporamine C is a robust stimulator of neuronal growth cone collapse. Neuroscience. 2006;139:1263–1274. doi: 10.1016/j.neuroscience.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 135.Temraz TA, Houssen WE, Jaspars M, Woolley DR, Wease KN, Davies SN, Scott RH. A pyridinium derivative from Red Sea soft corals inhibited voltage-activated potassium conductances and increased excitability of rat cultured sensory neurones. BMC Pharmacol. 2006;6:10. doi: 10.1186/1471-2210-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cragg GM, Newman DJ. Biodiversity: A continuing source of novel drug leads. Pure & Applied Chemistry. 2005;77(1):7–24. 77. 7–24. [Google Scholar]
- 137.Cragg GM, Newman DJ. International collaboration in drug discovery and development from natural sources. Pure Appl Chem. 2005;77:1923–1942. [Google Scholar]
- 138.Gul W, Hamann MT. Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–453. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jensen PR, Mincer TJ, Williams PG, Fenical W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek. 2005;87:43–48. doi: 10.1007/s10482-004-6540-1. [DOI] [PubMed] [Google Scholar]
- 140.Paterson I, Anderson EA. The renaissance of natural products as drug candidates. Science. 2005;310:451–453. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 141.Singh S, Kate BN, Banerjee UC. Bioactive compounds from cyanobacteria and microalgae: an overview. Crit Rev Biotechnol. 2005;25:73–95. doi: 10.1080/07388550500248498. [DOI] [PubMed] [Google Scholar]
- 142.Chin YW, Balunas MJ, Chai HB, Kinghorn AD. Drug discovery from natural sources. AAPS J. 2006;8:E239–E253. doi: 10.1007/BF02854894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fenical W, Jensen PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 144.Kim SK, Mendis E. Bioactive compounds from marine processing byproducts - A review [Review] Food Res Int. 2006;39:383–393. [Google Scholar]
- 145.Proksch P, Edrada R, Lin WH. Implications of Marine Biotechnology on Drug Discovery. In: Proksch P, Muller WEG, editors. Frontiers in Marine Biotechnology. Horizon Bioscience; 2006. pp. 1–19. [Google Scholar]
- 146.Brunel JM, Salmi C, Loncle C, Vidal N, Letourneux Y. Squalamine: a polyvalent drug of the future? Curr Cancer Drug Targets. 2005;5:267–272. doi: 10.2174/1568009054064642. [DOI] [PubMed] [Google Scholar]
- 147.Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Larsen TO, Smedsgaard J, Nielsen KF, Hansen ME, Frisvad JC. Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Nat Prod Rep. 2005;22:672–695. doi: 10.1039/b404943h. [DOI] [PubMed] [Google Scholar]
- 149.Bhadury P, Mohammad BT, Wright PC. The current status of natural products from marine fungi and their potential as anti-infective agents. J Ind Microbiol Biotechnol. 2006;33:325–337. doi: 10.1007/s10295-005-0070-3. [DOI] [PubMed] [Google Scholar]
- 150.Jude S, Roger S, Martel E, Besson P, Richard S, Bougnoux P, Champeroux P, Le Guennec JY. Dietary long-chain omega-3 fatty acids of marine origin: a comparison of their protective effects on coronary heart disease and breast cancers. Prog Biophys Mol Biol. 2006;90:299–325. doi: 10.1016/j.pbiomolbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 151.Bourguet-Kondracki ML, Kornprobst JM. Marine pharmacology: potentialities in the treatment of infectious diseases, osteoporosis and Alzheimer’s disease. Adv Biochem Eng Biotechnol. 2005;97:105–131. doi: 10.1007/b135824. [DOI] [PubMed] [Google Scholar]
- 152.De Souza MV. Marine natural products against tuberculosis. Sci World J. 2006;6:847–861. doi: 10.1100/tsw.2006.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martinez MJA, Del Olmo LMB, Benito PB. Antiviral Activities of Polysaccharides From Natural Sources. Bioact Nat Prod. 2005:393–418. [Google Scholar]
- 154.Laurent D, Pietra F. Antiplasmodial marine natural products in the perspective of current chemotherapy and prevention of malaria: a review. Mar Biotechnol (NY) 2006;8:433–447. doi: 10.1007/s10126-006-6100-y. [DOI] [PubMed] [Google Scholar]
- 155.Dubuisson ML, Rees JF, Marchand-Brynaert J. Coelenterazine (marine bioluminescent substrate): a source of inspiration for the discovery of novel antioxidants. Drug Dev Ind Pharm. 2005;31:827–849. doi: 10.1080/03639040500271803. [DOI] [PubMed] [Google Scholar]
- 156.Gomez-Paloma L, Monti MC, Terracciano S, Casapullo A, Riccio R. Chemistry and biology of anti-inflammatory marine natural products. Phospholipase A(2) inhibitors. Curr Org Chem. 2005;9:1419–1427. doi: 10.2174/092986706777585095. [DOI] [PubMed] [Google Scholar]
- 157.Heckrodt TJ, Mulzer J. Marine natural products from Pseudopterogorgia elisabethae: Structures, biosynthesis, pharmacology, and total synthesis. Top Curr Chem. 2005;244:1–41. [Google Scholar]
- 158.Terracciano S, Aquino M, Rodriquez M, Monti MC, Casapullo A, Riccio R, Gomez-Paloma L. Chemistry and biology of anti-inflammatory marine natural products: molecules interfering with cyclooxygenase, NF-kappaB and other unidentified targets. Curr Med Chem. 2006;13:1947–1969. doi: 10.2174/092986706777585095. [DOI] [PubMed] [Google Scholar]
- 159.Alcaraz MJ, Paya M. Marine sponge metabolites for the control of inflammatory diseases. Curr Opin Investig Drugs. 2006;7:974–979. [PubMed] [Google Scholar]
- 160.Fisch KM, Abdel-Lateff A, Konig GM. Antioxidant Metabolites From Marine Derived Fungi. In: Fingerman M, Nagabhushanam R, editors. Biomaterials from Aquatic and Terrestrial Organisms. Science Publishers; New Orleans: 2006. pp. 361–376. [Google Scholar]
- 161.Alonso D, Castro A, Martinez A. Marine compounds for the therapeutic treatment of neurological disorders. Expert Opinion Therap Pat. 2005;15:1377–1386. [Google Scholar]
- 162.Sharp D. Novel pain relief via marine snails. Lancet. 2005;366:439–440. doi: 10.1016/S0140-6736(05)67040-7. [DOI] [PubMed] [Google Scholar]
- 163.Sun MK, Alkon DL. Bryostatin-1: pharmacology and therapeutic potential as a CNS drug. CNS Drug Rev. 2006;12:1–8. doi: 10.1111/j.1527-3458.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bowman EJ, Bowman BJ. V-ATPases as drug targets. J Bioenerg Biomembr. 2005;37:431–435. doi: 10.1007/s10863-005-9485-9. [DOI] [PubMed] [Google Scholar]
- 165.Dias N, Vezin H, Lansiaux A, Bailly C. Topoisomerase inhibitors of marine origin and their potential use as anticancer agents. Top Curr Chem. 2005;253:89–108. [Google Scholar]
- 166.Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie Van Leeuwenhoek. 2005;87:59–63. doi: 10.1007/s10482-004-6544-x. [DOI] [PubMed] [Google Scholar]
- 167.Alonso D, Martinez A. Marine Compounds As a New Source for Glycogen Synthase Kinase 3 Inhibitors. In: Martinez A, Castro A, Medine M, editors. Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors: Drug Discovery and Development. John Wiley & Sons, Inc; 2006. pp. 307–331. [Google Scholar]
- 168.Williams JA, Day M, Heavner JE. Ziconotide: an update and review. Expert Opin Pharmacother. 2008;9:1575–1583. doi: 10.1517/14656566.9.9.1575. [DOI] [PubMed] [Google Scholar]
- 169.Bickmeyer U, Assmann M, Kock M, Schutt C. A secondary metabolite, 4,5-dibromopyrrole-2-carboxylic acid, from marine sponges of the genus Agelas alters cellular calcium signals. Environ Toxicol Pharmacol. 2005;19:423–427. doi: 10.1016/j.etap.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 170.Cao SG, Foster C, Brisson M, Lazo JS, Kingston DGI. Halenaquinone and xestoquinone derivatives, inhibitors of Cdc25B phosphatase from a Xestospongia sp. Bioorg Med Chem. 2005;13:999–1003. doi: 10.1016/j.bmc.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 171.Hedner E, Sjogren M, Frandberg PA, Johansson T, Goransson U, Dahlstrom M, Jonsson P, Nyberg F, Bohlin L. Brominated cyclodipeptides from the marine sponge Geodia barretti as selective 5-HT ligands. J Nat Prod. 2006;69:1421–1424. doi: 10.1021/np0601760. [DOI] [PubMed] [Google Scholar]
- 172.Bickmeyer U. Bromoageliferin and dibromoageliferin, secondary metabolites from the marine sponge Agelas conifera, inhibit voltage-operated, but not store-operated calcium entry in PC12 cells. Toxicon. 2005;45:627–632. doi: 10.1016/j.toxicon.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Robert F, Gao HQ, Donia M, Merrick WC, Hamann MT, Pelletier J. Chlorolissoclimides: new inhibitors of eukaryotic protein synthesis. RNA. 2006;12:717–725. doi: 10.1261/rna.2346806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mahale S, Aubry C, James WA, Jenkins PR, Marechal JD, Sutcliffe MJ, Chaudhuri B. CA224, a non-planar analogue of fascaplysin, inhibits Cdk4 but not Cdk2 and arrests cells at G0/G1 inhibiting pRB phosphorylation. Bioorg Med Chem Lett. 2006;16:4272–4278. doi: 10.1016/j.bmcl.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 175.Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, Pelletier J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol. 2006;2:213–220. doi: 10.1038/nchembio776. [DOI] [PubMed] [Google Scholar]
- 176.Marion F, Williams DE, Patrick BO, Hollander I, Mallon R, Kim SC, Roll DM, Feldberg L, Van SR, Andersen RJ. Liphagal, a Selective inhibitor of PI3 kinase alpha isolated from the sponge Aka coralliphaga: structure elucidation and biomimetic synthesis. Org Lett. 2006;8:321–324. doi: 10.1021/ol052744t. [DOI] [PubMed] [Google Scholar]
- 177.Manzanaro S, Salva J, de la Fuente JA. Phenolic marine natural products as aldose reductase inhibitors. J Nat Prod. 2006;69:1485–1487. doi: 10.1021/np0503698. [DOI] [PubMed] [Google Scholar]
- 178.Yamaki H, Sitachitta N, Sano T, Kaya K. Two new chymotrypsin inhibitors isolated from the Cyanobacterium Microcystis aeruginosa NIES-88. J Nat Prod. 2005;68:14–18. doi: 10.1021/np0401361. [DOI] [PubMed] [Google Scholar]
- 179.Bordeleau ME, Matthews J, Wojnar JM, Lindqvist L, Novac O, Jankowsky E, Sonenberg N, Northcote P, Teesdale-Spittle P, Pelletier J. Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc Natl Acad Sci U S A. 2005;102:10460–10465. doi: 10.1073/pnas.0504249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jung HA, Hyun SK, Kim HR, Choi JS. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fisheries Sci. 2006;72:1292–1299. [Google Scholar]
- 181.Zhu G, Yang F, Balachandran R, Hook P, Vallee RB, Curran DP, Day BW. Synthesis and biological evaluation of purealin and analogues as cytoplasmic dynein heavy chain inhibitors. J Med Chem. 2006;49:2063–2076. doi: 10.1021/jm051030l. [DOI] [PubMed] [Google Scholar]
- 182.Nam SJ, Ko H, Shin M, Ham J, Chin J, Kim Y, Kim H, Shin K, Choi H, Kang H. Farnesoid X-activated receptor antagonists from a marine sponge Spongia sp. Bioorg Med Chem Lett. 2006;16:5398–5402. doi: 10.1016/j.bmcl.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 183.Chernova MN, Vandorpe DH, Clark JS, Williams JI, Zasloff MA, Jiang L, Alper SL. Apparent receptor-mediated activation of Ca2+-dependent conductive Cl- transport by shark-derived polyaminosterols. Am J Physiol. 2005;289:R1644–R1658. doi: 10.1152/ajpregu.00098.2005. [DOI] [PubMed] [Google Scholar]
- 184.Jaimovich E, Mattei C, Liberona JL, Cardenas C, Estrada M, Barbier J, Debitus C, Laurent D, Molgo J. Xestospongin B, a competitive inhibitor of IP3-mediated Ca2+ signalling in cultured rat myotubes, isolated myonuclei, and neuroblastoma (NG108–15) cells. FEBS Lett. 2005;579:2051–2057. doi: 10.1016/j.febslet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 185.Ta TA, Feng W, Molinski TF, Pessah IN. Hydroxylated xestospongins block inositol-1,4,5-trisphosphate-induced Ca2+ release and sensitize Ca2+-induced Ca2+ release mediated by ryanodine receptors. Mol Pharmacol. 2006;69:532–538. doi: 10.1124/mol.105.019125. [DOI] [PubMed] [Google Scholar]
- 186.Cao S, Foster C, Lazo JS, Kingston DG. Four diterpenoid inhibitors of Cdc25B phosphatase from a marine anemone. Bioorg Med Chem. 2005;13:5830–5834. doi: 10.1016/j.bmc.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 187.Kubota T, Sakuma Y, Shimbo K, Tsuda M, Nakano M, Uozumi Y, Kobayashi J. Amphezonol A, a novel polyhydroxyl metabolite from marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006;47:4369–4371. [Google Scholar]
- 188.Seibert SF, Eguereva E, Krick A, Kehraus S, Voloshina E, Raabe G, Fleischhauer J, Leistner E, Wiese M, Prinz H, Alexandrov K, Janning P, Waldmann H, Konig GM. Polyketides from the marine-derived fungus Ascochyta salicorniae and their potential to inhibit protein phosphatases. Org Biomol Chem. 2006;4:2233–2240. doi: 10.1039/b601386d. [DOI] [PubMed] [Google Scholar]
- 189.Sung PJ, Chen YP, Hwang TL, Hu WP, Fang LS, Wu YC, Li JJ, Sheu JH. Briaexcavatins C–F, four new briarane-related diterpenoids from the Formosan octocoral Briareum excavatum (Briareidae) Tetrahedron. 2006;62:5686–5691. [Google Scholar]
- 190.Chen YP, Wu SL, Su JH, Lin MR, Hu WP, Hwang TL, Sheu JH, Fan TY, Fang LS, Sung PJ. Briaexcavatins G and H, two new briaranes from the octocoral Briareum excavatum. Bull Chem Soc Jpn. 2006;79:1900–1905. [Google Scholar]
- 191.Muller D, Krick A, Kehraus S, Mehner C, Hart M, Kupper FC, Saxena K, Prinz H, Schwalbe H, Janning P, Waldmann H, Konig GM. Brunsvicamides A–C: sponge-related cyanobacterial peptides with Mycobacterium tuberculosis protein tyrosine phosphatase inhibitory activity. J Med Chem. 2006;49:4871–4878. doi: 10.1021/jm060327w. [DOI] [PubMed] [Google Scholar]
- 192.Mao SC, Guo YW, Shen X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg Med Chem Lett. 2006;16:2947–2950. doi: 10.1016/j.bmcl.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 193.Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J Am Chem Soc. 2006;128:3148–3149. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]
- 194.von Elert E, Oberer L, Merkel P, Huhn T, Blom JF. Cyanopeptolin 954, a chlorine-containing chymotrypsin inhibitor of Microcystis aeruginosa NIVA Cya 43. J Nat Prod. 2005;68:1324–1327. doi: 10.1021/np050079r. [DOI] [PubMed] [Google Scholar]
- 195.Cao S, Foster C, Lazo JS, Kingston DG. Sesterterpenoids and an alkaloid from a Thorectandra sp. as inhibitors of the phosphatase Cdc25B. Bioorg Med Chem. 2005;13:5094–5098. doi: 10.1016/j.bmc.2005.04.070. [DOI] [PubMed] [Google Scholar]
- 196.Shao ZY, Li J, Sim CJ, Li JY, Li ZY, Nan FJ, Guo YW. O-methyl nakafuran-8 lactone, a new sesquiterpenoid from a hainan marine sponge Dysidea sp. J Asian Nat Prod Res. 2006;8:223–227. doi: 10.1080/10286020500383882. [DOI] [PubMed] [Google Scholar]
- 197.Sabry OM, Andrews S, McPhail KL, Goeger DE, Yokochi A, LePage KT, Murray TF, Gerwick WH. Neurotoxic meroditerpenoids from the tropical marine brown alga Stypopodium flabelliforme. J Nat Prod. 2005;68:1022–1030. doi: 10.1021/np050051f. [DOI] [PubMed] [Google Scholar]
- 198.Jayasuriya H, Herath KB, Ondeyka JG, Guan Z, Borris RP, Tiwari S, de Jong W, Chavez F, Moss J, Stevenson DW, Beck HT, Slattery M, Zamora N, Schulman M, Ali A, Sharma N, MacNaul K, Hayes N, Menke JG, Singh SB. Diterpenoid, steroid, and triterpenoid agonists of liver X receptors from diversified terrestrial plants and marine sources. J Nat Prod. 2005;68:1247–1252. doi: 10.1021/np050182g. [DOI] [PubMed] [Google Scholar]
- 199.Tsukamoto S, Yoshida T, Hosono H, Ohta T, Yokosawa H. Hexylitaconic acid: a new inhibitor of p53-HDM2 interaction isolated from a marine-derived fungus, Arthrinium sp. Bioorg Med Chem Lett. 2006;16:69–71. doi: 10.1016/j.bmcl.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 200.Tsukamoto S, Hirota H, Imachi M, Fujimuro M, Onuki H, Ohta T, Yokosawa H. Himeic acid A: a new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp. Bioorg Med Chem Lett. 2005;15:191–194. doi: 10.1016/j.bmcl.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 201.Yan XH, Zhu XZ, Yu JL, Jin DZ, Guo YW, Mollo E, Cimino G. 3-Oxo-axisonitrile-3, a new sesquiterpene isocyanide from the Chinese marine sponge Acanthella sp. J Asian Nat Prod Res. 2006;8:579–584. doi: 10.1080/10286020410001721096. [DOI] [PubMed] [Google Scholar]
- 202.Plaza A, Bewley CA. Largamides A–H, unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J Org Chem. 2006;71:6898–6907. doi: 10.1021/jo061044e. [DOI] [PubMed] [Google Scholar]
- 203.Yamada T, Doi M, Miura A, Harada W, Hiramura M, Minoura K, Tanaka R, Numata A. Absolute stereostructures of cell-adhesion inhibitors, peribysins A, E, F and G, produced by a sea hare-derived Periconia sp. J Antibiot (Tokyo) 2005;58:185–191. doi: 10.1038/ja.2005.21. [DOI] [PubMed] [Google Scholar]
- 204.Yamada T, Minoura K, Tanaka R, Numata A. Cell-adhesion inhibitors produced by a sea hare-derived Periconia sp. II. Absolute stereostructures of peribysins H and I. J Antibiot (Tokyo) 2006;59:345–350. doi: 10.1038/ja.2006.48. [DOI] [PubMed] [Google Scholar]
- 205.Carroll AR, Ngo A, Quinn RJ, Redburn J, Hooper JN. Petrosamine B, an inhibitor of the Helicobacter pylori enzyme aspartyl semialdehyde dehydrogenase from the Australian sponge Oceanapia sp. J Nat Prod. 2005;68:804–806. doi: 10.1021/np049595s. [DOI] [PubMed] [Google Scholar]
- 206.Levina EV, Kalinovsky AI, Andriyashenko PV, Dmitrenok PS, Aminin DL, Stonik VA. Phrygiasterol, a cytotoxic cyclopropane-containing polyhydroxysteroid, and related compounds from the pacific starfish Hippasteria phrygiana. J Nat Prod. 2005;68:1541–1544. doi: 10.1021/np049610t. [DOI] [PubMed] [Google Scholar]
- 207.Andrianasolo EH, France D, Cornell-Kennon S, Gerwick WH. DNA methyl transferase inhibiting halogenated monoterpenes from the Madagascar red marine alga Portieria hornemannii. J Nat Prod. 2006;69:576–579. doi: 10.1021/np0503956. [DOI] [PubMed] [Google Scholar]
- 208.Mori J, Iwashima M, Wakasugi H, Saito H, Matsunaga T, Ogasawara M, Takahashi S, Suzuki H, Hayashi T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem Pharm Bull (Tokyo) 2005;53:1159–1163. doi: 10.1248/cpb.53.1159. [DOI] [PubMed] [Google Scholar]
- 209.Xie Y, Liu L, Huang X, Guo Y, Lou L. Scalaradial inhibition of epidermal growth factor receptor-mediated Akt phosphorylation is independent of secretory phospholipase A2. J Pharmacol Exp Ther. 2005;314:1210–1217. doi: 10.1124/jpet.105.086520. [DOI] [PubMed] [Google Scholar]
- 210.Tsukamoto S, Koimaru K, Ohta T. Secomycalolide A: a new proteasome inhibitor isolated from a marine sponge of the genus Mycale. Marine Drugs. 2005;3:29–35. [Google Scholar]
- 211.Wang W, Okada Y, Shi H, Wang Y, Okuyama T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J Nat Prod. 2005;68:620–622. doi: 10.1021/np040199j. [DOI] [PubMed] [Google Scholar]