Summary
Eosinophils are usually considered as end-stage degranulating effector cells of innate immunity. However, accumulating evidence has revealed additional roles for eosinophils that are immunoregulatory in nature in both the adaptive and innate arms of immunity. Specifically, eosinophils have key immunoregulatory roles as professional antigen-presenting cells and as modulators of CD4+ T cell, dendritic cell, B cell, mast cell, neutrophil, and basophil functions. This review addresses the emerging immunoregulatory roles of eosinophils with a focus on recent data that support this new paradigm. Recognizing both the effector and immunoregulatory functions of eosinophils will enable a fuller understanding of the roles of eosinophils in allergic airways inflammation and may be pertinent to therapies that target eosinophils both for their acute and ongoing immunomodulatory functions.
Introduction
Eosinophils have been traditionally regarded as ‘end-stage’, terminally differentiated, non-replicating effector cells, playing a central potentially beneficial role in the clearance of parasitic infections, primarily through the exocytotic release of eosinophil granule-derived cytotoxic proteins. Eosinophils have potentially maladaptive roles in asthma and other atopic diseases, which have been viewed in the past singularly as actions of ‘end-stage’ effector cells releasing specific eicosanoid lipids, i.e. leukotriene C4, and cationic granule-derived proteins [1]. However, the traditional view of eosinophils has over time become more nuanced as mechanisms governing the regulation and chemotaxis of eosinophils are better understood. Data have now accumulated to a point where the old paradigm is no longer a sufficient model for describing the place of eosinophils in health and disease. The complex roles of eosinophils as initiators and perpetuators of asthma and allergic inflammation have gained attention, as noted in several reviews [2–4]. The substantial array of cytokines and chemokines that are produced by eosinophils has also gained increasing recognition, opening the door to a new understanding of eosinophils as more than a final end-point in inflammatory pathways [5–9]. The overlap between mediators that govern other components of the immune system, such as T cells, and those that are produced by eosinophils is also starting to attract attention [10]. Out of these threads of evidence and recognition, a new paradigm for eosinophils as true immunoregulatory cells has emerged and has been increasingly discussed in the literature [1, 11–15]. The purpose of this review is to discuss the various immunoregulatory roles of eosinophils as they are currently understood and to highlight the supporting data.
Eosinophils as antigen-presenting cells
Perhaps the most well-described immunoregulatory role of eosinophils, with developing lines of evidence over the last 3 decades, is their action as antigen-presenting cells (APCs) (Fig. 1). Despite the depth of the evidence supporting this important regulatory role, the notion of eosinophils as important players in antigen presentation remains somewhat novel. However, this role is becoming increasingly recognized and has been the subject of a prior review [16].
Fig. 1.
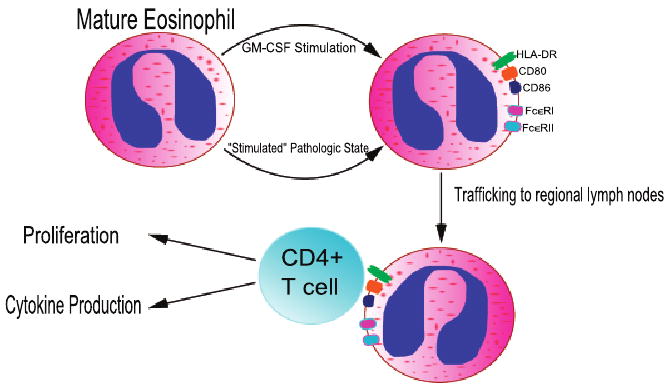
Illustration of a Model of Eosinophils as Antigen Presenting Cells. Eosinophils can be stimulated to express MHC Class II and co-stimulatory molecules by granulocyte-macrophage colony stimulating factor (GM-CSF) under experimental conditions. Eosinophils achieve the same expression of antigen presentation machinery in vivo in various disease states including asthma, chronic eosinophilic pneumonia, and parasitic infection. They then traffick to regional lymph nodes where they encounter CD4+ T cells, promoting proliferation and cytokine production (IL-4, IL-5, IL-13) by T cells.
As early as 1980, Ia antigen, the term used for the MHC Class II molecule HLA-DR at that time, was demonstrated to be expressed in early human eosinophils but not in mature eosinophils [17]. At that point Ia antigen was thought to primarily be a maturation marker in eosinophils and other haematopoietic cells. It was several years later, after the importance of HLA-DR in antigen processing and presentation was recognized, before it was demonstrated that mature eosinophils express MHC Class II when stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF) [18]. So while unstimulated mature eosinophils did not express MHC Class II in eight of the nine subjects studied, eosinophils could be stimulated in vitro with GM-CSF to express MHC Class II proteins [18]. Though this observation did not prove that eosinophils could actually function as APCs, it did show that they can produce the central MHC Class II proteins required for antigen presentation.
Eosinophils are known to express MHC Class II on their cell membrane in various pathologic processes in which they are ‘activated’. Sputum eosinophils from asthmatics not on glucocorticoids express HLA-DR, as opposed to blood eosinophils from the same subjects [19]. Similar observations have been made in comparing bronchoalveolar lavage (BAL) fluid eosinophils to peripheral blood eosinophils in a patient with chronic eosinophilic pneumonia [20]. Allergic rhinitis patients who were exposed to segmental lung allergen challenge demonstrated greater HLA-DR expression on airways recruited eosinophils recovered by BAL than on peripheral blood eosinophils [21]. In mice infected intraperitoneally (i.p.) with the helminthic parasite Brugia malayi, eosinophils recovered from the peritoneum had a high level of MHC Class II expression on the cell surface [22]. The difference between local vs. remote cells with respect to inflammation suggests that eosinophils can be induced by the local environment into an antigen-presentation phenotype. This phenomenon of separate compartments of activation has been reproduced in vitro utilizing a human pulmonary microvascular endothelial cell monolayer system. After peripheral blood eosinophils were incubated with endothelial cell monolayers, eosinophils that migrated through the monolayers had significantly increased HLA-DR expression compared with those that did not migrate through endothelial cells [23].
In addition to expression of MHC Class II, eosinophils can express necessary co-stimulatory molecules for antigen presentation on their cell membranes. Resting eosinophils from IL-5 transgenic mice express both CD80 and CD86, and the expression of both surface molecules is increased after stimulation with GM-CSF [24]. Mice subjected to allergic airways challenge also have CD80- and CD86-positive airway eosinophils [25, 26]. Additionally, human eosinophils can be stimulated to express CD86 by IL-3 [27]. The co-stimulatory protein CD40 has been recognized on peripheral blood eosinophils as well as on lesional allergic airways recruited eosinophils [28].
Beyond merely expressing the requisite surface molecules for antigen presentation, eosinophils have been shown to actually function as APCs in several different experimental settings. An early demonstration of their antigen-presenting capabilities featured the ability of eosinophils exposed to tetanus toxoid to induce T cell proliferation in co-culture [29]. In fact the ability was preserved if the eosinophils were fixed after exposure to antigen but not if they were fixed before exposure [29]. Similarly, peripheral blood eosinophils from normal subjects stimulate T cell proliferation in a HLA-DR and intercellular adhesion molecule-1 (ICAM-1) dependent fashion, as the presence of anti-HLA-DR and anti-ICAM-1 antibodies both inhibit the process [30]. Eosinophils also elicit T cell proliferation as presenters of staphylococcal superantigens [31]. Eosinophils from mice infected with Mesocestoides corti have been demonstrated to be capable of stimulating T cell proliferation [32]. Mouse eosinophils exposed to Strongyloides stercoralis antigens not only express high levels of MHC Class II and CD86 but also induce both naïve and primed CD4+ T cells to produce IL-5 in co-culture [33]. The ability of eosinophils to function as APCs in murine Strongyloides infection has also been demonstrated in vivo [34]. Strongyloides antigen-exposed eosinophils induce an antigen-specific T-helper type 2 (Th2) response in naïve mice and boost the response in previously-immunized mice [34]. Furthermore, in an ovalbumin (OVA) airways challenge model, eosinophils promote IL-4, IL-5, and IL-13 secretion in co-culture with in vitro polarized Th2 cells and promote IL-5 production in co-culture with antigen-specific CD4+ T cells [25, 35]. This ability of eosinophils is CD80 and CD86 dependent; monoclonal antibodies against the CD80 and CD86 surface co-stimulatory molecules are inhibitory [35].
Important in making the case for eosinophils as antigen-presenting cells are several studies that demonstrate their trafficking to regional lymph nodes after exposure to antigen in murine allergic airways models [26, 35–37]. Trafficking to draining thoracic lymph nodes occurs both with native eosinophils [36] and with eosinophils transferred intratracheally to sensitized mice [26, 35, 37]. Importantly, eosinophil migration to regional lymph nodes is independent of eotaxin, as both CCR3+ and CCR3 − cells are capable of migration [26]. Eosinophils also show a change in phenotype in their migration from airway to draining thoracic lymph nodes, expressing greater amounts of MHC Class II and CD86 in the lymph nodes than in lung tissue [36]. Eosinophils have been observed by fluorescence microscopy to interact directly with antigen-specific CD4+ T cells in draining thoracic lymph nodes [38].
There has been some controversy regarding whether eosinophils can truly function as ‘professional’ APCs, with the ability to stimulate previously naïve T cells. It had been reported that eosinophils isolated from the BAL fluid of OVA-challenged mice were incapable of priming T cells from naïve mice either in vivo or in vitro [37]. However, the eosinophils in this study had been exposed to ammonium chloride for the purposes of erythrocyte lysis. Ammonium chloride is an inhibitor of lysosomal acidification and therefore of antigen-processing [39, 40]. Further investigation has shown that when eosinophils are purified with alternative erythrocyte lysis methods, such as hypotonic saline, they do indeed function as true professional APCs [38]. In the absence of ammonium chloride during purification, eosinophils that were incubated with OVA and subsequently instilled intratracheally were able to induce activation, proliferation, and IL-4 cytokine production of OVA-specific CD4+ T cells in mice that had received infusions of these T cells in a naïve state [38]. The observation that eosinophils act as professional APCs when exposed to hypotonic saline but not when exposed to ammonium chloride indicates that eosinophils are able to intracellularly process and then present antigen [30, 32].
As professional APCs, eosinophils are similar to dendritic cells (DCs) in their potential to prime naïve T cells [33]. Padigel et al. [33] demonstrated that eosinophils pulsed with Strongyloides antigen primed naïve T cells to the same extent as DCs pulsed with Strongyloides antigen. Unpublished data from our laboratory also suggest that eosinophils may be as potent as lung DCs in their ability to stimulate T cells in a murine airways inflammation model. However, the finding that eosinophils are potent professional APCs does not diminish the role of DCs and other professional APCs; it does support the notion that eosinophils have an additional immunoregulatory role in modulating adaptive T cell responses. This is a novel concept given the traditional characterization of eosinophils as exclusively end-stage effector cells.
The presence of IgE receptors on the surface of eosinophils provides additional indirect evidence that they participate in antigen processing and presentation. The high-affinity IgE receptor, FcεRI, had been first noted on human eosinophils in the context of its cytotoxic role against parasites [41]. Subsequently, it was noted that eosinophils from the BAL fluid of human subjects after allergen airways challenge produce both FcεRI mRNA and protein, suggesting that FcεRI is involved in the role of eosinophils as mediators of airways inflammation [42]. Eosinophils also express the low-affinity FcεRII receptor, though there is little published data to support a role in antigen presentation [43]. However, unpublished data from colleagues in our laboratory indicate that both FcεRII- and FcεRI – facilitated uptake of antigen by eosinophils promote T cell proliferation in vivo in a murine airways inflammation model and in vitro as well, suggesting that IgE receptors are involved in the APC function of eosinophils.
Overall, there is now a robust store of evidence supporting the important immunoregulatory role of eosinophils as professional APCs.
T cell regulation and polarization
Eosinophils have the ability to regulate T cell function with respect to polarization of T cells to either the Th2 or Th1 pathway (Fig. 2). This section will discuss evidence that addresses the role of eosinophils in both pathways; subsequent sections will focus on evidence that specifically addresses each individual pathway. Important to establishing the immunoregulatory function of eosinophils toward T cells is the observation that they express both Th1- and Th2-associated cytokines [44, 45]. Intracellular flow cytometry and immunohistochemistry experiments show that human eosinophils express the Th1 cytokines IFN-γ and IL-2 as well as the Th2 cytokines IL-4, IL-5, IL-10 and IL-13 [44–46]. More recent evidence indicates that TNF-α has an important role in the production of both Th1- and Th2-type chemokines by human eosinophils [47]. Culture of eosinophils with IL-4 and TNF-α upregulates the production of CCL17 and CCL22, which attract Th2 cells; culture with IFN-γ and TNF-α upregulates the production of CXCL9 and CXCL10, which attract Th1 cells [47]. Furthermore, the same study demonstrated that TNF-α induces nuclear factor-κB, IL-4 induces STAT6, and IFN-γ induces STAT1 [47]. It is known that STAT6 is a key transcription factor in the Th2 pathway, while STAT1 is similarly important in the Th1 pathway [48, 49]. Therefore, not only are eosinophils capable of expressing Th1- and Th2-associated cytokines and chemokines, they are capable of eliciting transcription factors associated with each pathway. Colleagues in our laboratory have been able to show that eosinophils constitutively express Th1 and Th2 cytokines and are able to rapidly and differentially secrete each group of cytokines (unpublished data).
Fig. 2.
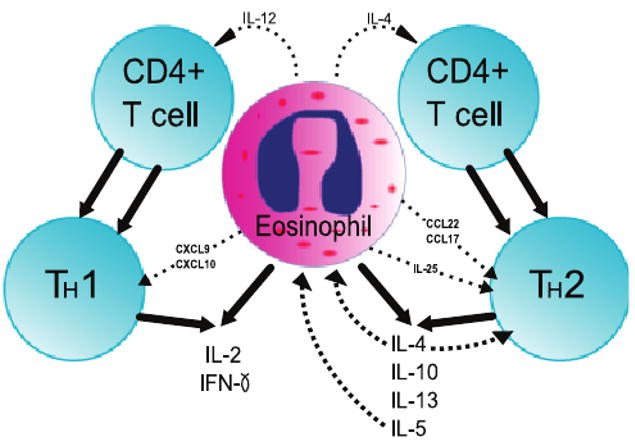
Illustration of a Model of Eosinophil Immunoregulation on CD4+ T cells. Eosinophils are capable of producing IL-12 and IL-4, which polarize T cells to Th1 cells and Th2 cells, respectively. Eosinophils express both Th1- and Th2-associated cytokines and chemokines. IL-25 is secreted by eosinophils and promotes Th2 activity. IL-4 has an amplifying effect on the Th2 response and promotes further eosinophil recruitment. IL-5 is a potent autocrine and paracrine growth and survival cytokine for eosinophils.
Not only do eosinophils produce T cell polarizing cytokines, the presence of eosinophils induces CD4+ T cells themselves to produce T cell polarizing cytokines [50]. CD4+ T cells, when placed in co-culture with staphylococcal superantigen and either blood or airway eosinophils, exhibit increased production of IL-5, IL-13, and IFN-γ [50].
It has also been demonstrated that eosinophils themselves may be subject to inhibition that could in turn affect their immunomodulatory effect on T cells [51]. They have been found to express the inhibitory receptor IRp60, and cross-linking of the receptor leads to decreased release of IFN-γ and IL-4 [51].
T-helper type 2 polarization
The presence of eosinophils in general has been considered an end-stage effect of Th2 polarization, but it can now be alternatively viewed as immunoregulatory, itself tilting T cell polarization toward a Th2 response. IL-5 is known as a Th2 cell product, but it is also a potent autocrine growth and survival cytokine produced by eosinophils themselves [52–55]. IL-5 is produced by eosinophils in a wide variety of pathologic settings in human disease [56–58]. In a mouse model of respiratory syncytial virus infection, inhibiting eosinophil chemotaxis by blocking eotaxin reduced both CD4+ T cell influx into the lungs and production of IL-5 [59].
Critical to making the case that eosinophils are able to cause Th2 polarization is the observation that eosinophils produce IL-4, a key Th2-polarizing cytokine [60, 61]. Sabin et al. [60] showed in a murine model of Schistosoma infection, a known potent Th2 stimulus, that there was a non-T cell population responsible for IL-4 production. They went on to demonstrate that in this model, IL-5 was produced and caused eosinophil recruitment. Eosinophils recruited to the i.p. site of Schistosoma egg injection proved to be responsible for local IL-4 production, suggesting that eosinophils are the primary early drivers of Th2 polarization in the immune response to murine schistosomiasis [61]. There appears to be an additional amplification effect of IL-4 as well; IL-4 itself recruits antigen-specific IL-4 producing eosinophils to the airways in a murine OVA-challenge system [62]. The recruited eosinophils had increased expression of IL-4 than local CD4+ T cells, suggesting that eosinophils are the dominant source of this Th2 cytokine [62]. Zhu et al. [63] observed that bone marrow progenitor cells stimulated with IL-5 differentiated into IL-4 producing cells, the majority of which were eosinophils.
Studies utilizing bicistronic IL-4 reporter mice, known as 4get mice, have also been important in establishing IL-4 production by eosinophils [64]. 4get mice infected with Nippostrongylus brasiliensis demonstrate recruitment of IL-4 producing eosinophils to infected lung tissue [65, 66]. Additionally, eosinophils from 4get mice constitutively express IL-4 and IL-13 transcripts, which are able to facilitate rapid cytokine production in response to stimulation [67]. Though further data from 4get mice infected with N. brasiliensis indicate a non-eosinophil, IL-4 producing innate immune cell is required for Th2 cell recruitment, the demonstration of IL-4 production by eosinophils furthers the case for important immunoregulatory function of eosinophils in Th2 polarization [68].
As noted previously, the presence of eosinophils promotes the production of Th2-associated cytokines from sensitized CD4+ T cells and from already polarized Th2 cells, pointing toward a regulatory role of eosinophils in amplifying and perpetuating allergic inflammation [25, 35]. Antigen-exposed eosinophils from a murine airways inflammation model are able to promote the production of IL-4, IL-13, and IL-5 by Th2 cells in co-culture [25]; the same group of cytokines is similarly produced by sensitized CD4+ T cells in co-culture with eosinophils [35]. Through these three cytokines respectively, eosinophils act as immunoregulators via CD4+ T cells to amplify Th2-polarization, amplify downstream Th2-mediated inflammation, and promote their own survival. The ability of eosinophils to affect Th2 responses via IL-4, IL-13, and IL-5 in vivo has been observed as well [35, 69]. Eosinophils that were transferred intratracheally from OVA-challenged mice to sensitized mice trafficked to draining paratracheal lymph nodes, where they promoted IL-4, IL-13, and IL-5 production by Th2 cells [35]. Significantly, this ability was dependent on CD80 and CD86 on eosinophils, providing a link between the antigen-presenting and Th2 polarization immunoregulatory roles of eosinophils in allergic inflammation [35]. A similar effect is seen with mice subjected to ragweed challenge, with airways eosinophils expressing IL-4, IL-5, and the Th2 transcription factor GATA-3 [70]. The demonstration of Th2 cytokine production in airways inflammation has been mirrored in murine schistosomiasis, in which eosinophils are an important source of IL-2, IL-4, and IL-5 in the schistosome granuloma [71].
It has been demonstrated that IL-4 is rapidly released in a vesicle-mediated fashion from human eosinophils in response to eotaxin and RANTES (regulated on activation, normal T cell expressed and secreted) [72]. Additionally, the rapid release of IL-4 that is stimulated by eotaxin is enhanced in the presence of IL-5, with the entire process being mediated by CCR3 receptors to eotaxin [72]. The eosinophil chemoattractant cytokine IL-16 promotes release of preformed IL-4 preferentially to the Th1-polarizing cytokine IL-12 via autocrine mechanisms acting on CCR3 receptors on eosinophils [73]. The fact that IL-4 is able to be released in such a manner provides a mechanism by which eosinophils are able to rapidly modulate T cell responses toward a Th2 phenotype.
New evidence indicates that eosinophils also regulate Th2 responses via secretion of IL-25 [74]. Eosinophils have now been found to secrete IL-25, and, in turn, promote amplification of the Th2 response by memory Th2 cells that have been stimulated by thymic stromic lymphopoietin-activated DCs [74]. The Th2 cells exposed to eosinophil-derived IL-25 have increased Th2 cytokine production, and Th2 cells exposed to exogenous IL-25 have increased proliferation and Th2 polarization; the effect of IL-25 is independent of IL-4 [74].
Eosinophils may act to promote Th2 polarization through inhibition of the Th1 pathway [75]. DCs transfected to produce indoleamine 2,3 dioxygenase (IDO), which breaks down tryptophan, have been noted in the past to have cytotoxic effects to T cells, presumably through the action of tryptophan metabolites [76]. Odemuyiwa et al. [75] demonstrated that human eosinophils express IDO and that IDO is upregulated by IFN-γ. An IFN-γ producing T cell line had decreased proliferation and increased apoptosis when co-cultured with eosinophils, while an IL-4 producing T cell line did not show the same effect [75]. These data suggest that eosinophils can mediate Th1 cell apoptosis via IDO when exposed to IFN-γ, causing polarization to tilt away from Th1 and toward Th2 [75]. Similarly, Jung et al. [77] have recently confirmed the finding that human eosinophils, both from peripheral blood and from an eosinophil cell line, can be stimulated to express IDO when stimulated with IFN-γ, IL-3, and GM-CSF.
Despite several lines of evidence that are in support of eosinophils actively regulating the Th2 response, there is evidence that suggests eosinophils may not be absolutely necessary to initiate and perpetuate Th2 inflammation. IL-5 knockout mice, which are eosinophil deficient (but not eosinophil devoid), continue to have an intact Th2 response after injection of antigen from the parasite Nippostrongylus [78]. Hypereosinophilic mice produced via an IL-5 gene-containing plasmid actually have reduced antigen-specific allergic inflammation when the gene is delivered before sensitization to allergen [79]. Furthermore, this effect appeared to be mediated by TGF-β produced by the eosinophils themselves [79]. However, a recent study by Jacobsen et al. [80] provides the most convincing evidence to date that eosinophils play a central and essential immunoregulatory function in the Th2 response characteristic of allergic pulmonary inflammation. They demonstrated that PHIL mice, which are lacking in eosinophils, have deficient recruitment of CD4+ T cells to the lung and airways in response to antigen challenge, as well as reduced levels of Th2-cytokines (IL-4, IL-5, and IL-13) and Th2-chemokines (CCL 17 and CCL22) [80]. These findings support prior data from another eosinophil-deficient strain (ΔdblGATA) and a strain deficient in eosinophil recruitment (CCR3 knockout) that both had decreased airway IL-4 and IL-13 in response to airways challenge [81]. Perhaps more significantly, Jacobsen et al. [80] also found that adoptive transfer of antigen-specific Th2-polarized T cells alone was not sufficient to generate a Th2 response to airways challenge in PHIL mice; adoptive transfer of eosinophils was needed as well to provoke Th2 inflammation. This finding strongly suggests that eosinophils are a requirement for Th2 polarization in allergic airways inflammation.
Though eosinophils have long been recognized as a component of allergic inflammation, the evidence reviewed in this section provides a basis for recognizing them as true regulators of the Th2 response, rather than as bystanders or simply end-stage effectors.
T-helper type 1 polarization
As discussed earlier, eosinophils are capable of producing Th1-associated cytokines. However, the evidence that they actually function to promote a Th1 phenotype is less extensive than that supporting its role in promoting a Th2 phenotype. The ability of eosinophils to differentially express Th1 cytokines under experimental conditions provides the best clue that they may act in vivo to effect Th1 polarization. Though there is no direct evidence of Th1-polarization by eosinophils, they do express the Th1-polarizing cytokine IL-12 [82, 83]. Ligation of CD28 in purified eosinophils appears to selectively cause the secretion of IFN-γ and IL-2, known Th1 products [84]. Stimulation of human eosinophils with IFN-γ results in the production of the Th1 cell-attracting chemokines CXCL9 and CXCL10; this effect was suppressed by the addition of IL-4 to the system [85]. The same study showed by immunohistochemistry that eosinophils in specimens from patients with Crohn's disease, a disorder characterized by Th1 inflammation, contain CXCL9 [85]. As is the case in Th2 inflammation, eosinophils can produce cytokines that cause Th1 polarization, cytokines that mediate Th1 inflammation, and chemokines that attract Th1 cells.
Another specific scenario in which the eosinophil as a Th1-promoting immunoregulatory cell has emerged is viral upper airways infection. Eosinophils incubated with rhinovirus have been found to present antigens to T cells in co-culture, leading to T cell proliferation and production of IFN-γ [86]. Though the presence of eosinophils is often associated with allergic inflammation in the setting of viral infection, the production of IFN-γ is indicative of eosinophils mediating a Th1-type response.
Regulation of dendritic cells
There is now increasing support for eosinophils exerting an immunoregulatory role on DCs as well. Yang et al. [87] found that eosinophil-derived neurotoxin (EDN), a component of eosinophil granules, acts as a chemotactic agent for DCs in vitro. Similarly, injection of mouse eosinophil-associated RNase 2, a mouse paralog of EDN, into air pouches of mice resulted in recruitment of DCs in vivo [87]. The same group also discovered that EDN is able to induce DCs to mature and activate [88]. They went on to demonstrate that EDN acts through the TLR2-MyD88 signaling pathway; DCs activated by EDN can then facilitate increased Th2 cytokine production (IL-5, IL-6, IL10, and IL-13) in response airways allergen challenge [89]. A recently published study demonstrates that eosinophils in co-culture with DCs in the presence of CpG DNA as a pathogen-associated molecular pattern molecule promote DC maturation [90]. The induction of maturation of DCs may be dependent on major basic protein (MBP) given that they are observed in this study to internalize MBP [90]. The fact that two prominent components of eosinophil granules, EDN and MBP, can have profound effects on DC maturation and function is suggestive that eosinophils may exert regulatory functions for DCs.
B cell priming
Prior work indicated that there exists a previously unrecognized Gr1+/CD11b+ IL-4-producing population of murine splenic myeloid cells that mediate priming of splenic B cell responses [91]. In this model, B cell priming was achieved with alum even in the absence of antigen [91]. Based on this observation, members of our laboratory have been able to show that the Gr1+/CD 11b+ IL-4 producing myeloid cells are eosinophils [92]. These eosinophils were capable of effecting alum-elicited B cell priming as demonstrated by MHC Class II-mediated calcium mobilization [92]. B cell priming was absent in eosinophil-deficient ΔdblGATA mice but present in wild-type mice and restored in knockout mice by adoptive transfer of wild-type eosinophils [92]. Furthermore, early antigen-specific IgM antibody responses were diminished in eosinophil-deficient ΔdblGATA mice but were fully restored by adoptive transfer of wild-type eosinophils [92].
Supporting the notion of eosinophils as immunoregulators of B cells is another recent study that observed that serial inoculation with eosinophils that had been previously pulsed with S. stercoralis antigen were able to induce antigen-specific IgM and IgG responses [34]. The data presented in these two recent studies strongly suggest that eosinophils are capable of playing key immunoregulatory roles in the priming of B cells.
Regulation of mast cells, basophils, and neutrophils
The previous sections discussed the immunoregulatory roles that eosinophils directly and indirectly play in adaptive immunity, itself a novel concept. There are also immunoregulatory functions of eosinophils within the context of their traditional home in the innate arm of immunity. Mast cells appear to be a target for immunoregulation by eosinophils, as has been reviewed elsewhere [15, 93–95]. Though mast cells themselves exert regulatory effects on eosinophils and have been demonstrated to mediate survival of eosinophils [96], a literature is emerging to support eosinophils as regulators of mast cells. Piliponsky et al. and colleagues showed that mast cells that had been previously activated in an IgE-dependent manner could be reactivated to release histamine in an IgE-independent manner by exposure to eosinophil sonicate or to purified MBP, a component of eosinophil granules [97, 98]. It had been previously observed that MBP causes histamine release not only from mast cells but from basophils as well [99]. Piliponsky et al. [100] went on to demonstrate that the ability of MBP to re-stimulate mast cells is dependent on the membrane form of stem cell factor (SCF) in a fibroblast co-culture system. Eosinophils themselves have been proven to be a source of SCF [101]. Taken together, these data support the hypothesis that eosinophils play a regulatory role in the perpetuation of late-stage mast cell-mediated inflammation, after early initiation in an IgE-dependent fashion.
There is some indirect evidence that nerve growth factor (NGF) plays some role in the immunoregulation of mast cells by eosinophils. NGF is known to promote mast cell survival [102], and eosinophils have been observed to be a source of NGF [103]. However, a direct line of evidence connecting eosinophil production of NGF to mast cell regulation has not yet been established.
Eosinophils have been observed to exert an immunoregulatory effect on neutrophils through the action of MBP. MBP has the ability to bind to human neutrophils and cause their activation, as measured by superoxide anion generation [104, 105]. The MBP released by eosinophils has also been demonstrated to stimulate IL-8 secretion by neutrophils [106].
Conclusion
A new understanding of eosinophils is beginning to emerge, with attention being drawn to eosinophils as candidate regulators of immune responses. This review has sought to compile the current evidence that supports the concept of eosinophils having key immunoregulatory roles as professional APCs and as modulators of CD4+ T cells, DCs, B cells, mast cells, neutrophils, and basophils. The immunoregulatory functions of eosinophils span both the adaptive and innate arms of immunity, expanding the niche of eosinophils beyond simply end-stage effectors in innate immunity. To varying degrees, understanding of the roles of eosinophils in the processes discussed remains incomplete, but evidence continues to accumulate. What is certain is that eosinophils are significantly more complex cells than had been conceived in the past and that eosinophils lie firmly entrenched in the interconnecting web of immune system interactions. Eosinophils can no longer be regarded as isolated entities working at the periphery of immune responses.
Acknowledgments
The research described from the Weller Laboratory was supported by Grant Number R01AI20241 and Grant Number R01AI51645 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 3.Shi HZ. Eosinophils in asthma. Chin Med J (Engl) 2004;117:792–4. [PubMed] [Google Scholar]
- 4.Wills-Karp M, Karp CL. Biomedicine. Eosinophils in asthma: remodeling a tangled tale. Science. 2004;305:1726–9. doi: 10.1126/science.1104134. [DOI] [PubMed] [Google Scholar]
- 5.Adamko D, Lacy P, Moqbel R. Eosinophil function in allergic inflammation: from bone marrow to tissue response. Curr Allergy Asthma Rep. 2004;4:149–58. doi: 10.1007/s11882-004-0061-x. [DOI] [PubMed] [Google Scholar]
- 6.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 7.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–55. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 8.Lacy P, Moqbel R. Immune effector functions of eosinophils in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2001;1:79–84. doi: 10.1097/01.all.0000010989.39379.49. [DOI] [PubMed] [Google Scholar]
- 9.Moqbel R, Lacy P. New concepts in effector functions of eosinophil cytokines. Clin Exp Allergy. 2000;30:1667–71. doi: 10.1111/j.1365-2222.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–75. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 11.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–94. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 12.Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. doi: 10.1111/j.1398-9995.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–20. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Weller PF, Lim K, Wan HC, et al. Role of the eosinophil in allergic reactions. Eur Respir J. 1996;22(Suppl):109s–15s. [PubMed] [Google Scholar]
- 15.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 16.Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76:520–7. doi: 10.1189/jlb.0404228. [DOI] [PubMed] [Google Scholar]
- 17.Koeffler HP, Billing R, Levine AM, Golde DW. Ia antigen is a differentiation marker on human eosinophils. Blood. 1980;56:11–4. [PubMed] [Google Scholar]
- 18.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci USA. 1989;86:1348–51. doi: 10.1073/pnas.86.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansel TT, Braunstein JB, Walker C, et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–7. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beninati W, Derdak S, Dixon PF, et al. Pulmonary eosinophils express HLA-DR in chronic eosinophilic pneumonia. J Allergy Clin Immunol. 1993;92:442–9. doi: 10.1016/0091-6749(93)90123-w. [DOI] [PubMed] [Google Scholar]
- 21.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–8. [PubMed] [Google Scholar]
- 22.Mawhorter SD, Pearlman E, Kazura JW, Boom WH. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi. Infect Immun. 1993;61:5410–2. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto H, Sedgwick JB, Vrtis RF, Busse WW. The effect of transendothelial migration on eosinophil function. Am J Respir Cell Mol Biol. 2000;23:379–88. doi: 10.1165/ajrcmb.23.3.3707. [DOI] [PubMed] [Google Scholar]
- 24.Tamura N, Ishii N, Nakazawa M, et al. Requirement of CD80 and CD86 molecules for antigen presentation by eosinophils. Scand J Immunol. 1996;44:229–38. doi: 10.1046/j.1365-3083.1996.d01-303.x. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–55. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 26.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–53. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celestin J, Rotschke O, Falk K, et al. IL-3 induces B7.2 (CD86) expression and costimulatory activity in human eosinophils. J Immunol. 2001;167:6097–104. doi: 10.4049/jimmunol.167.11.6097. [DOI] [PubMed] [Google Scholar]
- 28.Ohkawara Y, Lim KG, Xing Z, et al. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996;97:1761–6. doi: 10.1172/JCI118603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993;150:2554–62. [PubMed] [Google Scholar]
- 30.Hansel TT, De Vries IJ, Carballido JM, et al. Induction and function of eosinophil intercellular adhesion molecule-1 and HLA-DR. J Immunol. 1992;149:2130–6. [PubMed] [Google Scholar]
- 31.Mawhorter SD, Kazura JW, Boom WH. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology. 1994;81:584–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Del Pozo V, De Andres B, Martin E, et al. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–25. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 33.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–8. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padigel UM, Hess JA, Lee JJ, et al. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–51. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi HZ, Xiao CQ, Li CQ, et al. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–35. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 36.Duez C, Dakhama A, Tomkinson A, et al. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–5. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 37.van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–8. doi: 10.4049/jimmunol.171.7.3372. [DOI] [PubMed] [Google Scholar]
- 38.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–92. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loss GE, Jr, Sant AJ. Invariant chain retains MHC class II molecules in the endocytic pathway. J Immunol. 1993;150:3187–97. [PubMed] [Google Scholar]
- 40.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–8. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gounni AS, Lamkhioued B, Ochiai K, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–6. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 42.Rajakulasingam K, Till S, Ying S, et al. Increased expression of high affinity IgE (FcepsilonRI) receptor-alpha chain mRNA and protein-bearing eosinophils in human allergen-induced atopic asthma. Am J Respir Crit Care Med. 1998;158:233–40. doi: 10.1164/ajrccm.158.1.9708106. [DOI] [PubMed] [Google Scholar]
- 43.Gounni AS, Lamkhioued B, Masao M, et al. Molecular characterization of the low-affinity IgE receptor Fc epsilonRII/CD23 expressed by human eosinophils. Int Immunol. 1998;10:395–404. doi: 10.1093/intimm/10.4.395. [DOI] [PubMed] [Google Scholar]
- 44.Lamkhioued B, Gounni AS, Aldebert D, et al. Synthesis of type 1 (IFN gamma) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Ann N Y Acad Sci. 1996;796:203–8. doi: 10.1111/j.1749-6632.1996.tb32582.x. [DOI] [PubMed] [Google Scholar]
- 45.Woerly G, Roger N, Loiseau S, Capron M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Int Arch Allergy Immunol. 1999;118:95–7. doi: 10.1159/000024038. [DOI] [PubMed] [Google Scholar]
- 46.Levi-Schaffer F, Barkans J, Newman TM, et al. Identification of interleukin-2 in human peripheral blood eosinophils. Immunology. 1996;87:155–61. [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–8. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 48.Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med. 2001;193:1087–96. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikhak Z, Fleming CM, Medoff BD, et al. STAT1 in peripheral tissue differentially regulates homing of antigen-specific Th1 and Th2 cells. J Immunol. 2006;176:4959–67. doi: 10.4049/jimmunol.176.8.4959. [DOI] [PubMed] [Google Scholar]
- 50.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EA. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–97. doi: 10.1111/j.1398-9995.2006.01060.x. [DOI] [PubMed] [Google Scholar]
- 51.Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107:1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- 52.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73:1504–12. [PubMed] [Google Scholar]
- 53.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–24. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi Y, Hayashi Y, Sugama Y, et al. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–42. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi Y, Suda T, Suda J, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desreumaux P, Bloget F, Seguy D, et al. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis. Gastroenterology. 1996;110:768–74. doi: 10.1053/gast.1996.v110.pm8608886. [DOI] [PubMed] [Google Scholar]
- 57.Desreumaux P, Janin A, Colombel JF, et al. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992;175:293–6. doi: 10.1084/jem.175.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desreumaux P, Janin A, Dubucquoi S, et al. Synthesis of interleukin-5 by activated eosinophils in patients with eosinophilic heart diseases. Blood. 1993;82:1553–60. [PubMed] [Google Scholar]
- 59.Matthews SP, Tregoning JS, Coyle AJ, Hussell T, Openshaw PJ. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J Virol. 2005;79:2050–7. doi: 10.1128/JVI.79.4.2050-2057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabin EA, Pearce EJ. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol. 1995;155:4844–53. [PubMed] [Google Scholar]
- 61.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–8. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Grabowski KA, Xin JP, et al. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004;172:2059–66. doi: 10.4049/jimmunol.172.4.2059. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, Chen L, Huang Z, et al. Cutting edge: IL-5 primes Th2 cytokine-producing capacity in eosinophils through a STAT5-dependent mechanism. J Immunol. 2004;173:2918–22. doi: 10.4049/jimmunol.173.5.2918. [DOI] [PubMed] [Google Scholar]
- 64.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 65.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–9. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 66.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 67.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 68.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid-Grendelmeier P, Altznauer F, Fischer B, et al. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002;169:1021–7. doi: 10.4049/jimmunol.169.2.1021. [DOI] [PubMed] [Google Scholar]
- 70.Justice JP, Borchers MT, Lee JJ, Rowan WH, Shibata Y, Van Scott MR. Ragweed-induced expression of GATA-3, IL-4, and IL-5 by eosinophils in the lungs of allergic C57BL/6J mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L302–9. doi: 10.1152/ajplung.00158.2001. [DOI] [PubMed] [Google Scholar]
- 71.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–9. [PubMed] [Google Scholar]
- 72.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–7. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 73.Bandeira-Melo C, Sugiyama K, Woods LJ, et al. IL-16 promotes leukotriene C(4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–63. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 74.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odemuyiwa SO, Ghahary A, Li Y, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–13. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 76.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung YJ, Woo SY, Jang MH, et al. Human eosinophils show chemotaxis to lymphoid chemokines and exhibit antigen-presenting-cell-like properties upon stimulation with IFN-gamma, IL-3 and GM-CSF. Int Arch Allergy Immunol. 2008;146:227–34. doi: 10.1159/000115891. [DOI] [PubMed] [Google Scholar]
- 78.Holland MJ, Harcus YM, Balic A, Maizels RM. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC Class I-related receptors. Immunol Lett. 2005;96:93–101. doi: 10.1016/j.imlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Nakagome K, Dohi M, Okunishi K, et al. IL-5-induced hypereosinophilia suppresses the antigen-induced immune response via a TGF-beta-dependent mechanism. J Immunol. 2007;179:284–94. doi: 10.4049/jimmunol.179.1.284. [DOI] [PubMed] [Google Scholar]
- 80.Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA. 2006;103:16418–23. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grewe M, Czech W, Morita A, et al. Human eosinophils produce biologically active IL-12: implications for control of T cell responses. J Immunol. 1998;161:415–20. [PubMed] [Google Scholar]
- 83.Nutku E, Gounni AS, Olivenstein R, Hamid Q. Evidence for expression of eosinophil-associated IL-12 messenger RNA and immunoreactivity in bronchial asthma. J Allergy Clin Immunol. 2000;106:288–92. doi: 10.1067/mai.2000.107932. [DOI] [PubMed] [Google Scholar]
- 84.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999;190:487–95. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dajotoy T, Andersson P, Bjartell A, Lofdahl CG, Tapper H, Egesten A. Human eosinophils produce the T cell-attracting chemokines MIG and IP-10 upon stimulation with IFN-gamma. J Leukoc Biol. 2004;76:685–91. doi: 10.1189/jlb.0803379. [DOI] [PubMed] [Google Scholar]
- 86.Handzel ZT, Busse WW, Sedgwick JB, et al. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–84. [PubMed] [Google Scholar]
- 87.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 88.Yang D, Chen Q, Rosenberg HF, et al. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–42. doi: 10.4049/jimmunol.173.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang D, Chen Q, Su SB, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2 MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83:456–60. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 91.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–10. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 92.Wang HB, Weller PF. Pivotal Advance: Eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–21. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–75. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 94.Munitz A, Piliponsky AM, Levi-Schaffer F. IgE-independent activation of human mast cells indicates their role in the late phase reaction of allergic inflammation. Cell Tissue Bank. 2003;4:25–8. doi: 10.1023/A:1026307812980. [DOI] [PubMed] [Google Scholar]
- 95.Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: a new pathway for the perpetuation of allergic inflammation. Mol Immunol. 2002;38:1369–72. doi: 10.1016/s0161-5890(02)00090-1. [DOI] [PubMed] [Google Scholar]
- 96.Levi-Schaffer F, Temkin V, Malamud V, Feld S, Zilberman Y. Mast cells enhance eosinophil survival in vitro: role of TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol. 1998;160:5554–62. [PubMed] [Google Scholar]
- 97.Piliponsky AM, Pickholtz D, Gleich GJ, Levi-Schaffer F. Eosinophils activate mast cells to release histamine. Int Arch Allergy Immunol. 1999;118:202–3. doi: 10.1159/000024067. [DOI] [PubMed] [Google Scholar]
- 98.Piliponsky AM, Pickholtz D, Gleich GJ, Levi-Schaffer F. Human eosinophils induce histamine release from antigen-activated rat peritoneal mast cells: a possible role for mast cells in late-phase allergic reactions. J Allergy Clin Immunol. 2001;107:993–1000. doi: 10.1067/mai.2001.114656. [DOI] [PubMed] [Google Scholar]
- 99.O'Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med. 1983;157:1981–91. doi: 10.1084/jem.157.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piliponsky AM, Gleich GJ, Nagler A, Bar I, Levi-Schaffer F. Non-IgE-dependent activation of human lung- and cord blood-derived mast cells is induced by eosinophil major basic protein and modulated by the membrane form of stem cell factor. Blood. 2003;101:1898–904. doi: 10.1182/blood-2002-05-1488. [DOI] [PubMed] [Google Scholar]
- 101.Hartman M, Piliponsky AM, Temkin V, Levi-Schaffer F. Human peripheral blood eosinophils express stem cell factor. Blood. 2001;97:1086–91. doi: 10.1182/blood.v97.4.1086. [DOI] [PubMed] [Google Scholar]
- 102.Horigome K, Bullock ED, Johnson EM., Jr Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J Biol Chem. 1994;269:2695–702. [PubMed] [Google Scholar]
- 103.Solomon A, Aloe L, Pe'er J, et al. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol. 1998;102:454–60. doi: 10.1016/s0091-6749(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 104.Moy JN, Gleich GJ, Thomas LL. Noncytotoxic activation of neutrophils by eosinophil granule major basic protein. Effect on superoxide anion generation and lysosomal enzyme release. J Immunol. 1990;145:2626–32. [PubMed] [Google Scholar]
- 105.Shenoy NG, Gleich GJ, Thomas LL. Eosinophil major basic protein stimulates neutrophil superoxide production by a class IA phosphoinositide 3-kinase and protein kinase C-zeta-dependent pathway. J Immunol. 2003;171:3734–41. doi: 10.4049/jimmunol.171.7.3734. [DOI] [PubMed] [Google Scholar]
- 106.Page SM, Gleich GJ, Roebuck KA, Thomas LL. Stimulation of neutrophil interleukin-8 production by eosinophil granule major basic protein. Am J Respir Cell Mol Biol. 1999;21:230–7. doi: 10.1165/ajrcmb.21.2.3647. [DOI] [PubMed] [Google Scholar]


