Abstract
The principal components of both MHC class I and class II antigen processing and presentation pathways are well known. Within dendritic cells, these pathways are tightly regulated by Toll-like receptor signalling and include features, such as cross-presentation, that are not seen in other cell types. The exact mechanisms involved in the subcellular trafficking of antigens remain poorly understood and in some cases are controversial. Recent data suggest that diverse cellular machineries including autophagy participate in antigen processing and presentation, though their relative contributions remain to be fully elucidated. Here, we highlight some emerging themes of antigen processing and presentation that we believe merit further attention.
Since the discovery that T-cell immunity relies on ‘denatured, unfolded, sequential determinants’1 of proteins, whereas B-cell (that is, antibody) recognition of the same protein antigen is determined by its tertiary structure, immunologists have been fascinated with antigen processing and presentation. Decades of work have elucidated the pathways that generate peptide–MHC complexes. As a result, we can now explain most of the fundamental differences between T- and B-cell antigen recognition2,3 and such knowledge informs vaccine design and other immune-based interventions.
Central to T-cell activation, specific T-cell receptors recognize antigens in the peptide-binding groove of surface-expressed MHC class I and class II molecules. To fulfil their physiological function, MHC proteins must first acquire peptide antigens. The two major classes of MHC molecules execute this function differently. For MHC class I molecules, the goal is to report on intracellular events (such as viral infection, the presence of intracellular bacteria or cellular transformation) to CD8+ T cells4. MHC class I molecules are composed of heavy chains and an invariant light chain, known as β2-microglobulin. The events of the biosynthesis of MHC class I molecules may be summarized in six steps: one, acquisition of antigenic peptides; two, tagging of the antigenic peptide for destruction by ubiquitylation; three, proteolysis; four, delivery of peptides to the endoplasmic reticulum (ER); five, binding of peptides to MHC class I molecules; and six, display of peptide–MHC complexes on the cell surface (Figure 1). For MHC class II molecules, the goal is to sample the extracellular milieu and present antigens to CD4+ T cells4. Similar to MHC class I molecules, the α and β chain of MHC class II molecules are synthesized in the ER and associate with the invariant chain (Ii; also known as CD74) for proper folding, trafficking and protection of the antigen binding groove5. Newly assembled MHC class II molecules are then delivered by vesicular transport to endolysosomal compartments that supply peptide antigens. Following peptide loading, peptide–MHC class II complexes are delivered to the cell surface. Despite the involvement of different molecules and cellular compartments, the generation of peptide–MHC class II complexes can be stratified into the same six steps as for peptide–MHC class I complexes.
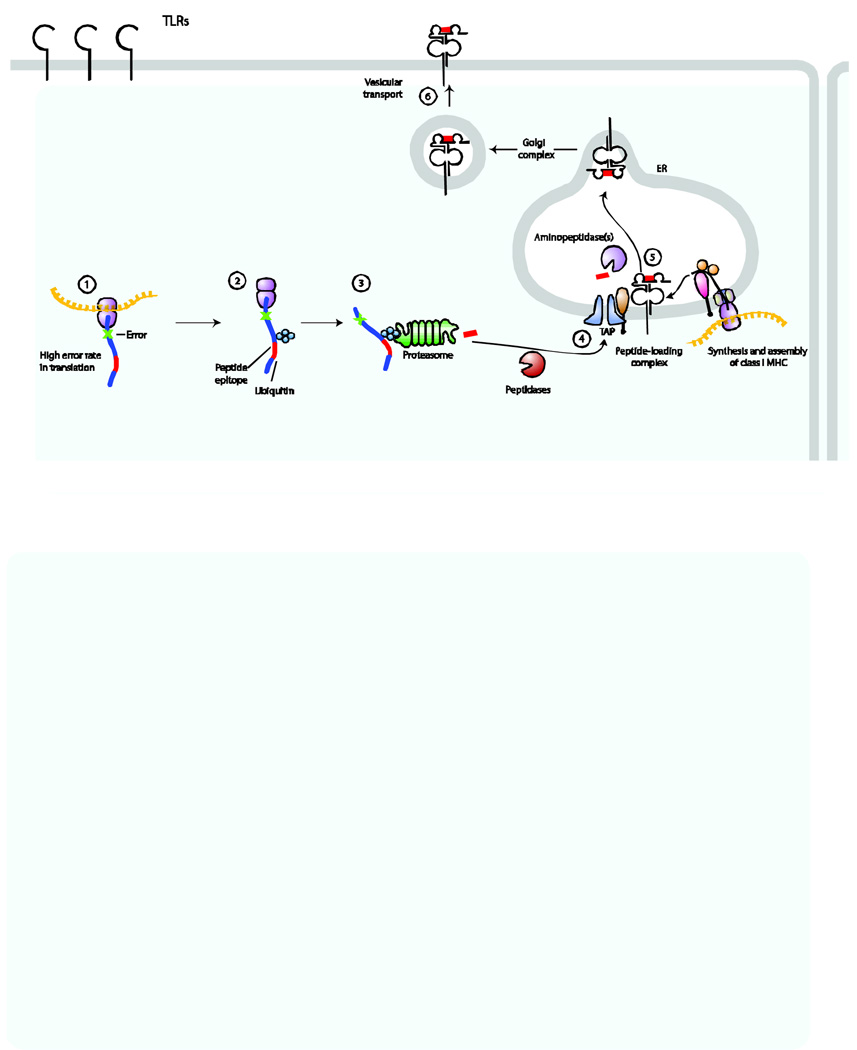
Figure 1. Six steps for loading and trafficking of MHC class I molecules to the cell surface.
Antigen processing and presentation by MHC class I molecules can be divided into six discrete steps. Step 1: acquisition of antigens from proteins with errors (for example, due to premature termination or misincorporation). Step 2: misfolded proteins are tagged with ubiquitin for degradation. Step 3: the proteasome degrades these ubiquitylated proteins into peptides. Step 4: the peptides are delivered to the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) complex. Step 5: peptide is loaded onto nascently formed MHC class I molecules; this process is facilitated by members of the peptide-loading complex, such as tapasin and two ‘housekeeping’ ER proteins, known as calreticulin and ERp57. Step 6: peptide-loaded MHC class I molecules are transported via the Golgi complex to the cell surface. The steps for MHC class II–peptide loading conceptually follow this same path.
The molecular expression of MHC class II molecules is mostly restricted to professional antigen-presenting cells (APCs), including macrophages and dendritic cells (DCs). DCs possess many unique features of antigen processing and presentation not seen in other cell types. Immature DCs reside in the tissue (for example, in the skin, lungs and gastrointestinal tract) and undergo remarkable transformation upon exposure to pathogens. Pathogen-associated molecular patterns and their vertebrate receptors, including Toll-like receptors (TLRs)6,7 influence the dynamics of antigen acquisition, cytoskeletal rearrangements and regulation of MHC biosynthesis, all of which affect antigen processing and presentation8,9. Likewise, the machinery of protein translation and degradation, which is required for generating antigenic peptides for presentation, is carefully regulated following DC activation10. Finally, DC activation by TLR ligands is required for the formation of endolysosomal tubules, which contain numerous proteins including MHC class II molecules, and deliver these proteins to the cell surface, where they are available to CD4+ T cells for potential activation11–13.
DCs have a central role in the activation of naive T cells and therefore direct the adaptive immune response against invading microorganisms. But how do DCs participate in the immune response to intracellular microorganisms that do not directly infect APCs? First, whole microorganisms may transiently exist in the extracellular space and be taken up by DCs into the endocytic pathway, where relevant antigens are loaded onto MHC class II molecules in endolysosomes. In addition, DCs possess the capacity to take these antigens and transfer them to the MHC class I pathway, a process referred to as cross-presentation, but the details of this process remain controversial. Another relevant question is how do antigens from the extracellular environment gain access to the MHC class I pathway, which is normally restricted to the presentation of intracellular antigens? Here, we review the evidence for hypotheses that invoke the involvement of the ER dislocation machinery and channel-independent pathways. The generation of peptides for both MHC class I and class II pathways had been viewed as the exclusive domains for the proteasome and lysosomal-associated proteases, respectively. Recent data indicate that additional pathways can participate in this process. The role of autophagy, a ubiquitous process by which cells remove damaged organelles, in the generation of peptides for MHC molecules will also be discussed. The pathways of antigen processing and presentation have been extensively reviewed recently4,14 and therefore, we focus here on aspects of antigen processing and presentation that are less well understood or that remain controversial.
MHC class II processing and presentation
Focused on the extracellular environment, the MHC class II antigen presentation pathway intersects with the endocytic pathway to sample antigens. Extracellular antigen is taken up by APCs and placed into a membrane-delimited compartment, known as the phagosome. This phagosomal compartment undergoes a series of modifications, in part dictated by its content, and finally fuses with lysosomes to form phagolysosomes, where this compartment interacts with MHC class II molecules (FIG. 2). Peptide-loaded MHC class II molecules are then transported to the cell surface where they engage antigen-specific CD4+ T cells. Despite the apparent simplicity of this pathway, important questions remain, including the nature of modifications made to the phagosome, modes of delivery of MHC class II molecules to the cell surface and the contribution of autophagy to the MHC class II pathway.
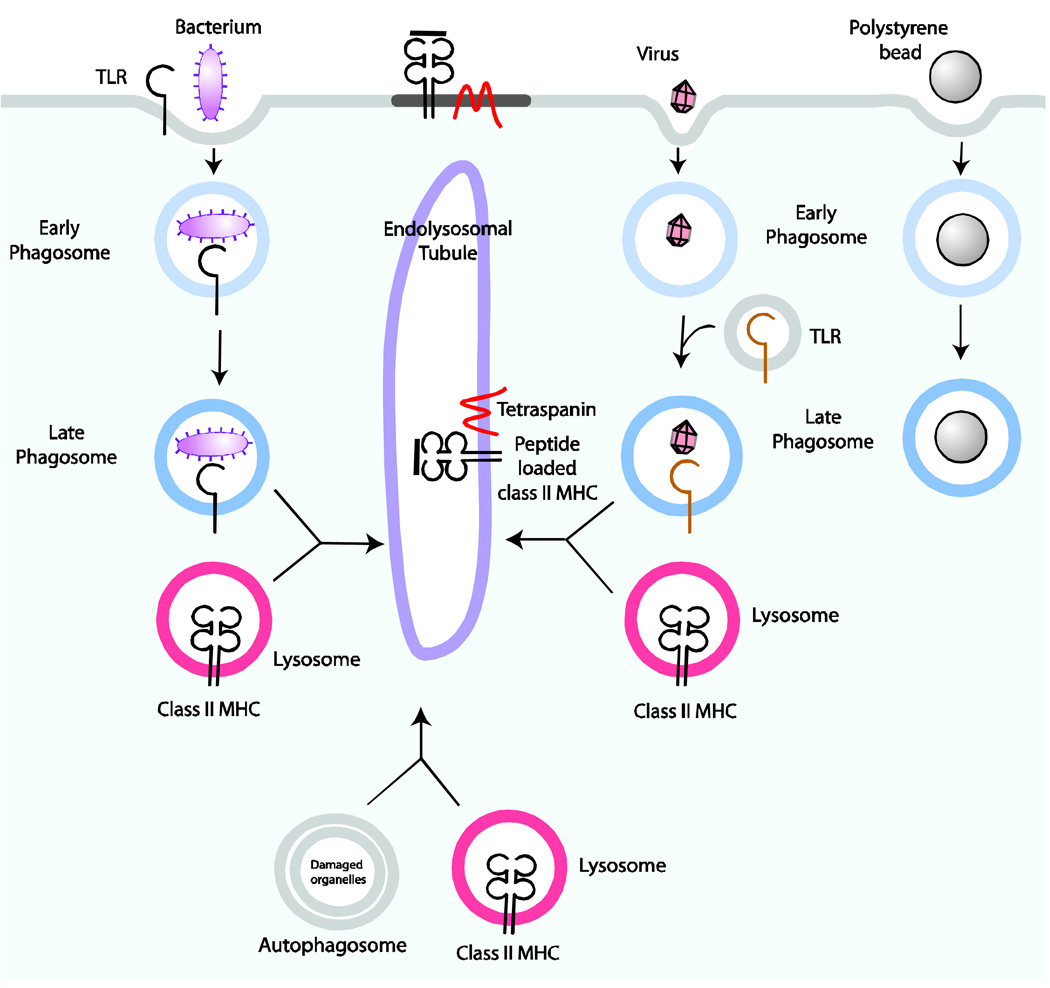
Figure 2. Contribution of pathogens and self-peptides to loading of MHC class II in DCs.
Following phagocytosis of bacteria, surface-expressed Toll-like receptors (TLRs) become activated and influence the nature of phagosome maturation. Similarly, viruses engage TLRs found in endocytic vesicles that recognize nucleic acids. Following the maturation of phagosomes, these structures fuse with lysosomes to form phagolysosomes. MHC class II molecules that are contained in the lysosomes are loaded with peptide fragments formed by lysosomal proteases. Autophagosomes also fuse with lysosomes and serve as an additional source of peptides, including endogenous peptides, for MHC class II presentation. MHC class II molecules, as well as a host of other lysosomal proteins including tetraspanins, are transported in endolysosomal tubules to the cell surface. Surface MHC class II molecules can be found in microdomains with other co-stimulatory proteins. Phagosomes that contain polystyrene beads, which fail to ligate TLRs, do not mature fully and probably contribute less to the antigen processing pathway for MHC class II molecules.
Phagosome maturation
Phagocytosis is restricted to professional APCs, responsible for the uptake of various particles, including microorganisms and apoptotic bodies, and serves as an important mechanism for antigen acquisition15. These particles are shuttled into phagosomes, which are then subjected to numerous modifications that serve to guide the recruitment of other proteins and subsequent fusion events with other vesicles16 (Fig. 2). The formation of phagosomes has been extensively reviewed16,17. Cellular membrane protein recruitment to the phagosome — and therefore the fate of the phagosome — appears to be regulated in part by the capacity to engage TLRs early in the course of phagosome formation. Indeed, antigen that is complexed with the TLR4 ligand lipopolysaccharide (LPS) and presented by DCs more potently activate T cells than antigen alone. Both MHC class II molecules and the tetraspanin member CD63 are specifically recruited to pathogen-containing phagosomes but not to phagosomes that contain polystyrene beads18 (many investigators use the term latex bead to refer to polystyrene beads; however, latex and polystyrene are distinct compounds). In addition, TLR4 signalling by LPS triggers the efficient loading of MHC class II molecules with peptide, whereas phagosomes devoid of LPS fail to contribute to the activation of antigen-specific T cells19. The connection between TLR signalling and the efficiency of MHC class II antigen presentation shows that the use of pathogens, rather than polystyrene beads, to induce phagocytic uptake will inform us on the pathways relevant for antigen processing and presentation.
Given the importance of phagosome maturation in antigen processing, pathogens have devised strategies to modify their fate within the phagosome20. Mycobacterium tuberculosis, for example, blocks phagosomal maturation, thereby enabling its own survival within macrophage phagosomes, from which it may then escape into the cytosol21. Legionella pneumophila, the causative agent of legionaire’s pneumonia escapes the degradative lysosomal pathway by intercepting vesicular traffic from the ER to form an ER-like compartment that avoids fusion with lysosomes22. Toxoplasma gondii can modify the compartment in which it resides to permit long-term growth and prevent death of the pathogen by forming a parasitophorous vacuole that resists fusion with vesicles of the endocytic pathway23,24.
Fusion with lysosomes to form phagolysosomes is the final event in the life of a phagosome. It seems that the intravesicular conditions of lysosomes found in DCs may be different from those in other cell types. DCs can be infected with HIV-1 through the cell-surface receptor DC-specific ICAM3-grabbing non-integrin (DC-SIGN) and HIV1 is internalized by endocytosis in a form that retains its viability and allows transfer to T cells, possibly through the immunological synapse25,26. The fact that the otherwise fragile HIV-1 virions remain infectious testifies to the rather innocuous environment to which they are initially delivered. The degradation of antigen within the endolysosomal compartment following the lysosomal acidification of endosomal compartments is tightly controlled in DCs in an activation-dependent manner10. Acidification of this compartment permits optimal activity of lysosomal acid hydrolases and cathepsins. RAB27, a protein involved in vesicular docking and secretion, is rapidly recruited to phagosomes in DCs and controls acidification of this compartment by the recruitment of the NADPH oxidase and by limiting the production of reactive oxygen species27. Phosphorylation of extracellular-signal regulated kinases 1 and 2 (ERK 1/2) in DCs is also controlled by the action of the lysosomal cysteine protease cathepsin K, which has recently been shown to regulate TLR9-induced signalling, but not that of other TLRs28. The inhibition or absence of cathepsin K in DCs greatly reduced TLR9-induced secretion of pro-inflammatory cytokines, linking cathepsin K activity specifically to the TLR9 signalling cascade and possibly affecting phagosome maturation. Thus, TLR activation serves in part to control the quality of lysosomes and therefore, the enzymatic activity of its proteases in DCs.
The contributions of autophagy to the MHC class II pathway
Much of the emphasis on antigen degradation has been on phagosomal maturation through the endocytic pathway and fusion with lysosomal vesicles. However, additional mechanisms have been recently implicated in antigen processing and presentation. Autophagy has an important role in maintaining cell homeostasis: it provides nutrients during periods of starvation and removes damaged organelles from the cytoplasm. There are at least three different types of autophagy: chaperone-mediated autophagy, microautophagy, and macroautophagy, of which the latter is the best characterized. At least thirty autophagy-related genes (ATGs) contribute to autophagy in yeast, many of which have orthologues in mammalian cells (supplementary information S1). Autophagosomal membrane formation and expansion is facilitated by two systems, the ATG8 (known as LC3 in mammals) system and ATG12 system, the details of which have been reviewed elsewhere29. Autophagy is now considered an important process for the delivery of antigens to MHC class II molecules.
Autophagy can target pathogens that reside in the cytosol or within phagosomes for lysosomal degradation and therefore participate in the effective elimination of viruses, bacteria, and parasites30–32 (Box 1). Autophagy might also contribute to several independent stages of antigen presentation, including the uptake of antigens from the cytosol or from phagosomes. This will influence the repertoire of peptides loaded onto MHC class II proteins, the delivery of antigen to the endolysosomal degradation pathway, the loading of MHC class II molecules with endogenous peptides, and the generation of functional, self-tolerant effector T cells. Importantly, TLR-derived signals appear to regulate autophagy in DCs and thus affect antigen processing and presentation.
Box 1: Pathogen interactions with the autophagy machinery
Autophagy can target pathogens that reside in the cytosol or within phagosomes for lysosomal degradation. Therefore, autophagy contributes to the effective elimination of viruses, bacteria, and parasites30–32. As discussed in the text, autophagy eliminates Mycobacterium tuberculosis-containing phagosomes. Similarly, clearance of pathogens such as Streptococcus pyogenes129, Salmonella enterica serovar Typhimurium130, and Toxoplasma gondii125,127 can also occur in an autophagy-dependent manner. Many pathogens strive to escape the autophagy machinery131,132. The herpes simplex virus type 1 (HSV-1) neurovirulence protein ICP34.5 antagonizes autophagy by interacting with the autophagy protein beclin-1133. Similarly, IcsB, a type III secretion effector protein from Shigella flexneri, escapes autophagy by competing with the autophagy protein ATG5 for binding to S. flexneri VirG134. The ability of pathogens to escape autophagy may permit survival within host cells and therefore represent a new mechanism for immune evasion. Understanding the interactions between pathogens and the autophagy machinery will clarify the role of autophagy in the immune response.
Autophagy and TLRs
The signals that induce autophagy during the immune response have only recently begun to be elucidated. Although professional APCs engage in constitutive autophagy33, cytokines can modulate this process. In addition to cytokines, TLR ligands, including LPS and the TLR7 ligands imiquimod and single stranded RNA, induce autophagy in macrophages, and enhance mycobacterial colocalization with autophagosomes and therefore the elimination of this pathogen34,35. Several other TLR ligands induce the recruitment of the autophagosomal marker beclin-1 to the phagosome, followed by the ATG5- and ATG7-dependent recruitment of ATG836. A failure to do so was shown to result in increased survival of engulfed Saccharomyces cerevisiae, further implicating TLR signalling in the induction of autophagy.
Conversely, autophagy also stimulates TLR signalling by delivery of viral replication intermediates to TLR7, which is present in the endosomes of plasmacytoid DCs37. TLR signalling is a well-known maturation stimulus for professional APCs. Autophagy therefore enhances antigen presentation by delivering TLR ligands to the endosome for functional maturation of DCs. The interplay between TLR signalling, autophagy, and antigen presentation merits further investigation.
Autophagy and the phagosome
Autophagosomal proteins participate in the maturation of phagosomes. The autophagosomal markers LC3 and beclin-1 translocate to the phagosomal membrane during early stages of phagocytosis in the presence of a TLR ligand36. However, the translocation of LC3 and beclin-1 to the phagosome was not associated with the formation of double-membrane structures36 and therefore this phenomenon might be distinct from conventional autophagy. A proteomic study of polystyrene-bead-containing phagosomes derived from cultured Drosophila melanogaster cells identified ATG9, another autophagosomal marker, as one of 617 phagosomal proteins38. However, other proteomic studies of phagosomes have not identified the presence of autophagosomal proteins on phagosomes39–41, perhaps because their association with the phagosomal membrane is transient36 or because TLRs might not have been suitably engaged.
Autophagosomes also converge with endosomes42,43 and deliver exogenous peptides to endolysosomal compartments for loading onto MHC class II molecules33. However, there is no direct evidence that autophagosomal degradation of pathogens in the phagolysosomal compartment results in enhanced MHC class II presentation of the corresponding pathogen-derived peptides and thus these two events have yet to be linked directly. The exact role of the recruitment of autophagy proteins to phagosomes remains to be determined, but it might affect antigen processing and presentation and T cell selection in the thymus (Box 2).
Box 2: Autophagy and T-cell selection and survival
Professional antigen-presenting cells (APCs) and interferon-γ (IFNγ)-stimulated epithelial cells show a high level of constitutive autophagy, continuous fusion between autophagosomes and MHC–class-II-expressing compartments, and efficient delivery of endogenous antigens to these compartments by autophagosomes33. Of note, the thymic compartment in transgenic mice in which LC3 was tagged with green fluorescent protein (GFP–LC3) shows a high level of constitutive autophagy in thymic epithelial cells, even under nutrient-rich conditions135. Medullary thymic epithelial cells (mTECs), which by promiscuous expression of tissue-restricted self antigens mirror virtually all tissues in the body, have an important role in the induction of central tolerance136. These poorly phagocytic cells might use autophagy for MHC class II-restricted antigen presentation for positive and negative selection of T cells. Similarly, the recently described autoimmune regulator (AIRE)+ fibroblast reticular-like cells in peripheral lymph nodes might use a similar mechanism to present self antigens to peripheral T cells137. Although not formally shown, in the absence of autophagy, endogenous peptides might not be presented by MHC class II molecules, and result in a failure to tolerize potentially self-reactive CD4+ T cells. Deficits in autophagy could therefore result in impaired clonal selection and defects in central or peripheral tolerance. Indeed, mutations in the autophagy gene ATG16L1, as well as the potentially autophagy-inducing immunity related GTPase family, M (IRGM) gene (a member of the p47 GTPases), have been associated with the development of autoimmunity in patients with Crohn’s disease138,139 140. How ATG16L1 or IRGM mutations influence autoimmunity is not known, but the possibility of defective presentation of the necessary self-peptides to allow maturation of CD4+ T cells in the absence of autophagy is a testable hypothesis. Autophagy influences many steps in the MHC class II pathway and has profound effects on T cell development, though further work is needed to understand the molecular interactions between the autophagy machinery and known components of the MHC class II pathway.
Autophagy and endogenous antigens
Autophagy can also deliver endogenous antigens to the MHC class II pathway30, as shown by its role in MHC class II presentation of a viral antigen (Epstein–Barr virus nuclear antigen 1 (EBNA1)) expressed at physiological levels44. As judged by its colocalization with the autophagosomal marker monodansylcadaverine (MDC), EBNA1 accumulates in autophagosomal structures when lysosomal acidification, and therefore autophagosome maturation, is blocked. In addition, EBNA1 was shown to be presented to MHC-class-II-restricted EBNA1-specific CD4+ T-cell clones. This presentation was abrogated by inclusion of 3-methyladenine and knockdown of ATG12, both of which inhibit autophagy. These studies demonstrate that cytosolic antigens degraded by autophagy can provide peptides to the MHC Class II pathway.
In addition to viral antigens, MHC-class-II-restricted antigen presentation of certain self45, tumour46, and model47 antigens depends on autophagy. A substantial number of peptides recovered in a complex with MHC class II molecules originate from proteins that are usually found in the cytosol or nucleus48–51 and the size of this fraction of peptides was increased following starvation-induced autophagy51. Therefore, by means of autophagy, the peptide repertoire presented by MHC class II molecules is extended from exogenous antigens to include some endogenous antigens.
Whereas the above examples all rely on macroautophagy, chaperone-mediated autophagy also contributes to MHC-class-II-restricted presentation of endogenous antigens52. In this case, lysosome-associated membrane protein 2a (LAMP2a), together with heat shock protein 70 (HSC70), may transport a cytoplasmic antigen to MHC class II molecules and contribute to antigen presentation52.
How the phagocytic uptake of antigens influences the balance between MHC-class-II-restricted presentation of endogenous versus exogenous antigens is not fully understood. One study briefly addressed this issue and showed that exogenous antigen does not compete with endogenous antigen for MHC–class-II-restricted presentation in mature DCs53. The maturation status of APCs might also affect their ability to present endogenous antigens. Autophagy is a constitutive process in both immature and mature human monocyte-derived DCs33. However, TLR4 and TLR7 stimulation in RAW macrophages increases autophagy34, 35, suggesting that maturation of APCs may enhance MHC-class-II-restricted antigen presentation of endogenous proteins. While autophagy is clearly involved in the MHC class II pathway; further work is needed to define the extent by which autophagy influences the MHC class II peptide repertoire during an immune response to pathogens.
Delivery of MHC class II molecules to the cell surface
The final step in antigen processing and presentation is the transport of vesicles that contain MHC class II molecules and proteins usually found in the immunological synapse from the endolysosomal compartment to the cell surface (Figure 2). The transformation of MHC-class-II-containing compartments into tubular structures that are directed towards the site of T-cell interaction at the plasma membrane has been proposed as a mechanism of transport to the cell surface as determined by direct visualization of primary DCs (Figure 2)54,55. Tubulation of MHC-class-II-expressing endosomal compartments requires loading of DCs with antigen, maturation of DCs in response to a TLR ligand, and T cells specific for the antigen presented by MHC class II molecules. The direction of the MHC-class-II-containing tubules towards the interacting T cell is proposed to promote the clustering of MHC class II molecules at the site of T-cell contact, and might assist in controlling the formation of the immunological synapse54. MHC-class-II-containing endolysosomal tubules use microtubule-based movement with RAB7 and RAB- interacting lysosomal protein (RILP) involved in the engagement of the necessary motor proteins56. The composition of the immunological synapse on the DC side has not been fully identified. Spinophilin, a scaffolding protein of neuronal dendritic spines that regulates synaptic transmission, has been found in DCs and is directed dynamically to contact sites in an antigen-dependent manner57. Other proteins involved in the polarized movement of these compartments to the cell surface remain to be identified.
At the plasma membrane, MHC class II molecules cluster in lipid rafts or tetraspanin-rich microdomains58. Although incorporation of MHC class II molecules into these microdomains is believed to be functionally important, it remains to be defined how exactly microdomains participate in effective MHC-class-II-mediated antigen presentation. In addition, it is unclear what controls the incorporation of MHC class II molecules into microdomains. The MHC class II β-chain cytoplasmic tail is ubiquitylated in mouse immature DCs59,which is essential for recycling MHC class II molecules from the cell surface in immature DCs. The number of ubiquitylated MHC class II molecules in DCs decreases following maturation, resulting in the accumulation of MHC class II molecules at the cell surface. However, no motif in the MHC class II molecule has been described to date for microdomain sorting and the functional importance of this ubiquitylation reaction remains to be established. Analysis of mice in which the endogenous MHC class II β locus is replaced with a mutated version that cannot be ubiquitinylated should prove informative.
The translocation of MHC class II molecules from tubular compartments to specialized membrane subdomains (that is, lipid rafts, tetraspanin-rich microdomains and the immunological synapse) following DC maturation is suggestive of a highly controlled and polarized transport mechanism. Given its role in polarized exocytosis, the exocyst complex is an attractive candidate to mediate MHC-class-II-mediated transport to the plasma membrane. The 743 kD exocyst complex is composed of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo8460 and is involved in the docking of secretory vesicles to specific sites at the plasma membrane, before soluble-N-ethylmaleimide-sensitive-factor accessory-protein receptor (SNARE)-mediated fusion takes place61. In addition, proteins of the exocyst complex have been found on phagosomes from the Drosophila melanogaster S2 cell line38. This expression of the exocyst complex in DCs might also allow for the regulated fusion of this compartment with the plasma membrane following the completion of the peptide–MHC class II loading reactions, though direct evidence of this role remains to be established.
MHC class I processing and presentation
Antigenic peptides derived from cytosolic proteins intersect the MHC class I biosynthetic pathway in the ER where the MHC class I heavy chains and β2-microglobulin are synthesized. Proteins destined for degradation undergo ubiquitylation and are then processed by the proteasome. In some viral infections, interferon-γ production leads to the recruitment of distinct proteins to the proteasome to permit increased production of octamers to decamer peptides, suitable for binding to MHC class I molecules. These peptides are transported from the cytoplasm into the ER via the transporter associated with antigen processing (TAP) molecular complex where they associate with nascently produced MHC class I molecules and β2-microglobulin. The trimeric complex of MHC class I heavy chain, β2-microglobulin and peptide allow for optimal folding, glycosylation and delivery to the cell surface.
Cross-presentation
Important intracellular changes in non-immune cells, which can be induced by events such as viral infection or malignant transformation, must be reported to the immune system to ensure the induction of a CD8+ T-cell response. DCs possess the unique capacity to stimulate naive T cells and can take up and degrade infected non-immune cells or cell-derived fragments and subsequently delivery the peptide fragments to the MHC class I pathway for display on the cell surface to CD8+ T cells62. This property is atypical, because most cells exclusively present peptides derived from endogenous proteins on MHC class I molecules. This process of presenting exogenous peptides on MHC class I molecules is known as cross-presentation or cross priming, was first described by Bevan in 197663, 64 and requires that the requisite peptide precursors gains access to the cytosol for processing by the proteasome, followed by active transport of peptides into the ER where newly assembled MHC class I molecules are found. DCs appear to be uniquely equipped for cross-presentation65. Nonetheless, the routes by which exogenous antigens access newly formed MHC class I molecules remain unclear.
A tool that has been used to examine the delivery of exogenous antigens to the cytosol is ICP47 (infected cell protein 47), which is produced by human herpes simplex virus 1 and is a potent inhibitor of TAP66,67. ICP47 can be reduced to a 35-residue peptide without loss of its inhibitory potency67. This ICP47 peptide can freely access the DC cytosol after phagocytic uptake where it can interfere with the cytoplasmic face of the human TAP complex and thus inhibit peptide translocation into the ER. Exposure of the human DC-like cell line KG-1 to the ICP47 fragment blocked TAP-dependent maturation of the mouse MHC class I molecule H-2Kb introduced into these cells, indicating that extracellular proteins can access the cytosol in DCs, though the subcellular route remains unclear68.
The lipid bilayer is not a passive barrier that separates one compartment from another but actively participates in the translocation of substrates. The transport of peptide antigens across the lipid bilayers can principally occur either in a protein-dependent or -independent manner. During protein-dependent transport across lipid bilayers, there is a precedent for the translocation of proteins from the ER into the cytoplasm for the purposes of degradation. The ER dislocation machinery serves as a quality control complex that is responsible for removing misfolded proteins for degradation. Alternatively, peptides may be able to pass across membranes without the need for dedicated protein channels (see later). We explore the possible role of protein-dependent and protein-independent transport of exogenous proteins across lipid bilayers to gain access to MHC class I molecules for cross-presentation.
Is the ER dislocon complex involved in cross-presentation?
How do extracellular peptides escape from the phagosome to the cytosol? Delivery of proteins through a membrane pore to the cytosol may use the same protein complex responsible for transporting misfolded proteins out of the ER. The ER dislocon is a complex, incompletely identified collection of ER resident proteins, the principal function of which appears to be ejection of misfolded proteins from the ER to the cytosol for degradation. Dislocated proteins are deglycosylated, ubiquitinylated, and eventually degraded by the proteasome in a pathway known as the ERAD (endoplasmic reticulum associated degradation) pathway69. Some proteins involved in dislocation are now known (see below and Figure 3), and include several ubiquitin ligases, members of the derlin family, which are the human homologues of the Saccharomyces cerevisiae protein Der1 (degradation in the ER 1) and the AAA (ATPases associated with various cellular activities) ATPase p97.
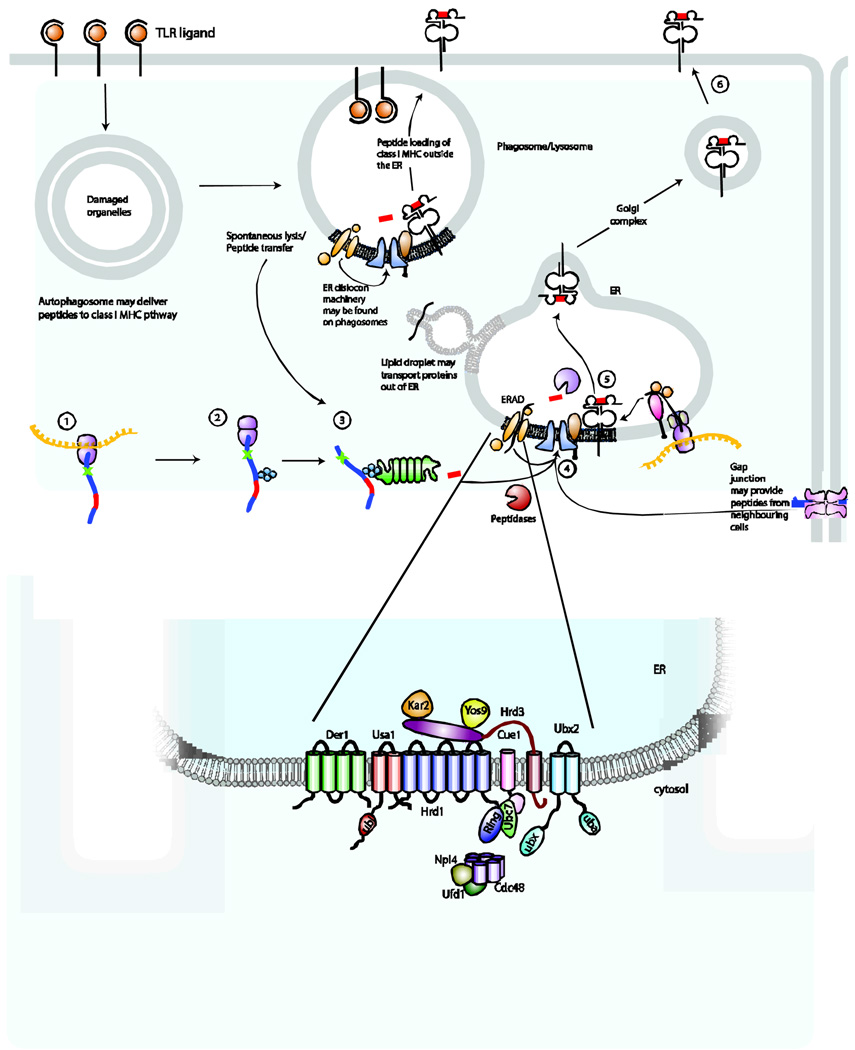
Figure 3. Additional pathways that may be relevant for antigen processing and presentation by MHC class I molecules in TLR-stimulated DCs.
How peptides traffic of peptides to MHC class I molecules during cross-presentation in DCs is unknown. We propose several possibilities that could permit peptides to access MHC class I molecules. Autophagosomes containing both endogenous peptides and pathogen-derived proteins could potentially serve as a source of peptides for the MHC class I pathway. Peptides may transit from the phagosome through leaky membranes, following spontaneous lysis of the phagosomal compartment or by traversing the lipid bilayer. Components of the peptide-loading complex and the endoplasmic reticulum (ER) dislocation machinery may be shuttled from the ER to the phagosome, perhaps involving lipid droplets for transport. Transfer of these components to the phagosome may permit loading of MHC class I molecules with peptides at this site rather than in the ER. Gap junctions may serve as conduits to allow neighbouring cells to donate peptide epitopes for loading of MHC class I molecules. The yeast ERAD protein complex is illustrated in greater detail on the panel below.
Some data exists to suggest that ER-derived proteins may reside on the phagosomal membrane. Proteomic analysis of the phagosome revealed the presence of several ER-derived proteins, such as the chaperones calnexin, calreticulin, GRP78 (also known as BIP) and Sec6139 70. In addition, Gagnon et al. claimed that the ER (or a subcomponent thereof) fused with nascent phagosomes, referred to as the ER–phagosome fusion model71, and it was proposed that the presence of ER-derived components in phagosomes would allow the ER dislocon to participate in cross-presentation at the phagosomal membrane by allowing the escape of proteins from the phagosomes into the cytosol72–74. While not definitive, these data suggest that the ERAD machinery may be involved in translocating peptides from the phagosome to the cytosol.
However, the ER–phagosome fusion model70,71 is controversial because potential ER contamination remains a confounding problem, despite rigorous efforts to purify phagosomes75. In addition, biochemical experiments that fail to control for post-lysis associations with ER proteins have limited use in understanding the nature of protein–protein interactions. Nonetheless, we cannot consider the question of whether the ER fuses with phagosomes settled without an exact replication of the original report71. Cell-type differences are a factor worthy of consideration as well.
The ERAD protein Sec61 (also referred to as the translocon) is a protein channel responsible for the transport of newly synthesized proteins into the ER and also has been proposed to dislocate proteins from the ER to the cytoplasm with the help of other ERAD proteins76. In addition, Sec61 has been shown to localize to bead-containing phagosomes in macrophages71 and therefore, Sec61 might be involved in the dislocation of proteins from the phagosome into the cytosol that in turn access the cross-presentation pathway. It has been proposed that the Pseudomonas aeruginosa bacteria exotoxin A inhibits the function of the Sec61 channel and has been used to block Sec61-dependent protein transport across the ER and phagosome membranes68,77. Incubation of cells with ovalbumin in the presence of exotoxin A failed to induce interleukin-2 (IL-2) production by a peptide-specific CD8+ T-cell hybridoma, suggesting a role for Sec61 in cross-presentation68. However, evidence that exotoxin A binds to Sec61 is quite indirect and rests on an immunoprecipitation–immunoblotting protocol that does not exclude exotoxin A targets other than Sec6177, including the potent inhibitory effect of exotoxin A on protein synthesis. In fact, there is no evidence for a direct role for Sec61 in the escape of any bacterial toxins from the ER. More recent work suggests that cholera toxin uses ER luminal proteins that participate in quality control, as well as a derlin-1-dependent step for escape from the ER78.
The complete molecular composition of the ER dislocon remains to be resolved but several components of the ER dislocation machinery, in addition to Sec61 and ATPase p97, might have a role in the cross-presentation of exogenous antigen. For example, mammalian cells possess several ubiquitin E3 ligases that reside in the ER lumen, such as gp78 (glycoprotein 78), HRD1 (HMG-CoA reductase degradation 1), MARCH VI (membrane-associated ring finger (C3HC4) 6), SCF (SKP1–cullin–F-box), CHIP (C-terminus of HSC70-interacting protein), and Parkin (implicated in Parkinson’s disease), all of which are involved in tagging proteins that are destined for degradation69. Other components of the dislocation machinery include the homologue of yeast Hrd3p, SEL1L79, signal peptide peptidase (SPP)80 and the newly identified Ubc6e, Auf1 and UBxD881, but the role of these components in the context of antigen presentation remain to be investigated.
Proteomic studies of polystyrene-bead-containing phagosomes identified only a small number of dislocation components on the phagosome38–41. However, the nature of the polystyrene bead and the presence or absence of TLR ligands may lead to differential recruitment of the dislocation machinery to the phagosome. An analysis of the composition and distribution of components of the dislocation machinery in professional APCs is in its infancy, and no systematic attempt has been made to link most of these components to cross-presentation.
Even in the absence of ER–phagosome fusion, ER components might participate in the dislocation of proteins from the phagosome. The ER and the endocytic pathway might be connected by a specific transport pathway that shuttles cargo between them and in this way components of the ER could redistribute to phagolysosomal compartments following TLR stimulation. In primary immature DCs, TLR3, TLR7, TLR9 and TLR13 move from the ER to endolysosomes82 under the control of UNC93B1 in response to stimulation with TLR agonists83. This phenomenon, if extendable to other ER proteins, could allow for the activation-dependent trafficking of the components of the ER dislocon complex to an endosomal or phagosomal compartment. The evidence that the ER dislocon complex contributes to cross-presentation is limited and further experiments are needed to address whether the ER dislocon complex is truly involved and if so, how it arrives at the phagosome.
How do ER resident proteins arrive on the phagosome? One possible mode of transport of proteins from the ER to the phagosomes may involve lipid droplets. We have proposed a model whereby lipid droplets may form on the surface of organelles to deliver lipids and other protein cargo to different destinations within the cell, consistent with the idea that lipid bilayers are not passive participants in antigen processing and presentation84. In this manner, the lipid droplets could serve as an escape hatch for both proteins and viruses, such as hepatitis C virus85. The model does not exclude other protein-based pathways for antigen translocation and almost certainly requires proteins for its formation. Whether the model is relevant for antigen processing and presentation remains to be determined. The presence of ubiquitylated apolipoprotein B (ApoB), a protein that is usually secreted from the cell, on lipid droplets targets the protein for proteasomal degradation and provides at least one documented example that would support such a role of substrate transport86.
Alternatives to membrane channels for cross-presentation
In addition to ER-derived protein channels, other hypotheses of how peptides and/or proteins travel from the phagosome to the cytoplasm for cross-presentation exist. The delivery of hydrophilic substrates across membranes typically requires protein channels, but this is not universally true. Here, we present three models by which peptides may traverse the lipid bilayer and gain access to another subcellular compartment without the need for a conventional energy-dependent protein channel.
The first model proposes that proteins might ‘leak’ from the phagosomes into the cytosol. The HIV-1 Tat protein is an example of a polypeptide that can spontaneously traverse a lipid bilayer87. A decamer of the HIV-1 Tat protein (47YGRKKRRQRR57) can translocate itself — and cargo of various sizes appended to it — across the membrane in an energy-independent manner. But how can a highly charged peptide traverse a largely hydrophobic boundary? Models to explain this transport suggests that charged residues of the Tat decamer interact with the phosphate groups on either side of membrane, and that the peptide may form small pores (∼3 nm in diameter) to permit additional peptides to pass through the membrane87. Other endogenous peptides might have similar features, negating the need for a dedicated peptide transporter in the plasma membrane. The penetration of non-enveloped viruses88, or the action of certain microbial peptides89 and proteins90 could also affect host membranes, such that the permeability barrier for proteins is compromised or lost completely. The effects of such pathogen-derived products could also be limited to certain intracellular compartments, such as endosomes or the ER (as reported for listeriolysin91 and papova virus, respectively92,93). Thus, peptide antigens might simply leak out of phagosomes and thereby gain access to the cytoplasm.
A second possibility for peptide escape into the cytosol is the rupture of the phagolysosomal membrane. Peptides might thereby be released from the phagolysosome and directly interact with the cytoplasmic processing machinery. Phagosomes that contain Cryptococcus neoformans lose membrane integrity, which relates in part to the de novo synthesis of the polysaccharide capsule following phagocytosis94. C. neoformans slips out of these compartments and is extruded from the cell, leaving the APC intact and the fungal organism viable95,96. Equally interesting is the proposal that M. tuberculosis may not remain confined to the endocytic structures in which it initially resides. M. tuberculosis can escape from phagolysosomes into the cytosol21. Both the C. Neoformans-containing and the M. tuberculosis-containing compartments, if not perfectly sealed while the microorganisms are in transit, may release other phagosomal content into the cytoplasm.
In addition, peptides may travel out of the phagolysosomal compartment as a result of rupture of this compartment. It should not be presumed that the lysosomal membrane is stable. Indeed, the notion of the lysosome as a ‘suicide bag’ could lead to the assumption that the release of lysosomal compartments into the intracellular milieu leads to cell death97. Because only a few hundred protons are sufficient to maintain the topical lysosomal pH in these modestly sized organelles, the rupture of such a compartment may not be as disastrous as it would seem at first glance98: the appropriate cytoplasmic pH can be rapidly restored. Indeed, osmotic lysis of pinosomes is an effective means of introducing endocytosed soluble materials into the cytoplasm for presentation by MHC class I molecules99 and may mimic normal disruption as it might occur in the phagolysosomal compartment.
Lysosomal membrane stability may vary. Sphingosine affects lysosomal membrane permeability in a dose-dependent manner100. Other host proteins such as apolipoprotein L-1 and SRP6 (serpin 6) can also perturb the lysosomal membrane101. SRP6 regulates proteolytic activity of lysosomal enzymes and hypotonic shock of Caenorhabditis elegans that lack SRP6 results in cell necrosis by release of lysosomal contents101. The extent to which these changes might occur in a cell-type specific manner (for example in DCs) is not known. In any case, such perturbations may permit peptides to gain access to the cytosol and therefore to the MHC–class-I-–peptide-loading machinery.
A third possibility is that MHC class I molecules might be loaded with peptide outside of the ER. The biosynthetic pathway of MHC class I molecules may intersect with the phagosome, permitting MHC class I peptide loading in the endolysosomal compartments themselves. Another pathway may permit internalized antigens to be exported to the cytosol and processed by the proteasome before translocation back into the phagosomes in a TAP-dependent manner73,74. In addition, surface MHC class I molecules internalized during phagocytic uptake of cargo may end up in phagolysosomal compartments (Figure 3).
The idea that MHC class I molecules may traffic to different compartments for peptide loading is not new102. MHC class I molecules may visit phagolysosomal compartments to acquire peptides prior to their surface display103–105. The conserved tyrosine residue in the cytoplasmic tail of the heavy chains of MHC class I molecules is important for the internalization of this complex and may assist in trafficking MHC class I molecules into the phagolysosomal compartment106. MHC class I molecules that lack this tyrosine residue were found to be inferior in their capacity to present antigens to CD8+ T cells during viral infections106. However, presentation of endogenous antigens to CD8+ T cells was unaffected. In this model, it is proposed that internalization of MHC class I molecules is therefore a prerequisite for cross-presentation106.
Alternate sources of cytosolic peptides
As described above, the bulk of peptides for MHC class I molecules are thought to be derived from proteasomal breakdown of cytosolic proteins. However, emerging data suggest that this proteasome-mediated process may not be the exclusive provider of such antigens. Both intercellular protein channels and autophagy may have a role.
Gap junctions are intercellular channels that connect the cytoplasms of two adjacent cells by generating a functional channel, composed of six connexins on each side107, and may facilitate the transport of extracellular peptides to the cytosol. Although the role of these channels in the translocation of peptides into the cytosol for presentation by the MHC class I pathway is intriguing, to date the data that support this mechanism is limited108. Gap junctions may serve to transport peptides from infected cells into neighbouring cells in an effort by the immune system to inflict damage on adjacent cells and so limit the spread of a viral infection109 (Figure 3). This proposed mechanism for the prevention of viral dissemination is therefore crucially dependent on adequate levels of peptide–MHC complexes and antigen-specific CD8+ T cells. A single peptide–MHC complex will suffice to induce the cytotoxic activity of CD8+ T cells and so the criterion may easily be met110. The availability of gene-targeted mice with defects in gap junctions will likely clarify their involvement in antigen presentation.
The role, if any, of autophagy in MHC-class-I-restricted antigen presentation remains to be determined. Autophagy clears ubiquitylated cytoplasmic protein aggregates111. Indeed, in hepatocytes and neurons of mice deficient for ATG5 or ATG7, such cytoplasmic protein aggregates accumulate in the cytoplasm112–114. DC aggresome-like structures (DALIS) contain polyubiquitylated proteins, and peptides derived from DALIS can be presented by MHC class I molecules115. Aggresome-like structures (ALIS) similar to DALIS are observed in many immune and non-immune cells in response to stress, and these ALIS serve as substrates for autophagy116. Thus one might speculate that autophagy-mediated clearance of DALIS may serve as a source for MHC class I-restricted peptides.
H2–M3a (histocompatibility 2, M region locus 3), a member of the non-polymorphic class I-b MHC family, presents N-formylated peptides to CD8+ T cells117. These peptides are present only in mitochondria and in prokaryotes, such as Listeria monocytogenes and M. tuberculosis. Given that autophagy is implicated in the clearance of these organisms117, 118 (as well as mitochondria), H2–M3a might acquire its peptides by an autophagy-dependent process.
Presentation of DALIS, ubiquitylated proteins or N-formylated peptides require the access of autophagosomal degradation products to the MHC-class-I–peptide-loading complex. The autophagosomal membrane has been proposed to originate from the ER119,120. In addition, during ER stress and the unfolded protein response (UPR), autophagy is induced and consumes portions of the ER, serving as an alternative to ER-associated degradation of proteins119,121–123. The MHC-class-I–peptide-loading complex and/or dislocation machinery may therefore be present on the autophagosome. Indeed, Brucella abortus, Legionella pneumophila, and Porphyromonas gingivalis can be found in autophagosomes containing ER proteins, including Sec61124. Alternatively, peptides might ‘escape’ from autophagosomes, and be transported into the ER in a TAP-dependent manner.
Although a role for autophagy in MHC class I presentation seems plausible, treatment with Wortmannin or 3-methyladenine (3-MA), which inhibit autophagy by inhibiting PI3K activity, as well as knockdown of ATG12 failed to affect MHC-class-I-restricted presentation of an endogenous antigen by Epstein–Barr virus (EBV)-transformed B cells44,47. In addition, colocalization between MHC class I molecules and green fluorescent protein-labelled –LC3 was not observed in epithelial cells33. Although B cells and epithelial cells express MHC class I molecules, their behaviour and regulation might differ from DCs. In both immature and mature DCs, the MHC-class-I-restricted presentation of a peptide derived from influenza matrix protein 1 (MP1) fused to LC3 to direct the peptide–MHC class I complex to autophagosomes remains unaltered when compared to unfused cytosolic MP133. However, the cytosolic MP1 is presented very efficiently and almost saturates the MHC class I pathway. These features may mask any contribution of the autophagosomal MP1–LC3 complex to cross-presentation.
Several invasive bacteria and parasites that can be cleared through autophagy induce a CD8+ T-cell response. Toxoplasma gondii is cleared after invasion of macrophages through autophagy in a process that requires either IFNγ-inducible p47 GTPases and disruption of the parasitophorous vacuole that encloses the parasite, or CD40 ligation125–127. Protective immunity from this organism requires the induction of a CD8+ T-cell response, and therefore cross-presentation is essential128. But how do peptides derived from T. gondii gain access to the MHC class I peptide loading complex? Perhaps, the fusion of ER membrane with the autophagosome in T. gondii-infected cells might bring the MHC class I loading complex into close contact with peptides derived from the parasitophorous vacuoles. However, the net contribution of autophagy to the generation of most of the peptide–MHC class I complexes found on the surface of an infected cell remains to be determined.
Concluding remarks
The rules that govern antigen processing and processing in DCs are complex. Numerous important cellular changes take place following exposure of DCs to TLR ligands. Although the pathways of cross-presentation, autophagy and ER dislocation have been established as important for antigen processing and presentation, many questions remain unanswered. Future work that elucidates the molecular details of these pathways in DCs will need to be put into context with relates to TLR stimulation. The established importance of TLR signals for antigen processing and presentation requires us to focus on the interaction of whole microorganisms and their products with DCs to establish relevant insights on the pathways used by antigens to gain access to MHC molecules. It is likely that the observations summarized in this Review are just the tip of the iceberg.
Online summary.
Antigen processing and presentation is the mechanism by which whole antigens are degraded and loaded onto MHC molecules for display on the cell surface for T cells.
Both macrophages and dendritic cells are considered professional antigen presenting cells, though DCs possess the unique capacity to activate naïve T cells.
DCs phagocytose antigens and whole microorganisms and places them into membrane-delimited compartments termed phagosomes. These structures are modified over time and ultimately fuse with lysosomes.
Cross presentation permits DCs to take up antigens from the extracellular environment and present onto MHC class I molecules for CD8+ T cell activation. While the exact trafficking patterns of antigens are not known, many hypotheses have been generated including the recruitment of the ER dislocation machinery and protein-independent passing of antigens into the cytosol.
The autophagy machinery appears to play an important role in the development of peptide antigens for MHC molecules. More evidence for MHC class II peptides exists than for MHC class I molecules.
Acknowledgements
We thank all members of the Ploegh laboratory for their thoughtful discussions and Katerina Artavanis-Tsakonas, Eva Frickel and Annette Pollington for their critical review of this manuscript. We thank Britta Mueller for permission to use her figure on the ER dislocation machinery and Tom DiCesare for assistance with artwork.
JMV is funded by a NIH K08 AI57999 and MGH DOM start-up funds; AGV is funded by the Boehringer Ingelheim Fonds; HLP is funded by R01 GM062502, RO1 AI34893, R01 AI057182 and R37 AI33456.
Terms
- Phagolysosome
An intracellular compartment that results from the fusion of phagosomes, which enclose extracellular material that has been ingested, and lysosomes, which contain lytic enzymes.
- pathogen-associated molecular pattern
A molecular pattern that is found in pathogens but not mammalian cells. Examples include terminally mannosylated and polymannosylated compounds, which bind the mannose receptor, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA, which bind Toll-like receptors.
- endolysosomal tubules
Highly dynamic subcellular structures that eminate from late endocytic/lysosomal and/or phagolysosomal compartments. They contain, at least, MHC class II molecules, CD63, CD82 and LAMP1, and require microtubules for movement.
- Endocytic pathway
A trafficking pathway used by all cells for the internalization of molecules from the plasma membrane to lysosomes.
- Endosome
A membrane delimited compartment containing material ingested by endocytosis. Some material will be recycled to the cell surface while some cargo transit to late endosomes and eventually fuse with lysosomes (endolysosomes). Endosomes may also fuse with phagosomes to permit maturation of the phagosomal compartment.
- Endolysosome/endolysosomal compartment
Endosomes that have fused with lysosomes. This acidic environment permits degradation of antigens.
- Cross-presentation
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens onto MHC class I molecules. Cross-presentation is important for the initiation of immune responses to viruses that do not infect antigen-presenting cells.
- Autophagy
An evolutionarily conserved process in which acidic double-membrane vacuoles known as autophagosomes sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes. This process does not involve direct transport through the endocytic or vacuolar protein sorting pathways.
- Phagosome
Phagosomes are membrane-delimited compartments that confine ingested material such as microorganisms following phagocytosis. Unless counteracted by a pathogen survival strategy, the phagosome matures into a hostile environment by fusion with lysosomes that is designed to kill and digest microorganisms.
- Tetraspanins
A family of transmembrane proteins that have four transmembrane domains and two extracellular domains of different sizes, which are defined by several conserved amino acids in the transmembrane domains. Their function is not known clearly, but they seem to interact with many other transmembrane proteins and to form large multimeric protein networks.
- Immunological synapse
A region that can form between two cells of the immune system in close contact. The immunological synapse refers to the interaction between a T cell or natural killer cell and an antigen-presenting cell. This interface involves adhesion molecules, as well as antigen receptors and cytokine receptors.
- RAB proteins
Cytosolic proteins that have GTPase activity, which, in their GTP-bound form, associate with membranes. Different RAB proteins associate with different intracellular compartments — for example, RAB5 associates with early endosomes, RAB7 with late endosomes, and RAB11 with recycling endosomes.
- Chaperone-mediated autophagy
The import and degradation of soluble cytosolic proteins by chaperone-dependent, direct translocation across the lysosomal membrane.
- Microautophagy
The uptake and degradation of cytoplasm by invagination of the lysosomal membrane.
- Macroautophagy
(Also known as autophagy). The largely non-specific autophagic sequestration of cytoplasm into a double- or multiple-membrane-delimited compartment (an autophagosome) of non-lysosomal origin. Note that certain proteins, organelles and pathogens may be selectively degraded via macroautophagy.
- Lysosomal degradation
The digestion of macromolecules in lysosomal organelles, which are the terminal organelles of degradative pathways, such as phagosomal or endosomal and autophagy pathways.
- Lipid rafts
Lipid rafts are microdomains of the cell membrane that are enriched in sphingolipids. Several membrane-associated signalling molecules are concentrated in these rafts.
- central tolerance
Deletion of self-reactive T cells in the thymus.
- Exocytosis
The release of material contained within vesicles by fusion of the vesicles with the plasma membrane.
- SNARE proteins
(Soluble-N-ethylmaleimide-sensitive-factor accessory-protein receptor proteins). A class of proteins that is required for membrane fusion events that occur in the course of vesicle trafficking and secretion.
- peripheral tolerance
Control of self-reactive T cells in the periphery.
- transporter associated with antigen processing
(TAP). TAP1 and TAP2 form a heterodimer in the membrane of the endoplasmic reticulum. The TAP1–TAP2 complex transports peptides from the cytoplasm to the endoplasmic reticulum, where peptides can be loaded onto MHC class I molecules. Without these peptides, MHC class I molecules are unstable and are much less likely to transit to the cell surface or to remain there.
- Aggresome
proteinaceous inclusion body that forms when cellular degradation machinery is impaired or overwhelmed, leading to an accumulation of protein for disposal.
- Unfolded-protein response
A response that increases the ability of the endoplasmic reticulum to fold and translocate proteins, decreases the synthesis of proteins, and causes the arrest of the cell cycle and apoptosis.
- Cathepsins
Proteases that are mostly located in lysosomes and lysosome-like organelles and can be divided into cysteine, aspartate and serine cathepsin subgroups according to their active-site amino acid.
- Sphingosine
An amino alcohol that can be linked to a fatty acid via the amino group to form the basic structure of sphingolipids.
- Necrosis
Forms of cell death that result from a decline in cellular ATP or ADP levels below the concentration that is required to maintain cellular organization and integrity.
Biographies
Jatin M. Vyas, MD PhD is an Instructor in Medicine at the Harvard Medical School and a faculty member of the Division of Infectious Disease, Department of Medicine at the Massachusetts General Hospital. His laboratory studies the immune response to microorganisms including fungal pathogens and the molecular details of phagosome maturation.
Annemarthe G. Van der Veen is a graduate student from the Vrije Universiteit in Amsterdam (The Netherlands) in the laboratory of Hidde L. Ploegh, where she studies different aspects of antigen processing and presentation. She is a Boehringer Ingelheim Fonds fellow.
Hidde L. Ploegh, PhD is a member of the Whitehead Institute for Biomedical Research and Professor of Biology at the Massachusetts Institute of Technology. His laboratory focuses on the molecular mechanisms of antigen processing and presentation, ER quality control and development of chemical probes as reagents to probe the immune system.
Reference
- 1.Gell PGH, Benacerraf B. Studies on Hypersensitivity. II. Delayed Hypersensitivity to Denatured Proteins in Guinea Pigs. Immunology. 1959;2:64–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Call ME, Wucherpfennig KW. THE T CELL RECEPTOR: Critical Role of the Membrane Environment in Receptor Assembly and Function. Annual Review of Immunology. 2005;23:101–125. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- 3.Martin F, Chan AC. B CELL IMMUNOBIOLOGY IN DISEASE: Evolving Concepts from the Clinic. Annual Review of Immunology. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 4.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 5.Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Current Opinion in Immunology. 2004;16:96–102. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. The Dorsoventral Regulatory Gene Cassette spatzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 7.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Seminars in Immunology. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Garrett WS, et al. Developmental Control of Endocytosis in Dendritic Cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 9.West MA, et al. Enhanced Dendritic Cell Antigen Capture via Toll-Like Receptor-Induced Actin Remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. Upon TLR stimulation, DCs initially increase macropinocytosis and directs dramatic actin remodeling. [DOI] [PubMed] [Google Scholar]
- 10.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of Lysosomal Function During Dendritic Cell Maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 11.Kleijmeer M, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol. 2001;155:53–64. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boes M, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 13.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 14.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunological Reviews. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 15.Henry RM, Hoppe AD, Joshi N, Swanson JA. The uniformity of phagosome maturation in macrophages. J. Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 17.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas K, Love JC, Ploegh HL, Vyas JM. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci U S A. 2006;103:15945–15950. doi: 10.1073/pnas.0607528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. Antigen complexed with TLR ligands are more efficiently presented to T cells. [DOI] [PubMed] [Google Scholar]
- 20.Meresse S, et al. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 21.van der Wel N, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. Mycobacterial gene products CFP-10 and ESAT-6 are required for M. tuberculosis and M. leprae to escape from phagolysosomes in human DCs and macrophages. [DOI] [PubMed] [Google Scholar]
- 22.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 23.Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 24.Yap GS, Shaw MH, Ling Y, Sher A. Genetic analysis of host resistance to intracellular pathogens: lessons from studies of Toxoplasma gondii infection. Microbes and Infection. 2006;8:1174–1178. doi: 10.1016/j.micinf.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 26.Geijtenbeek TB, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 27.Jancic C, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. Limiting acidification in phagolysosomes in DCs permit antigen cross presentation as shown by Rab27a-deficient mice which demonstrate increased phagosome acidification and antigen degradation and a defect in antigen cross-presentation. [DOI] [PubMed] [Google Scholar]
- 28.Asagiri M, et al. Cathepsin K-Dependent Toll-Like Receptor 9 Signaling Revealed in Experimental Arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. Cathespin K is required for TLR9-dependent signaling in DCs. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 30.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. In DCs, autophagosomes formed by macroautophagy fuse with MHC class II-containing compartments. In this model, influenza-derived peptides are presented on MHC class II molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6:1837–1849. doi: 10.4161/cc.6.15.4511. [DOI] [PubMed] [Google Scholar]
- 32.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. TLR4 ligands induce autophagy in macrophages, but do not serve as a signal for the cell to undergo apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. Embo J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 37.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 38.Stuart LM, et al. A systems biology analysis of the Drosophila phagosome. Nature. 2007;445:95–101. doi: 10.1038/nature05380. The most comprehensive list of proteins associated with the phagosome to date. Interesting relationships of proteins are shown in the systems analysis. [DOI] [PubMed] [Google Scholar]
- 39.Garin J, et al. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jutras I, et al. Modulation of the phagosome proteome by interferon-gamma. Mol Cell Proteomics. 2007 doi: 10.1074/mcp.M700267-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Burlak C, Whitney AR, Mead DJ, Hackstadt T, Deleo FR. Maturation of human neutrophil phagosomes includes incorporation of molecular chaperones and endoplasmic reticulum quality control machinery. Mol Cell Proteomics. 2006;5:620–634. doi: 10.1074/mcp.M500336-MCP200. [DOI] [PubMed] [Google Scholar]
- 42.Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 44.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. A gene product of EBV, EBNA1 is delivered by autophagy to the lysosome where it intersects the MHC class II pathway. [DOI] [PubMed] [Google Scholar]
- 45.Brazil MI, Weiss S, Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur J Immunol. 1997;27:1506–1514. doi: 10.1002/eji.1830270629. [DOI] [PubMed] [Google Scholar]
- 46.Dorfel D, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 48.Chicz RM, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dongre AR, et al. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur J Immunol. 2001;31:1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Muntasell A, et al. HLA-DR4 molecules in neuroendocrine epithelial cells associate to a heterogeneous repertoire of cytoplasmic and surface self peptides. J Immunol. 2002;169:5052–5060. doi: 10.4049/jimmunol.169.9.5052. [DOI] [PubMed] [Google Scholar]
- 51.Dengjel J, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou D, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Zwickey HL, Unternaehrer JJ, Mellman I. Presentation of self-antigens on MHC class II molecules during dendritic cell maturation. Int Immunol. 2006;18:199–209. doi: 10.1093/intimm/dxh363. [DOI] [PubMed] [Google Scholar]
- 54.Boes M, et al. T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner. J Immunol. 2003;171:4081–4088. doi: 10.4049/jimmunol.171.8.4081. [DOI] [PubMed] [Google Scholar]
- 55.Vyas JM, et al. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordens I, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Current Biology. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 57.Bloom O, et al. Spinophilin participates in information transfer at immunological synapses. J. Cell Biol. 2008;181:203–211. doi: 10.1083/jcb.200711149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiltbold EM, Poloso NJ, Roche PA. MHC Class II-Peptide Complexes and APC Lipid Rafts Accumulate at the Immunological Synapse. J Immunol. 2003;170:1329–1338. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 59.Shin J-S, et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. Regulation of MHC class II cell surface expression in DCs is achieved in part by control of MHC II beta chain cytoplasmic tail ubiquitination. Upon DC maturation, the rate of this post-translational modification decreased, resulting in the accumulation of MHC II at the cell surface. [DOI] [PubMed] [Google Scholar]
- 60.Hsu S-C, TerBush D, Abraham M, Guo W. The Exocyst Complex in Polarized Exocytosis. International Review of Cytology. 2004;Volume 233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 61.Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 63.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bevan MJ. Minor H Antigens Introduced on H-2 Different Stimulating Cells Cross-React at the Cytotoxic T Cell Level during in Vivo Priming. J Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 65.Guermonprez P, Amigorena S. Pathways for antigen cross presentation. Springer Semin Immunopathol. 2005;26:257–271. doi: 10.1007/s00281-004-0176-0. [DOI] [PubMed] [Google Scholar]
- 66.Hill A, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 67.Galocha B, et al. The Active Site of ICP47, a Herpes Simplex Virus-encoded Inhibitor of the Major Histocompatibility Complex (MHC)-encoded Peptide Transporter Associated with Antigen Processing (TAP), Maps to the NH2-terminal 35 Residues. J. Exp. Med. 1997;185:1565–1572. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. The ER dislocation machinery participates in cross presentation in DCs. Using an cell-free model, p97, an protein involved in ER dislocation, was the only cytosolic cofactor required for protein export from isolated phagosomes. [DOI] [PubMed] [Google Scholar]
- 69.Loureiro J, Ploegh HL. Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions. Adv Immunol. 2006;92:225–305. doi: 10.1016/S0065-2776(06)92006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol. 2003;3:280–291. doi: 10.1038/nri1053. [DOI] [PubMed] [Google Scholar]
- 71.Gagnon E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. Using electron microscopy, ER components are found on the surface of phagosomes, suggesting that the ER is directly involved in phagosome formation and maturation. [DOI] [PubMed] [Google Scholar]
- 72.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. Cross-presentation occurs in a specialized compartment composed of a mix of ER and phagosome components. Here, peptides are transported via TAP and access MHC class I molecules. [DOI] [PubMed] [Google Scholar]
- 74.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. The phagosome is endowed with the ability to load MHC class I molecules with peptides to engage CD8+ T cells. [DOI] [PubMed] [Google Scholar]
- 75.Touret N, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. Using biochemical, fluorescence imaging, and electron microscopy techniques, the plasma membrane donated most of the membrane of newly-formed phagosomes and contrasted sharply the conclusions of Gagnon et al. [DOI] [PubMed] [Google Scholar]
- 76.Wiertz EJ, et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 77.Koopmann JO, et al. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13:117–127. doi: 10.1016/s1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 78.Bernardi KM, Forster ML, Lencer WI, Tsai B. Derlin-1 facilitates the retro-translocation of cholera toxin. Mol Biol Cell. 2008;19:877–884. doi: 10.1091/mbc.E07-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mueller B, Lilley BN, Ploegh HL. SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER. J Cell Biol. 2006;175:261–270. doi: 10.1083/jcb.200605196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loureiro J, et al. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature. 2006;441:894–897. doi: 10.1038/nature04830. [DOI] [PubMed] [Google Scholar]
- 81.Mueller B, Klemm EJ, Spooner E, Claessen JH, Ploegh H. An extended mammalian membrane protein complex that mediates glycoprotein dislocation. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0805371105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 83.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 84.Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 85.Barba G, et al. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proceedings of the National Academy of Sciences. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic Lipid Droplets Are Sites of Convergence of Proteasomal and Autophagic Degradation of Apolipoprotein B. Mol. Biol. Cell. 2006;17:2674–2683. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herce HD, Garcia AE. Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes. Proc Natl Acad Sci U S A. 2007;104:20805–20810. doi: 10.1073/pnas.0706574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith AE, Helenius A. How Viruses Enter Animal Cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 89.Peisajovich SG, Epand RF, Epand RM, Shai Y. Sendai virus N-terminal fusion peptide consists of two similar repeats, both of which contribute to membrane fusion. European Journal of Biochemistry. 2002;269:4342–4350. doi: 10.1046/j.1432-1033.2002.03132.x. [DOI] [PubMed] [Google Scholar]
- 90.Rosenbluh J, et al. Translocation of Histone Proteins Across Lipid Bilayers and Mycoplasma Membranes. Journal of Molecular Biology. 2005;345:387–400. doi: 10.1016/j.jmb.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 91.Darji A, Chakraborty T, Wehland J, Weiss S. TAP-dependent major histocompatibility complex class I presentation of soluble proteins using listeriolysin. Eur J Immunol. 1997;27:1353–1359. doi: 10.1002/eji.1830270609. [DOI] [PubMed] [Google Scholar]
- 92.Lilley BN, Gilbert JM, Ploegh HL, Benjamin TL. Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J Virol. 2006;80:8739–8744. doi: 10.1128/JVI.00791-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daniels R, Rusan NM, Wadsworth P, Hebert DN. SV40 VP2 and VP3 Insertion into ER Membranes Is Controlled by the Capsid Protein VP1: Implications for DNA Translocation out of the ER. Molecular Cell. 2006;24:955–966. doi: 10.1016/j.molcel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proceedings of the National Academy of Sciences. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alvarez M, Casadevall A. Phagosome Extrusion and Host-Cell Survival after Cryptococcus neoformans Phagocytosis by Macrophages. Current Biology. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 96.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of Live Pathogenic Yeast by Macrophages. Current Biology. 2006;16:2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 97.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 98.Van Dyke RW. Acidification of rat liver lysosomes: quantitation and comparison with endosomes. Am J Physiol. 1993;265:C901–C917. doi: 10.1152/ajpcell.1993.265.4.C901. [DOI] [PubMed] [Google Scholar]
- 99.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ. Tumor necrosis factor-alpha -associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol. 2002;283:G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- 101.Luke CJ, et al. An Intracellular Serpin Regulates Necrosis by Inhibiting the Induction and Sequelae of Lysosomal Injury. Cell. 2007;130:1108–1119. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pfeifer JD, et al. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 103.Bachmann MF, et al. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739–1743. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 104.Liu T, et al. TAP peptide transporter-independent presentation of heat-killed Sendai virus antigen on MHC class I molecules by splenic antigen-presenting cells. J Immunol. 1997;159:5364–5371. [PubMed] [Google Scholar]
- 105.Schirmbeck R, Bohm W, Reimann J. Stress protein (hsp73)-mediated, TAP-independent processing of endogenous, truncated SV40 large T antigen for Db-restricted peptide presentation. Eur J Immunol. 1997;27:2016–2023. doi: 10.1002/eji.1830270828. [DOI] [PubMed] [Google Scholar]
- 106.Lizee G, et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 2003;4:1065–1073. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 107.Yeager M, Harris AL. Gap junction channel structure in the early 21st century: facts and fantasies. Current Opinion in Cell Biology. 2007;19:521–528. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neijssen J, Pang B, Neefjes J. Gap junction-mediated intercellular communication in the immune system. Progress in Biophysics and Molecular Biology. 2007;94:207–218. doi: 10.1016/j.pbiomolbio.2007.03.008. Gap junctions supply peptides of a relative molecular mass of up to approximately 1,800 daltons to neighboring cells. [DOI] [PubMed] [Google Scholar]
- 109.Neijssen J, et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 110.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a Single Peptide-MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 111.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Komatsu M, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 114.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 115.Pierre P. Dendritic cells, DRiPs, and DALIS in the control of antigen processing. Immunol Rev. 2005;207:184–190. doi: 10.1111/j.0105-2896.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 116.Szeto J, et al. ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy. 2006;2:189–199. doi: 10.4161/auto.2731. [DOI] [PubMed] [Google Scholar]
- 117.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 118.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. Phagosomes containing M. tuberculosis evade fusion with lysosomes. Autophagy pathways overcome this block and may show a general pathway for pathogen control in APCs. [DOI] [PubMed] [Google Scholar]
- 119.Yorimitsu T, Klionsky DJ. Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol. 2007;17:279–285. doi: 10.1016/j.tcb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 120.Mijaljica D, Prescott M, Devenish RJ. Endoplasmic reticulum and Golgi complex: Contributions to, and turnover by, autophagy. Traffic. 2006;7:1590–1595. doi: 10.1111/j.1600-0854.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 121.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogata M, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Bacterial interactions with the autophagic pathway. Cell Microbiol. 2002;4:1–10. doi: 10.1046/j.1462-5822.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 125.Ling YM, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75:4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luder CG, Seeber F. Toxoplasma gondii and MHC-restricted antigen presentation: on degradation, transport and modulation. Int J Parasitol. 2001;31:1355–1369. doi: 10.1016/s0020-7519(01)00260-0. [DOI] [PubMed] [Google Scholar]
- 129.Nakagawa I, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 130.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 131.Orvedahl A, Levine B. Viral evasion of autophagy. Autophagy. 2008;4:280–285. doi: 10.4161/auto.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ogawa M, Sasakawa C. Bacterial evasion of the autophagic defense system. Curr Opin Microbiol. 2006;9:62–68. doi: 10.1016/j.mib.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 133.Orvedahl A, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. The HSV-1 protein ICP34.5 binds to the mammalian autophagy protein Beclin 1 and inhibits its autophagy function, indicating that microorganisms target key molecules in autophagy to evade immunity. [DOI] [PubMed] [Google Scholar]
- 134.Ogawa M, et al. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 135.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 137.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 138.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 139.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parkes M, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]