1. Introduction
1.1. The ubiquitin-proteasome pathway
Ubiquitin is a highly conserved, 76-amino acid protein that is ubiquitously expressed in eukaryotic cells 1. This small protein controls almost all aspects of a cell's life and death, through its covalent modification of other cellular proteins in a process known as ubiquitination 2,3. The enzymatic cascade of ubiquitination begins with ubiquitin activation by an E1 (ubiquitin-activating enzyme), followed by transfer of the activated ubiquitin to an E2 (ubiquitin-conjugating enzyme, also known as Ubc), and ends with conjugation of ubiquitin to a target protein through the formation of an isopeptide bond between the carboxyl terminus of ubiquitin and an ε-amino group of a lysine residue on the protein substrate. The last step requires a member of a very large family of ubiquitin-protein ligases (E3), which, together with E2s, determine substrate specificity. E3s can be divided into two categories, depending on whether they contain a HECT (homology to E6AP C-terminus) or RING (really interesting new gene) domain. The HECT domain E3s contain an active-site cysteine, which can accept ubiquitin from an E2 and transfer the ubiquitin to a target protein. In contrast, the RING domain E3s do not contain a conventional enzyme active site, but they promote ubiquitination by binding to both protein substrates and E2s, facilitating the conjugation of ubiquitin to specific protein targets. Ubiquitination reactions are reversed by members of a large family of deubiquitination enzymes (DUBs, also known as isopeptidases)4,5. Thus, ubiquitination is a reversible covalent modification, similar to phosphorylation.
Ubiquitin has seven lysines, each of which can be conjugated by another ubiquitin to form a polyubiquitin chain 6. The topology of polyubiquitin chains can influence the fate of target proteins. For example, polyubiquitin chains linked through lysine 48 (K48) of ubiquitin normally target a protein for degradation by the proteasome, whereas K63 polyubiquitin chains have functions independent of proteolysis, including protein kinase activation, DNA repair and membrane trafficking. Monoubiquitination usually does not lead to proteasomal degradation; instead, it regulates important cellular functions such as chromatin remodeling and vesicle trafficking.
1.2. The NF-κB pathway
Both the proteolytic and non-proteolytic functions of ubiquitin are critically important for the regulation of nuclear factor kappa B (NF-κB), a family of transcription factors actively involved in the regulation of immunity, inflammation, and cell survival 7. The NF-κB /Rel family includes RelA (p65), c-Rel, RelB, p50 and p52. They share an N-terminal Rel homology domain (RHD), which mediates dimerization, nuclear translocation, DNA binding and association with the inhibitory proteins IκBs. p50 and p52 are generated from their precursors p105 and p100, respectively, through proteasomal degradation of the C-terminal IκB-like ankyrin repeats.
The NF-κB activation pathways are classified into canonical and non-canonical pathways; the canonical pathway leads to the degradation of IκB, whereas the non-canonical pathway involves the processing of p100 to the mature subunit p52 8. The canonical pathway is activated by most NF-κB stimulatory ligands, including proinflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β), and microbial ligands such as bacterial lipopolysaccharides (LPS) and viral nucleic acids. These ligands bind to their receptors and trigger distinct signaling pathways that converge on a large kinase complex consisting of the catalytic subunits IKKα and IKKβ (IκB kinase α and β), and an essential regulatory subunit NEMO (NF-κB essential modulator, also known as IKKγ or IKKAP). The IKK complex phosphorylates IκBs, and targets these inhibitors for polyubiquitination and subsequent degradation by the proteasome. The liberated NF-κB enters the nucleus to turn on the transcription of target genes. The non-canonical pathway is activated by a subset of receptors in B cells, such as CD40 and B cell activating factor receptor (BAFF-R). These receptors initiate a signaling cascade leading to activation of IKKα, which phosphorylates p100. Phosphorylated p100 is polyubiquitinated and then its C-terminus is selectively degraded by the proteasome, sparing the N-terminal Rel homology domain to generate the mature p52 subunit. p52 forms a dimer with RelB and the dimeric complex enters the nucleus to turn on the expression of genes that are important for B cell maturation and activation.
In both canonical and non-canonical pathways, IKK is key to NF-κB activation. Mounting evidence shows that ubiquitination and deubiquitination play a central role in IKK regulation by diverse NF-κB signaling pathways 9,10. In particular, K63 polyubiquitination mediates the activation of IKK and mitogen-activated protein kinases (MAPKs) through a proteasome-independent mechanism. In this chapter, we will discuss recent progress in understanding the roles of ubiquitin in three steps of the NF-κB pathway: IκB degradation, processing of NF-κB precursors and activation of IKK and other kinases. In addition, we will discuss how deubiquitination enzymes negatively regulate the NF-κB pathway, and how dysfunction of these enzymes may lead to human diseases.
2. Ubiquitin in IκB degradation
IκB is a family of ankyrin repeats-containing proteins, including IκBα, IκBβ, IκBε, IκBζ, IκBNS and Bcl-3 (B-cell lymphoma 3) 7. IκBα, IκBβ and IκBε bind to and sequester NF-κB in the cytoplasm, whereas IκBζ, IκBNS and Bcl-3 are localized in the nucleus and cooperate with NF-κB to activate transcription. The cytoplasmic IκB proteins are rapidly phosphorylated, ubiquitinated and degraded upon stimulation of cells with ligands such as TNFα or IL-1β. The E2 enzyme involved in ubiquitination of IκBα belongs to the Ubc4/Ubc5 family, and the E3 in this process is a complex consisting of Skp1, Cul1, Roc1 (also called Rbx1) and the F-box protein Slimb/βTrCP (SCF-βTrCP) 11. Slimb was first identified in a genetic screen as a negative regulator of the Hedgehog (Hh) and Wnt/Wingless (Wg) pathways in Drosophila 12. Subsequent experiments showed that βTrCP, the mammalian homologue of Slimb, is responsible for the ubiquitination of IκBα, IκBβ, p105, p100 and several other proteins involved in cellular processes ranging from circadian rhythm to cell cycle progression 13. In all of these cases, βTrCP binds to phosphorylated, but not unphosphorylated, forms of substrates through its C-terminal WD40 repeats, which recognize a degron motif with a consensus sequence of DpSGXXpS, where pS represents phosphorylated serine. βTrCP also contains an N-terminal F-box, which binds to Skp1. Skp1 forms a complex with Cul1 and the RING domain protein Roc1, which binds to an E2, such as Ubc5. Therefore, the SCF-βTrCP complex brings phosphorylated IκBα and Ubc5 together and stimulates the activity of Ubc5 to catalyze polyubiquitination of IκBα at two N-terminal lysines (K21 and K22). Ubiquitinated IκBα remains bound to NF-κB but is selectively degraded by the 26S proteasome 14. The mechanism by which the proteasome selectively degrades ubiquitinated IκBα but not NF-κB is still not well understood. Two mammalian βTrCP genes, βTrCP1 and βTrCP2, have been identified. Although IκBα degradation still occurs in βTrCP1-deficient cells stimulated with TNFα, IL-1β or LPS, silencing of both βTrCP1 and βTrCP2 by RNAi in HeLa cells efficiently stabilize IκBα upon stimulation 15, suggesting that βTrCP1 and βTrCP2 function redundantly in IκB degradation.
3. Ubiquitin in the processing of NF-κB precursors
The p50 and p52 subunits of NF-κB are generated from their larger precursors, p105 and p100, respectively, through limited processing by the proteasome 8,16. Both p50 and p105 are present in unstimulated cells, indicating that p105 is processed constitutively. However, the processing of p105 to p50 can be enhanced by stimulation of cells with phorbol ester 17. Although it is generally agreed that the proteasome is important for processing of p105 to p50, whether or not ubiquitination plays a role has been a subject of debate 18. According to one model, p50 is generated by co-translational processing of p105 through a proteasome-dependent but ubiquitination-independent mechanism 19. However, several other groups have shown that p105 is processed to p50 post-translationally, and that this processing depends on polyubiquitination, both under basal and stimulated conditions 16,20,21. Stimulation of cells with LPS or TNFα also leads to complete degradation of p105 in a manner that depends on IKK-mediated phosphorylation and βTrCP-mediated polyubiquitination 22,23. Interestingly, degradation of p105 liberates the MAP kinase kinase kinase (MAP3K) Tpl2/Cot (Tumor progression locus 2/Cancer Osaka thyroid), resulting in the activation of ERK (Extracellular signal regulated kinase), which is important for TNFα production in response to LPS stimulation 24. A recent study showed that phosphorylation and degradation of p105 are also important for T cell receptor signaling 25.
The processing of p100 to p52 is tightly regulated by the non-canonical pathway of NF-κB activation. p100 processing requires the NF-κB inducing kinase (NIK), which is normally degraded by the proteasome such that its level is maintained at a very low level in unstimulated B cells 26. NIK degradation requires the cellular inhibitors of apoptosis, cIAP1 and cIAP2 (cellular inhibitor of apoptosis 1 and 2), which are RING domain E3s that catalyze NIK ubiquitination 27. Stimulation of B cells through certain receptors of the TNFR superfamily, including CD40, BAFF-R and lymphotoxin-β receptor, leads to the degradation of TRAF3 (TNF receptor-associated factor 3), a key negative regulator of the non-canonical NF-κB pathway 28-30. In the absence of TRAF3, cIAPs fail to ubiquitinate NIK, resulting in its accumulation. NIK then phosphorylates and activates IKKα, which in turn phosphorylates p100, leading to its polyubiquitination by SCF-βTrCP 31. Ubiquitinated p100 is then processed by the proteasome to generate p52.
How does the proteasome selectively degrade the C-termini of p100 and p105 while leaving the N-terminal Rel homology domain intact? Jentsch and colleagues propose a model that sheds light on this intriguing question 9,32,33. According to this model, the proteasome is recruited to substrates through binding to polyubiquitin chains. This allows an unstructured region near the ubiquitination site to insert into the proteasome as a hairpin-like loop. Inside the catalytic chamber of the proteasome, the polypeptide of the hairpin loop is degraded in both N- and C-terminal directions. In the case of p100 and p105, degradation towards the C-terminus proceeds to completion, whereas degradation towards the N-terminus comes to a halt when the proteasome encounters a glycine-rich region (GRR) followed by the Rel homology domain, which forms a tightly folded dimeric structure. This structure may be resistant to unfolding by the ATPase subunits of the 19S proteasome, allowing the N-terminal fragments (p50 and p52) to escape from the proteasome.
4. Ubiquitin in protein kinase activation by diverse NF-κB signaling pathways
A prerequisite for the degradation of IκBs and processing of p100 and p105 is the phosphorylation of these proteins by the IKK complex. Thus, a key question in the field is how IKK is regulated by a large variety of stimulatory signals. Unexpectedly, it was found in 1996 that polyubiquitination could activate a large kinase complex capable of site-specific phosphorylation of IκBα in vitro 34. The activation of this kinase complex, later known as IKK, requires E1, an E2 of the Ubc4/5 family and ubiquitin, but not the proteasome. This in vitro activation is prevented by methylated ubiquitin, but not the K48R mutant of ubiquitin, suggesting that polyubiquitination through another lysine of ubiquitin is important for IKK activation. However, as these experiments were carried out in vitro, the relevance of this finding to IKK activation under physiological conditions was not clear. In particular, there was no known connection between ubiquitination and upstream regulators of IKK at the time. Such a connection was established later when it was found that TRAF6, a key regulator of IKK, is a RING domain ubiquitin ligase 35.
TRAF6 belongs to a family of seven proteins; all but TRAF1 contain an N-terminal RING domain 36. TRAF1-6 also contain a highly conserved C-terminal TRAF/MATH (meprin and TRAF Homology) domain, whereas the C-terminus of TRAF7 contains seven WD40 repeats. TRAF proteins are critically involved in NF-κB signaling by various cell surface and intracellular receptors. For example, TRAF6 is essential for NF-κB and MAP kinase activation by interleukin-1 receptor (IL-1R) and Toll-like receptors (TLR), whereas TRAF2 and TRAF5 are important for signaling by TNF receptors. TRAF3, on the other hand, is required for the activation of another transcription factor, interferon-regulatory factor 3 (IRF3). In all of these cases, the TRAF proteins function as ubiquitin ligases to activate the protein kinases involved in different pathways. Recent advances in understanding the role of ubiquitination in protein kinase activation in several signaling pathways will be discussed below, with emphasis on the biochemical mechanisms involved.
4.1. Interleukin-1 receptor/Toll-like receptors (IL-1R/TLR)
IL-1β is a potent inflammatory cytokine that activates NF-κB and other signaling pathways that are important for effective immune responses against microbial infection 37. IL-1R contains an intracellular signaling domain that is homologous to the intracellular domain of TLRs, which represent a major class of pattern recognition receptors that recognize conserved microbial-derived molecules, such as LPS and viral nucleic acids. Upon stimulation of cells with IL-1β or a TLR ligand such as LPS, the intracellular signaling domain, termed Toll and IL-1 Receptor (TIR) domain, recruits the adaptor protein MyD88 (myeloid differentiation primary response gene 88), which also contains a TIR domain. MyD88 in turn recruits the IL-1 receptor associated kinases, IRAK4 and IRAK1. IRAK4 phosphorylates IRAK1, releasing IRAK1 into the cytosol, where it forms a complex with TIFA (TRAF-interacting protein with a forkhead-associated domain) and TRAF6. Genetic experiments have shown that TRAF6 is essential for NF-κB and MAP kinase activation by IL-1, CD40, LPS and other TLR agonists.
In an effort to understand how TRAF6 activates IKK, a cell-free system of TRAF6-regulated IKK activation was established 35. Fractionation of cytosolic extracts led to the discovery of two TRAF6-regualted IKK activators, TRIKA1 and TRIKA2 (TRAF6-regulated IKK activators 1 and 2). TRIKA1 is a ubiquitin-conjugating enzyme complex consisting of Ubc13 and a Ubc-like protein termed Uev1A (also known as Mms2; 35,38), whereas TRIKA2 is a complex containing the TGFβ-activated kinase (TAK1) and the adaptor proteins TAB1 and TAB2 (TAK1 binding protein 1 and 2) 39. Biochemical studies showed that TRAF6 and Ubc13/Uev1A catalyze the synthesis of a unique polyubiquitin chain linked through K63 of ubiquitin. This polyubiquitination activates the TAK1 kinase complex through a proteasome-independent mechanism (Figure 1). TAK1 then phosphorylates IKKβ, resulting in activation of the IKK complex. TAK1 also phosphorylates MAP kinase kinases (MKKs; e.g, MKK6 and MKK7), leading to activation of JNK (Jun N-terminal kinase) and p38 kinase.
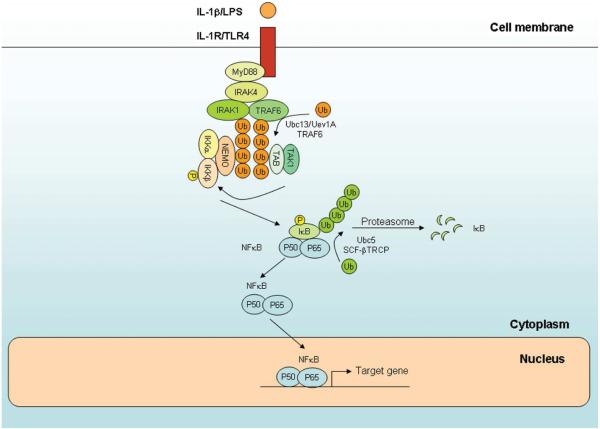
Figure 1. Roles of ubiquitin in NF-κB activation by IL-1 receptor and Toll-like receptors (IL-1R/TLR).
Stimulation of IL-1R and TLRs by their ligands leads to recruitment of the adaptor protein MyD88, protein kinases IRAK4 and IRAK1 and ubiquitin ligase TRAF6. In the presence of the E2 Ubc13/Uev1A, TRAF6 catalyzes K63 polyubiquitination of IRAK1 and TRAF6 itself. The polyubiquitin chains bind to TAB2 and TAB3 and activate the TAK1 kinase complex. The polyubiquitin chains also serve as a scaffold to recruit the IKK complex through NEMO, facilitating the phosphorylation of IKKβ by TAK1. IKK is activated to phosphorylate IκB proteins, which are subsequently ubiquitinated by the SCF-βTRCP ubiquitin E3 complex. The ubiquitinated IκBs are degraded by the proteasome, leading to NF-κB nuclear translocation and activation of downstream target genes.
Subsequent studies provide mechanistic insights into how K63 polyubiquitination activates the TAK1 kinase complex. It turns out that TAB2 and its homologue TAB3 contain a highly conserved C-terminal zinc finger domain (NZF) that binds preferentially to K63 polyubiquitin chains 40. Mutations within this domain that disrupt the binding of TAB2 or TAB3 to ubiquitin also abrogate its ability to mediate activation of TAK1 and IKK. Conversely, replacement of the NZF domain with another ubiquitin-binding domain restores the ability of TAB2 and TAB3 to support TAK1 and IKK activation. Although TAB2-deficient MEF cells can still activate NF-κB normally in response to stimulation by IL-1β or TNFα, RNAi of both TAB2 and TAB3 prevent IKK activation, suggesting that these two proteins have redundant functions in the NF-κB pathway 40-43. TAB1 is dispensable for NF-κB and MAP kinase activation by cytokines, but required for the activation of TAK1 by osmotic stress 44.
The activation of IKK by TAK1 requires NEMO, the essential regulatory subunit of the IKK complex 45. NEMO also binds preferentially to K63 polyubiquitin chains through a C-terminal coiled-coil domain termed NUB (Nemo-ubiquitin binding; also known as UBAN, CoZi or NOA domain)46-50. A recent structural study of the NUB domain of NEMO reveals that it forms a dimeric coiled-coil, with the ubiquitin-binding residues clustering in the leucine zipper region 51. Mutations within this region that disrupt ubiquitin binding also impair IKK activation. Some of these mutations have been found in human patients with anhydrotic ectodermal dysplasia with immunodeficiency (EDA-ID)52. Therefore, a critical role of NEMO in IKK activation may be explained at least in part by its ability to recognize K63 polyubiquitin chains. The polyubiquitin chains may function as a scaffold to recruit the TAK1 and IKK complexes, allowing TAK1 to phosphorylate and activate IKK.
Several proteins in the IL-1R/TLR pathway have been shown to be the targets of K63 polyubiquitination by TRAF6. These proteins include IRAK1, NEMO and TRAF6 itself 39,53-56. A specific lysine on TRAF6 (K124) appears to be the major site of polyubiquitination, and a point mutation of K124 abrogates its ability to rescue IL-1β and RANK signaling in TRAF6-deficient cells 57,58. However, another recent study showed that TRAF6 lacking all lysines is still capable of supporting IKK activation in response to IL-1 stimulation, suggesting that TRAF6 autoubiquitination is dispensable for IKK activation59. IL-1β also triggers K63 polyubiquitination of IRAK1, and mutations of two lysines (K134 and K180) impaired IRAK1 ubiquitination as well as NF-κB activation by IL-1β and TLR ligands 55,56. A recent study suggests that NEMO is conjugated at two lysines by a linear polyubiquitin chains in which ubiquitin is linked from “head to tail”. Although two ubiquitin genes are known to encode linear polyubiquitin, it is believed that polyubiquitin precursors are rapidly cleaved by cellular DUBs to generate the mature ubiquitin proteins. The de novo synthesis of linear polyubiquitin and its conjugation to NEMO are catalyzed by a ubiquitin E3 complex consisting of two RING domain proteins, HOIL-1L and HOIP 60. Mouse cells lacking HOIL-1L are partially defective in IKK activation by TNFα, suggesting that linear polyubiquitination of NEMO might play a role in IKK activation. However, an independent study showed that HOIL-1L (also known as RBCK1) targets TAB2 and TAB3 for proteasomal degradation, thereby inhibiting IKK activation by TNFα and IL-1β61. Thus, more work is needed to determine whether and how linear polyubiquitination of NEMO plays a role in IKK activation.
Although the RING domain of TRAF6 is clearly important for its ubiquitin ligase activity, conflicting results concerning the role of this domain in vivo have been reported. While one report showed that TRAF6 lacking the RING domain could still activate NF-κB in response to IL-1β62, several other reports found that deletion or mutation of the RING domain that impaired the E3 activity of TRAF6 blocked IKK activation by IL-1β and RANK 55,57,58. Therefore, further research, perhaps through the use of knock-in mouse models, is needed to clarify the in vivo function of TRAF6 as a ubiquitin ligase.
4.2. Tumor necrosis factor receptor (TNFR)
TNFα binds to two members of TNF receptor superfamily, TNFR1 and TNFR2, and initiates signaling cascades leading to activation of NF-κB and MAP kinases, as well as apoptosis 63,64. The signaling pathways initiated by TNFR1 have been most extensively studied (Figure 2). Upon binding to trimeric TNFα, TNFR1 becomes trimerized and recruits the adaptor protein, TRADD (TNFR1-associated death domain). TRADD further recruits the RING domain ubiquitin ligases TRAF2, TRAF5, cIAP1 and cIAP2, as well as the protein kinase RIP1 (receptor interacting protein kinase 1). RIP1 then activates IKK in a manner independent of its kinase activity.
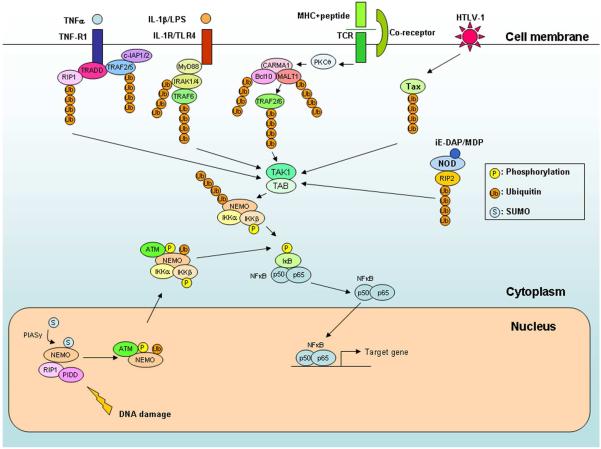
Figure 2. Expanding role of ubiquitination in the activation of TAK1 and IKK by diverse NF-κB signaling pathways.
NF-κB is activated by many different signaling pathways that converge on TAK1 and IKK complexes. These pathways include those emanating from cell surface receptors, including TNF receptor (TNF-R1), IL-1 receptor and Toll-like receptors (IL-1R/TLR), and T cell receptors (TCR), as well as those from intracellular receptors, such as NOD1 and NOD2. In addition, viral proteins such as Tax of human T cell leukemia virus-1 (HTLV-1) activate TAK1 and IKK in the cytosol. All of these pathways employ one or more TRAF proteins as the ubiquitin ligase(s) to catalyze K63 polyubiquitination of various signaling proteins, which activate the TAK1 kinase complex, leading to the activation of IKK and NF-κB. DNA damage in the nucleus can also activate IKK in the cytosol through a mechanism involving sequential sumoylation, phosphorylation and ubiquitination of NEMO (see text for details).
Like TRAF6, TRAF2 acts as a ubiquitin ligase in the TNFR pathway and itself can be a target of ubiquitination. Overexpression of a catalytically inactive mutant (C87A) of Ubc13 inhibits TRAF2- and TNFα-induced NF-κB activation 35. Polyubiquitination of TRAF2 requires Ubc13/Uev1A, and ubiquitinated TRAF2 is important for JNK activation 65,66. However, deletion of one or both copies of Ubc13 does not completely block NF-κB activation by TNFα, suggesting that another E2 may compensate for the loss of Ubc13 67-69. Deletion of both TRAF2 and TRAF5, but not TRAF2 alone, impairs NF-κB activation by TNFα, indicating that TRAF2 and TRAF5 function redundantly in the TNFα pathway 70-72.
RIP1 is rapidly polyubiquitinated at a specific lysine (K377) following TNFα stimulation 46,73. A point mutation of RIP1 at K377 abolishes its ubiquitination as well as its ability to recruit the TAK1 and IKK complexes to TNFR1, and prevents IKK activation. Ubiquitination of RIP1 is impaired in TRAF2-deficient cells; however, there is no evidence that TRAF2 can directly catalyze polyubiquitination of RIP1 74. Recent studies suggest that cIAP1 and cIAP2 may be more directly involved in the ubiquitination of RIP1. In fact, cIAPs have been shown to promote both K48 and K63 polyubiquitination of RIP1 in conjunction with Ubc5 in vitro 75. Consistent with the role of cIAPs as the RIP1 E3, reducing the expression of both c-IAP1 and c-IAP2 by RNAi attenuates polyubiquitination of RIP1 and activation of NF-κB.
In addition to activating NF-κB, polyubiquitination of RIP1 protects cells from TNFα-induced apoptosis through both NF-κB-dependent and -independent mechanisms 76,77. After the formation of the TNFR1-associated complex containing TRADD, TRAF2 and RIP1 (complex I), which triggers IKK activation, this complex dissociates from the receptor and forms another complex with Fas-associated death domain protein (FADD) and procaspase-8 in the cytoplasm (complex II; Figure 2) 78. Within this complex, procaspase-8 undergoes autocleavage to generate mature caspase-8, which initiate the apoptosis cascade. However, as a result of NF-κB activation, several anti-apoptotic proteins are induced. For example, cellular FLICE-inhibitory protein (c-FLIP) inhibits procaspase-8 activation, thereby preventing apoptosis. As polyubiquitination of RIP1 is required for NF-κB activation, it plays a critical role in blocking apoptosis. Polyubiquitination of RIP1 also plays a more direct role in preventing apoptosis by inhibiting the transition of RIP1 from the receptor-associated complex to the cytosolic death-inducing complex. When ubiquitination of RIP1 is blocked, through mutation of the ubiquitination site, depletion of cIAPs or by deubiquitination (catalyzed by CYLD; to be further discussed in section 5), the formation of the death-inducing complex is enhanced to promote apoptosis 76,77.
4.3. T cell receptors (TCR)
T cells, which are the central mediator of adaptive immune responses, are activated through the engagement of T cell receptors (TCRs) by antigenic peptides presented by major histocompatibility complexes (MHCs) on the surface of antigen presenting cells. Stimulation of TCRs activates a tyrosine kinase cascade, leading to activation of the serine/threonine kinase PKCθ (protein kinase θ). PKCθ triggers the formation of a complex termed CBM, which contains CARMA1 (caspase recruitment domain-containing membrane-associated guanylate kinase 1), BCL10 (B-cell lymphoma 10) and MALT1 (mucosa-associated lymphoid tissue lymphoma translocation gene 1)79,80. The CBM complex is essential for the activation of IKK through a mechanism involving K63 polyubiquitination (Figure 2). MALT1 contains binding sites for TRAF2 and TRAF6, and the binding of MALT1 to TRAF6 promotes the oligomerization of TRAF6, leading to activation of its ubiquitin E3 activity 54. TRAF6 then functions together with Ubc13/Uev1A to catalyze K63 polyubiquitination of target proteins including BCL10, MALT1, NEMO and TRAF6 itself 54,81-83. One or more of these polyubiquitination events activates the TAK1 kinase complex, which in turn activates IKK. Genetic experiments have demonstrated that conditional deletion of Ubc13 or TAK1 blocks the activation of IKK and JNK by TCR stimulation 68,84-86. However, T cells lacking TRAF6 can still activate NF-κB 87. This may be due to the redundant functions of TRAF2 and TRAF6, as RNAi of both TRAF2 and TRAF6 in T cells causes a more severe defect in NF-κB activation and IL-2 production than RNAi of either TRAF alone 54. It has also been reported that MALT1 directly catalyzes K63 polyubiquitination of NEMO at K399 81. However, MALT1 does not contain a known ubiquitin ligase domain, such as RING or HECT. In addition, a recent mouse “knock-in” study showed that T cells carrying a K392R mutation of NEMO (equivalent to K399 in human) are fully capable of activating IKK and NF-κB in response to TCR stimulation 88. Thus, the role of NEMO ubiquitination in the TCR pathway remains to be clarified.
4.4. NOD-like receptors (NLR)
NOD1 and NOD2 (nucleotide-binding oligomerization domain 1 and 2) belong to a large family of evolutionarily conserved proteins containing nucleotide-binding domain (NBD) and leucine-rich repeats (NLR) 89. Both NOD1 and NOD2 contain N-terminal CARD domains that are important for NF-κB activation in response to intracellular bacterial infection. NOD1 is activated by iE-DAP (γ-glutamyl-meso-diaminopimelic acid), a peptidoglycan derived from Gram-negative bacteria, whereas NOD2 is activated by muramyl dipeptide (MDP), a peptidoglycan commonly found in all bacteria. The activation of NF-κB by NOD1 and NOD2 requires RIP2, a protein kinase containing a C-terminal CARD (caspase recruitment domain) domain. NOD2 mutations have been closely linked to human Crohn's disease, an autoimmune inflammatory disorder of the gastrointestinal tract. NOD2 mutations associated with Crohn's disease impair its ability to activate NF-κB, resulting in faulty production of both pro- and anti-inflammatory cytokines.
Recent studies have shown that K63 polyubiquitination plays a key role in NF-κB activation by NOD1 and NOD2 (Figure 2). Abbott et al first showed that NOD2 and RIP2 promote K63-linked polyubiquitination of NEMO at a specific lysine (K285), and that the Crohn's disease-associated mutations diminish the ability of NOD2 to bind RIP2 and promote NEMO ubiquitination 90. RIP2 has also been found to be polyubiquitinated at a specific lysine by NOD1 and NOD2, and a mutation of the ubiquitination site in RIP2 impairs NF-κB activation 91-93. Polyubiquitinated RIP2 recruits the TAK1 kinase complex to activate IKK 91,92,94. Cells lacking TAK1 or Ubc13 fail to activate NF-κB in response to NOD1 or NOD2 ligands. It is still not clear what the E3 for RIP2 ubiquitination is. While one study showed that RIP2 ubiquitination is abrogated in TRAF6-deficient cells, another study found that TRAF2 and TRAF5 are responsible for RIP2 ubiquitination 91,92.
4.5. RIG-I-like receptors (RLR)
Retinoic acid inducible gene I (RIG-I) is a cytosolic RNA helicase that binds to viral RNA and activates a signaling cascade leading to the induction of type-I interferons, such as IFN-β95. Members of the RIG-I-like receptor (RLR) family include melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2). RIG-I and MDA5 contain N-terminal tandem CARD domains that activate the transcription factors NF-κB, AP1 and IRF3, which form an enhanceosome complex to induce IFN-β. A key adaptor protein that links RIG-I and MDA5 to the downstream signaling cascade is MAVS (mitochondrial antiviral signaling; also known as IPS-1, VISA or CARDIF), a CARD domain protein localized to the mitochondrial outer membrane 96. MAVS contains binding sites for TRAF2, TRAF3 and TRAF6. While TRAF2 and TRAF6 are likely to be important for IKK activation, TRAF3 mediates the activation of the IKK-like kinases, TBK1 and IKKε, which phosphorylate and activate IRF3. An intact RING domain of TRAF3 is required for IRF3 activation, implying that ubiquitination is important for IRF3 activation by MAVS (Figure 3)97.
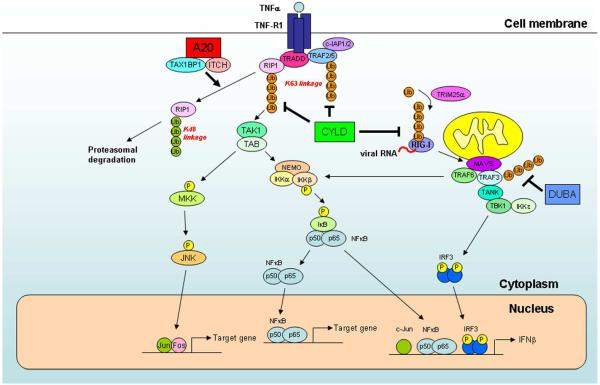
Figure 3. Negative regulation of NF-κB and IRF3 by deubiquitination enzymes.
Binding of TNFα to its receptor induces the recruitment of signaling proteins, including the adaptor TRADD, ubiquitin ligases TRAF2, TRAF5, cIAP1 and cIAP2, and protein kinase RIP1. RIP1 and TRAFs are polyubiquitinated by TRAFs and/or cIAPs, and the polyubiquitin chains recruit and activate the TAK1 and IKK complexes, leading to activation of NF-κB and MAP kinases (e.g, JNK). To shut down the signaling cascades, the deubiquitination enzymes A20 and CYLD remove the polyubiquitin chains from RIP1 and TRAFs. In addition, A20 forms a ubiquitin-editing complex with the ubiquitin-binding protein TAX1BP1 and the HECT domain ubiquitin ligase ITCH. This complex targets RIP1 for K48 polyubiquitination and proteasomal degradation. Ubiquitination and deubiquitination also regulate the RIG-I pathway, which detect viral infection in the cytosol. Binding of RIG-I to viral RNA triggers its K63 polyubiquitination by the RING domain E3 TRIM25. RIG-I then interacts with the mitochondrial adaptor protein MAVS, which recruits TRAF6 and TRAF3 to activate IKK and TBK1, respectively. TBK1 phosphorylates IRF3, leading to induction of type-I interferons. CYLD and DUBA are deubiquitination enzymes that remove K63 polyubiquitin chains from RIG-I and TRAF3, respectively, thereby damping the antiviral response.
Upstream of MAVS, the activation of RIG-I requires K63 polyubiquitination by TRIM25, a member of the tripartite motif (TRIM) proteins containing a RING domain, a B box/coiled-coil domain and a SPRY domain98. The SPRY domain binds to the first CARD domain of RIG-I and promotes polyubiquitination of RIG-I at a specific lysine (K172) within the second CARD domain. TRIM25-deficient cells are compromised in their ability to induce IFN-β in response to RNA virus infection. RIG-I and MAVS are also negatively regulated by another E3 ubiquitin ligase, RNF125, which target RIG-I and MAVS for degradation by the proteasome 99.
4.6. DNA damage
DNA damage is known to activate NF-κB, which promotes cell survival and provides a time window for DNA repair to proceed. NF-κB activation by DNA damage may also contribute to resistance of cancer cells to chemo- and radiation therapies. Recent studies show that DNA damage, which occurs in the nucleus, activates the IKK complex in the cytosol through a complex mechanism involving modification of NEMO by ubiquitin and small ubiquitin-like modifier-1 (SUMO-1; Figure 2). Upon genotoxic stress, nuclear NEMO, which does not form a complex with IKKα and IKKβ, binds to PIDD (p53-inducible death-domain-containing protein) and RIP1 and is modified by SUMO at two lysines (K277 and K309) by PIASy (putative protein inhibitor of activated STAT Y), a nuclear matrix-associated SUMO E3 ligase 100-102. Sumoylated NEMO is phosphorylated by the kinase ATM (ataxia telangiectasia mutated) at Ser-25. Phosphorylated NEMO is then mono-ubiquitinated by unknown ubiquitination enzymes at the same lysines used for sumoylation 103. It is not clear whether SUMO is removed from NEMO before subsequent ubiquitination at the same sites, or ubiquitination occurs on the fraction of NEMO which is not sumoylated. In any case, fusion of SUMO to a NEMO mutant that cannot be ubiquitinated restores phosphorylation of NEMO by ATM, but it does not restore IKK activation by DNA damage, indicating that ubiquitination of NEMO is important for IKK activation. Ubiquitinated NEMO together with ATM enter the cytoplasm where they associate with IKKα, IKKβ and ELKS (a protein rich in glutamate, leucine, lysine and serine). IKK is then activated by monoubiquitinated NEMO and ATM, but the biochemical mechanism remains to be elucidated.
4.7. Human T cell leukemia virus-1 (HTLV-1) Tax protein
Many microbial pathogens can usurp the host ubiquitination machinery for their own benefits. An example is provided by Human T-cell leukemia virus type-1 (HTLV-1), a retrovirus that infects T cells and causes adult T cell leukemia (ATL)104. The Tax protein of HTLV-1 constitutively activates NF-κB and plays a critical role in T cell transformation. Tax physically interacts with NEMO and triggers the catalytic activity of IKK complex 105. Both ubiquitination and sumoylation of Tax have been reported (Figure 2). Ubiquitinated Tax associates with the IKK complex in the cytoplasm, whereas sumoylated Tax binds to RelA in the nucleus 106,107. Tax-mediated NF-κB activation is abolished in Ubc13 deficient cells 108. Furthermore, reducing ubiquitination of Tax by Ubc13 RNAi disrupts the interaction between Tax and NEMO, suggesting that NEMO binds to the polyubiquitin chains on Tax. Overexpression of TRAF2, 5 or 6 strongly induces Tax ubiquitination, but it is not clear whether any of these TRAF proteins is required for Tax ubiquitination 109. TAK1 is constitutively activated in HTLV-1-infected T cells and is required for Tax-mediated IKK activation 110. Tax binds to and activates TAK1 through the adapter protein TAB2 109. These studies suggest that polyubiquitination of Tax leads to the recruitment of both TAK1 and IKK complexes, thereby facilitating the phosphorylation of IKK by TAK1. The mechanism of IKK activation by Tax is very similar to that employed by the TCR pathway, in which polyubiquitination of BCL10, MALT1 and/or TRAF6 leads to the activation of TAK1 and IKK. Tax has an additional ability to cause persistent activation of NF-κB through its binding to Tax1BP1 111. Tax1BP1 is a component of a ubiquitin-editing complex that includes the deubiquitination enzyme A20 and the HECT domain ubiquitin ligase ITCH, which inhibit IKK activation (see next section for details). The binding of Tax to Tax1BP1 disrupts the formation of the A20 editing complex, leading to persistent activation of NF-κB, which causes T cell transformation and leukemia.
5. Negative regulation of protein kinases by deubiquitination enzymes
Like phosphatases that reverse protein phosphorylation, a large number of deubiquitination enzymes (DUBs) trim polyubiquitin chains or remove ubiquitin from target proteins. The human genome encodes close to 100 DUBs, which can be divided into five categories: ubiquitin C-terminal hydrolases (UCH), ubiquitin-specific proteases (USP), ovarian tumor-type proteases (OTU), Machado-Joseph Disease proteases (MJDs), and JAMM motif proteases (JAMMs) 4,5. The JAMM domain proteases are metalloproteases, whereas all the other DUBs are cysteine proteases. These DUBs function alone or with other regulatory subunits to control a large variety of cellular functions.
The regulatory role of polyubiquitination in protein kinase activation is strongly supported by the recent discovery of several DUBs that function as inhibitors of IKK and TBK1 (TANK binding kinase1) in the NF-κB and IRF3 pathways, respectively. In this section, we will focus on the role of three DUBs, CYLD, A20 and DUBA, in the negative regulation of protein kinases and their relevance to human diseases (Figure 3).
5.1. CYLD
The cylindromatosis gene Cyld encodes a tumor suppressor protein involved in the development of familial cylindromatosis, a benign skin tumor 112. The CYLD protein contains a USP domain at the C-terminus, and many of the tumor-associated mutations identified in human patients impair the DUB activity of CYLD. Overexpression of CYLD inhibits IKK activation, whereas reducing CYLD expression has the opposite effect 53,113,114. CYLD contains three CAP-Gly domains (Cytoskeleton-associated proteins (CAP) glycine-rich domains) at the N-terminus, which mediate binding to TRAF2 and NEMO. CYLD specifically cleaves K63-linked polyubiquitin chains from target proteins including NEMO, TRAF2 and TRAF6. CYLD also inhibits viral induction of type-I interferons by removing K63 polyubiquitin chains from RIG-I 115,116. The crystal structure of the USP domain of CYLD reveals a unique sequence insertion forming a loop that contributes to its specificity in cleaving K63 polyubiquitin chains 117.
Cyld-deficient mice have been generated in several laboratories 118. Although the phenotypes of these mice show some variations, a common theme is that loss of CYLD leads to hyperactivation of NF-κB in multiple tissues, leading to inflammatory diseases and tumor. Consistent with the role of CYLD in reversing ubiquitination of signaling proteins upstream of IKK, polyubiquitination of TRAF2, TRAF6, TAK1 and NEMO are enhanced in Cyld-deficient mice under different experimental conditions. These genetic studies also reveal additional targets of CYLD, such as BCL3 119. In the absence of CYLD, K63 polyubiquitination of BCL3 is enhanced, resulting in its nuclear translocation as a complex with p50 and p52. The nuclear complexes containing polyubiquitinated BCL3 apparently have increased activity to induce the expression of cyclin D1, which drives proliferation of keratinocytes, resulting in skin tumors. It has also been suggested that CYLD can remove K48 polyubiquitin chains from certain proteins such as LCK (Lymphoid specific cytosolic protein tyrosine kinase) and TRAF2, thereby stabilizing these proteins 120. However, it is difficult to reconcile these results with biochemical studies, which show that CYLD specifically cleaves K63, but not K48, polyubiquitin chains.
5.2. A20
A20 is rapidly induced by NF-κB and it potently inhibits NF-κB to provide a negative feedback loop 121. Genetic deletion of A20 in mice causes persistent activation of NF-κB in response to TNFR and TLR stimulation, leading to multi-organ inflammation, cachexia and neonatal lethality 122. A20 contains an N-terminal OTU domain and seven zinc finger domains at the C-terminus 123. It has been proposed that the OTU domain of A20 inhibits IKK by removing K63 polyubiquitin chains from target proteins including RIP1 and TRAF6 124,125. However, A20 mutants lacking the N-terminal OTU domain remain capable of inhibiting NF-κB in overexpression studies 126. In addition, structural studies showed that the A20 OTU domain is not specific for K63 polyubiquitin chains but rather recognizes specific substrates modified by ubiquitin 127,128. A second mechanism of IKK inhibition by A20 is mediated through its C-terminal zinc finger domains, which function as an E3 to promote K48-linked polyubiquitination of RIP1 and target it for degradation by the proteasome 125.
A20 does not act alone as an inhibitor of IKK. Instead, it forms a complex together with the ubiquitin-binding protein TAX1BP1 and ubiquitin ligase ITCH 111. Cells lacking TAX1BP1 or ITCH display persistent activation of IKK and JNK after stimulation with TNFα or IL-1β. TAX1BP1 contains a zinc finger-type ubiquitin-binding domain (UBZ) that is required for binding to polyubiquitinated TRAF6 129. Thus, a function of TAX1BP1 is to recruit A20 to deubiquitinate TRAF6 in the IL-1R/TLR pathways. TAX1BP1 also binds to ITCH through its PPXY motif, and recruits ITCH to promote K48 polyubiquitination of RIP1 111. In ITCH-deficient cells, K63 polyubiquitination of RIP1 is enhanced, resulting in persistent IKK activation. The HECT domain of ITCH is required for RIP1 ubiquitination, suggesting that ITCH may function as an E3 to catalyze RIP1 ubiquitination.
Other A20-interacting proteins include ABIN1, 2 and 3 130 (A20 binding inhibitor of NF-κB 1, 2 and 3). In addition to the A20-binding domain, the ABIN proteins contain a NEMO-binding domain as well as a ubiquitin-binding domain similar to the NUB domain found in NEMO 50. Overexpression of ABIN-1 inhibits IKK activation by facilitating deubiquitination of NEMO by A20. However, genetic deletion of ABIN-1 in mice does not enhance or reduce IKK activation, but rather facilitates TNFα-induced apoptosis by promoting the interaction between FADD and caspase-8 131. The NUB domain of ABIN-1 is required for its interaction with polyubiquitinated RIP1 and for protecting cells from apoptosis. ABIN-2 forms a complex with p105 and Tpl2, and it is required for the stabilization of Tpl2 132. T cells lacking ABIN-2 have impaired ERK activation due to low level of Tpl2 133. ABIN-3 is a target gene of NF-κB and its overexpression also inhibits NF-κB activation, but its physiological function has not been investigated through genetic studies 134. Given the structural and functional similarities of ABIN proteins, some functional redundancy might exist among these proteins.
5.3. DUBA
DUBA (Deubiquitinating enzyme A) is an OTU-type DUB that functions as a negative regulator of type-I interferon production triggered by several pattern recognition receptors, including TLRs, RIG-I and MDA-5 135. RNAi of DUBA enhances IRF3 activation, whereas overexpression of DUBA has the opposite effect. DUBA cleaves K63, but not K48, polyubiquitin chains in vitro, and its catalytic activity is required for IRF3 inhibition in transfection experiments. DUBA interacts with TRAF3 and removes K63 polyubiquitin chains from TRAF3 in a manner that depends on its ubiquitin-interaction motif (UIM). These results suggest that DUBA inhibits IRF3 by functioning as a K63-specific DUB that antagonizes the function of TRAF3.
6. Conclusions and Perspectives
The study of NF-κB pathways has provided a paradigm for understanding multiple roles of ubiquitin in cell signaling, including signal-dependent degradation of an inhibitor that leads to rapid activation of a signaling cascade, limited proteolysis of a precursor protein by the proteasome, and activation of protein kinases. Conversely, the study of ubiquitin signaling has provided key insights into the regulation of NF-κB and immune responses in general. It is now firmly established that the ubiquitin system is not merely a garbage disposal, but it plays a pivotal role at multiple steps in diverse signaling cascades leading to NF-κB activation. The pervasive role of ubiquitin in NF-κB pathways represents one of the best examples that the regulatory potential of ubiquitination and deubiquitination rivals that of phosphorylation and dephosphorylation.
A common mechanism underlying regulation by ubiquitination and phosphorylation is the presence of many types of domains or motifs that recognize ubiquitin, polyubiquitin chains or phosphorylated peptides. Close to 20 different types of ubiquitin-binding domains have been discovered 136. These domains differ in their structures and binding affinities, and they are present in a large variety of cellular proteins that execute distinct functions in response to signals from different ubiquitinated proteins. In the NF-κB pathway, the NZF domain in TAB2 and TAB3, and the NUB domain in NEMO, allow the TAK1 and IKK complexes to detect and respond to upstream polyubiquitinated proteins such as RIP1, IRAK1 and TRAFs. However, further research is required to fully elucidate the biochemical mechanism by which TAK1 and IKK are activated as a result of binding to polyubiquitinated proteins. In this regard, high-resolution structures of the TAK1 and IKK complexes bound to polyubiquitinated ligands would be very informative.
Another important question that needs to be resolved is why certain polyubiquitinated proteins are targeted for degradation by the proteasome, while others are spared. One widely held model is that polyubiquitin chains of different topology dictate the fate of the target proteins; e.g, K48 polyubiquitin chains target protein degradation by the proteasome, whereas K63 polyubiquitin chains escape proteasomal degradation. However, in vitro biochemical experiments have shown that proteins conjugated by K63 polyubiquitin chains can be efficiently degraded by the proteasome 137,138. In addition, K48 polyubiquitin chains have been shown to regulate the function of certain proteins without involving proteolysis 139. Therefore, whether a protein is targeted for proteasomal degradation may not be determined solely by the topology of polyubiquitin chains. Structural properties of ubiquitinated proteins, such as their unfolding propensity, and the presence of other ubiquitin-binding domains that may compete with the proteasome are likely to determine whether a protein is ultimately degraded by the proteasome. Nevertheless, early genetic experiments in yeast clearly show that a point mutation of K48 of ubiquitin has far more severe phenotypes than mutations of any other lysine, indicating that polyubiquitin chains linked through different lysines of ubiquitin have distinct functions 140,141. Indeed, K63 polyubiquitination has been linked to protein kinase activation and DNA repair, and so far there is no evidence that K63 polyubiquitination directly targets a protein for proteasomal degradation in vivo 142. Of course, all proteins are eventually degraded, and most are degraded by the proteasome. An example that a protein conjugated by K63 polyubiquitin chains is eventually degraded by the proteasome is provided by RIP1. TNFα stimulation triggers a very rapid K63 polyubiquitination of RIP1, which is important for NF-κB activation and cell survival. Subsequently, RIP1 is conjugated by K48-linked polyubiquitin chains, which targets it for degradation by the proteasome, thereby down regulating the NF-κB pathway 143.
It is remarkable that many proteins involved in the NF-κB signaling cascades are ubiquitination and deubiquitination enzymes as well as ubiquitin-binding proteins. For example, most proteins recruited to TNF receptor are involved in the ubiquitin pathway. It is unlikely that extensive involvement of the ubiquitin system in cell signaling is limited to the NF-κB pathway. Indeed, many proteins involved in DNA repair pathways are linked to the ubiquitin system, including enzymes involved in the synthesis of K63 polyubiquitin chains and receptors that bind specifically to these chains 144,145. In light of very large families of ubiquitin ligases, deubiquitination enzymes and ubiquitin-binding proteins in the mammalian proteome, and recent technological breakthroughs such as mass spectrometry and RNAi, the next few years should witness a rapid expansion of both proteolytic and non-proteolytic roles of ubiquitin in most, if not all, cell signaling pathways.
Acknowledgements
We thank Brian Skaug (UT Southwestern) for critically reading the manuscript. Research in our laboratory is supported by grants from the NIH, the Welch Foundation and Howard Hughes Medical Institute.
Biographies
Biography

Zhijian `James' Chen received his B.S. degree in Biology in 1985 from Fujian Normal University in China. His doctoral research on the biochemistry of the ubiquitin system was conducted under the direction of Cecile Pickart at the State University of New York at Buffalo. After receiving his Ph.D degree in 1991, he carried out his postdoctoral research on NF-κB in the laboratory of Inder Verma at the Salk Institute. He spent several years in the biotech industry before joining the faculty at the University of Texas Southwestern Medical Center at Dallas in 1997. His research focuses on the role of ubiquitin in NF-κB signaling and innate immunity.

Yu-Hsin Chiu was born in Taipei, Taiwan. She received her B. S. degree in zoology and M. S. degree in immunology from National Taiwan University. She characterized the functions of a novel ubiquitin E1-like protein (E1-L2) in the laboratory of Dr. Zhijian `James' Chen at the University of Texas Southwestern Medical Center at Dallas, and earned her Ph.D. degree in 2008. Currently she is investigating DNA -mediated innate immune response in the Chen lab.

Meng Zhao received her B.S. degree in Biological Science from Wuhan University in China in 2004. She came to Dallas to pursue her doctoral degree at UT-Southwestern Medical Center. She is currently a Ph.D student in Dr. Zhijian `James' Chen's laboratory and her doctoral research is focused on NF-κB activation pathways.
References
- (1).Wilkinson KD. Cell. 2004;119:741. doi: 10.1016/j.cell.2004.12.001. [DOI] [PubMed] [Google Scholar]
- (2).Hershko A. Cell. 1983;34:11. doi: 10.1016/0092-8674(83)90131-9. [DOI] [PubMed] [Google Scholar]
- (3).Pickart CM. Cell. 2004;116:181. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- (4).Amerik AY, Hochstrasser M. Biochim Biophys Acta. 2004;1695:189. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- (5).Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. Cell. 2005;123:773. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- (6).Pickart CM. Mol Cell. 2001;8:499. doi: 10.1016/s1097-2765(01)00347-1. [DOI] [PubMed] [Google Scholar]
- (7).Hayden MS, Ghosh S. Cell. 2008;132:344. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- (8).Pomerantz JL, Baltimore D. Mol Cell. 2002;10:693. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- (9).Chen ZJ. Nat Cell Biol. 2005;7:758. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Krappmann D, Scheidereit C. EMBO Rep. 2005;6:321. doi: 10.1038/sj.embor.7400380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Maniatis T. Genes Dev. 1999;13:505. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- (12).Jiang J, Struhl G. Nature. 1998;391:493. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- (13).Frescas D, Pagano M. Nat Rev Cancer. 2008;8:438. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Genes Dev. 1995;9:1586. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- (15).Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L, Pagano M. Dev Cell. 2003;4:799. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- (16).Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. Cell. 1994;78:773. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- (17).Mercurio F, DiDonato JA, Rosette C, Karin M. Genes Dev. 1993;7:705. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- (18).Ciechanover A, Gonen H, Bercovich B, Cohen S, Fajerman I, Israel A, Mercurio F, Kahana C, Schwartz AL, Iwai K, Orian A. Biochimie. 2001;83:341. doi: 10.1016/s0300-9084(01)01239-1. [DOI] [PubMed] [Google Scholar]
- (19).Lin L, DeMartino GN, Greene WC. Cell. 1998;92:819. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- (20).Cohen S, Achbert-Weiner H, Ciechanover A. Mol Cell Biol. 2004;24:475. doi: 10.1128/MCB.24.1.475-486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cohen S, Lahav-Baratz S, Ciechanover A. Biochem Biophys Res Commun. 2006;345:7. doi: 10.1016/j.bbrc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- (22).Beinke S, Ley SC. Biochem J. 2004;382:393. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Belich MP, Salmeron A, Johnston LH, Ley SC. Nature. 1999;397:363. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- (24).Waterfield MR, Zhang M, Norman LP, Sun SC. Mol Cell. 2003;11:685. doi: 10.1016/s1097-2765(03)00070-4. [DOI] [PubMed] [Google Scholar]
- (25).Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, Seddon B, Ley SC. Nat Immunol. 2009;10:38. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- (26).Xiao G, Harhaj EW, Sun SC. Mol Cell. 2001;7:401. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- (27).Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. Cell. 2007;131:669. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- (28).Liao G, Zhang M, Harhaj EW, Sun SC. J Biol Chem. 2004;279:26243. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- (29).Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nat Immunol. 2008;9:1364. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Nat Immunol. 2008;9:1371. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Science. 2001;293:1495. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- (32).Piwko W, Jentsch S. Nat Struct Mol Biol. 2006;13:691. doi: 10.1038/nsmb1122. [DOI] [PubMed] [Google Scholar]
- (33).Rape M, Jentsch S. Biochim Biophys Acta. 2004;1695:209. doi: 10.1016/j.bbamcr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- (34).Chen ZJ, Parent L, Maniatis T. Cell. 1996;84:853. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- (35).Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Cell. 2000;103:351. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- (36).Chung JY, Park YC, Ye H, Wu H. J Cell Sci. 2002;115:679. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- (37).Dunne A, O'Neill LA. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- (38).Hofmann RM, Pickart CM. Cell. 1999;96:645. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- (39).Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. Nature. 2001;412:346. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- (40).Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. Mol Cell. 2004;15:535. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- (41).Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Embo J. 2003;22:6277. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sanjo H, Takeda K, Tsujimura T, Ninomiya-Tsuji J, Matsumoto K, Akira S. Mol Cell Biol. 2003;23:1231. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K. Mol Cell. 2000;5:649. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- (44).Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, Yamada G, Matsumoto K, Mishina Y, Ninomiya-Tsuji J. J Biol Chem. 2008;283:33080. doi: 10.1074/jbc.M807574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israel A. Cell. 1998;93:1231. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- (46).Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Mol Cell. 2006;22:245. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- (47).Israel A. Trends Immunol. 2006;27:395. doi: 10.1016/j.it.2006.07.003. [DOI] [PubMed] [Google Scholar]
- (48).Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Nat Cell Biol. 2006;8:398. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- (49).Bloor S, Ryzhakov G, Wagner S, Butler PJ, Smith DL, Krumbach R, Dikic I, Randow F. Proc Natl Acad Sci U S A. 2008;105:1279. doi: 10.1073/pnas.0706552105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wagner S, Carpentier I, Rogov V, Kreike M, Ikeda F, Lohr F, Wu CJ, Ashwell JD, Dotsch V, Dikic I, Beyaert R. Oncogene. 2008;27:3739. doi: 10.1038/sj.onc.1211042. [DOI] [PubMed] [Google Scholar]
- (51).Lo YC, Lin SC, Rospigliosi CC, Conze DB, Wu CJ, Ashwell JD, Eliezer D, Wu H. Mol Cell. 2009 doi: 10.1016/j.molcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israel A, Courtois G, Casanova JL. Nat Genet. 2001;27:277. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- (53).Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. Nature. 2003;424:793. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- (54).Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. Mol Cell. 2004;14:289. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- (55).Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Mol Cell Biol. 2008;28:3538. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Windheim M, Stafford M, Peggie M, Cohen P. Mol Cell Biol. 2008;28:1783. doi: 10.1128/MCB.02380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. J Biol Chem. 2007;282:4102. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Lamothe B, Webster WK, Gopinathan A, Besse A, Campos AD, Darnay BG. Biochem Biophys Res Commun. 2007;359:1044. doi: 10.1016/j.bbrc.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. PLoS ONE. 2008;3:e4064. doi: 10.1371/journal.pone.0004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Nat Cell Biol. 2009;11:123. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- (61).Tian Y, Zhang Y, Zhong B, Wang YY, Diao FC, Wang RP, Zhang M, Chen DY, Zhai ZH, Shu HB. J Biol Chem. 2007;282:16776. doi: 10.1074/jbc.M701913200. [DOI] [PubMed] [Google Scholar]
- (62).Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Embo J. 2001;20:1271. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Tartaglia LA, Goeddel DV. Immunol Today. 1992;13:151. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- (64).Chen G, Goeddel DV. Science. 2002;296:1634. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- (65).Shi CS, Kehrl JH. J Biol Chem. 2003;278:15429. doi: 10.1074/jbc.M211796200. [DOI] [PubMed] [Google Scholar]
- (66).Habelhah H, Takahashi S, Cho SG, Kadoya T, Watanabe T, Ronai Z. Embo J. 2004;23:322. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Yamamoto M, Okamoto T, Takeda K, Sato S, Sanjo H, Uematsu S, Saitoh T, Yamamoto N, Sakurai H, Ishii KJ, Yamaoka S, Kawai T, Matsuura Y, Takeuchi O, Akira S. Nat Immunol. 2006;7:962. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- (68).Yamamoto M, Sato S, Saitoh T, Sakurai H, Uematsu S, Kawai T, Ishii KJ, Takeuchi O, Akira S. J Immunol. 2006;177:7520. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- (69).Fukushima T, Matsuzawa S, Kress CL, Bruey JM, Krajewska M, Lefebvre S, Zapata JM, Ronai Z, Reed JC. Proc Natl Acad Sci U S A. 2007;104:6371. doi: 10.1073/pnas.0700548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. Immunity. 1997;7:703. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- (71).Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Immunity. 1997;7:715. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- (72).Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H. J Biol Chem. 2001;276:36530. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- (73).Li H, Kobayashi M, Blonska M, You Y, Lin X. J Biol Chem. 2006;281:13636. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- (74).Lee TH, Shank J, Cusson N, Kelliher MA. J Biol Chem. 2004;279:33185. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- (75).Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. Mol Cell. 2008;30:689. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- (76).O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Curr Biol. 2007;17:418. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Wang L, Du F, Wang X. Cell. 2008;133:693. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- (78).Micheau O, Tschopp J. Cell. 2003;114:181. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- (79).Rawlings DJ, Sommer K, Moreno-Garcia ME. Nat Rev Immunol. 2006;6:799. doi: 10.1038/nri1944. [DOI] [PubMed] [Google Scholar]
- (80).van Oers NS, Chen ZJ. Science. 2005;308:65. doi: 10.1126/science.1110902. [DOI] [PubMed] [Google Scholar]
- (81).Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Nature. 2004;427:167. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- (82).Oeckinghaus A, Wegener E, Welteke V, Ferch U, Arslan SC, Ruland J, Scheidereit C, Krappmann D. Embo J. 2007;26:4634. doi: 10.1038/sj.emboj.7601897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Wu CJ, Ashwell JD. Proc Natl Acad Sci U S A. 2008;105:3023. doi: 10.1073/pnas.0712313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Liu HH, Xie M, Schneider MD, Chen ZJ. Proc Natl Acad Sci U S A. 2006;103:11677. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Sato S, Sanjo H, Tsujimura T, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Takeuchi O, Akira S. Int Immunol. 2006;18:1405. doi: 10.1093/intimm/dxl082. [DOI] [PubMed] [Google Scholar]
- (86).Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. Nat Immunol. 2006;7:851. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- (87).King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y. Nat Med. 2006;12:1088. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- (88).Ni CY, Wu ZH, Florence WC, Parekh VV, Arrate MP, Pierce S, Schweitzer B, Van Kaer L, Joyce S, Miyamoto S, Ballard DW, Oltz EM. J Immunol. 2008;180:7107. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Kanneganti TD, Lamkanfi M, Nunez G. Immunity. 2007;27:549. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- (90).Abbott DW, Wilkins A, Asara JM, Cantley LC. Curr Biol. 2004;14:2217. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- (91).Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. Embo J. 2008;27:373. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. J Biol Chem. 2007;282:36223. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- (93).Hitotsumatsu O, Ahmad RC, Tavares R, Wang M, Philpott D, Turer EE, Lee BL, Shiffin N, Advincula R, Malynn BA, Werts C, Ma A. Immunity. 2008;28:381. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Biochem J. 2007;404:179. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Yoneyama M, Fujita T. Immunity. 2008;29:178. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- (96).Seth RB, Sun L, Chen ZJ. Cell Res. 2006;16:141. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- (97).Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, Shahangian A, Zarnegar B, Shiba TL, Wang Y, Cheng G. Embo J. 2006;25:3257. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. Nature. 2007;446:916. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- (99).Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Proc Natl Acad Sci U S A. 2007;104:7500. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Cell. 2003;115:565. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- (101).Janssens S, Tinel A, Lippens S, Tschopp J. Cell. 2005;123:1079. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- (102).Mabb AM, Wuerzberger-Davis SM, Miyamoto S. Nat Cell Biol. 2006;8:986. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- (103).Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Science. 2006;311:1141. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- (104).Yoshida M. Oncogene. 2005;24:5931. doi: 10.1038/sj.onc.1208981. [DOI] [PubMed] [Google Scholar]
- (105).Sun SC, Ballard DW. Oncogene. 1999;18:6948. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- (106).Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, de The H, Pique C, Bazarbachi A. Blood. 2006;107:4021. doi: 10.1182/blood-2005-09-3572. [DOI] [PubMed] [Google Scholar]
- (107).Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Mol Cell Biol. 2005;25:10391. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. J Virol. 2007;81:13735. doi: 10.1128/JVI.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Yu Q, Minoda Y, Yoshida R, Yoshida H, Iha H, Kobayashi T, Yoshimura A, Takaesu G. Biochem Biophys Res Commun. 2008;365:189. doi: 10.1016/j.bbrc.2007.10.172. [DOI] [PubMed] [Google Scholar]
- (110).Wu X, Sun SC. EMBO Rep. 2007;8:510. doi: 10.1038/sj.embor.7400931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW. Nat Immunol. 2008;9:254. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- (112).Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Nat Genet. 2000;25:160. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- (113).Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Nature. 2003;424:797. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- (114).Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. Nature. 2003;424:801. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- (115).Friedman CS, O'Donnell MA, Legarda-Addison D, Ng A, Cardenas WB, Yount JS, Moran TM, Basler CF, Komuro A, Horvath CM, Xavier R, Ting AT. EMBO Rep. 2008 doi: 10.1038/embor.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Zhang M, Wu X, Lee AJ, Jin W, Chang M, Wright A, Imaizumi T, Sun SC. J Biol Chem. 2008;283:18621. doi: 10.1074/jbc.M801451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Komander D, Lord CJ, Scheel H, Swift S, Hofmann K, Ashworth A, Barford D. Mol Cell. 2008;29:451. doi: 10.1016/j.molcel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- (118).Sun SC. Nat Rev Immunol. 2008;8:501. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cell. 2006;125:665. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- (120).Xue L, Igaki T, Kuranaga E, Kanda H, Miura M, Xu T. Dev Cell. 2007;13:446. doi: 10.1016/j.devcel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- (121).Krikos A, Laherty CD, Dixit VM. J Biol Chem. 1992;267:17971. [PubMed] [Google Scholar]
- (122).Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Science. 2000;289:2350. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ. J Biol Chem. 2003;278:23180. doi: 10.1074/jbc.M301863200. [DOI] [PubMed] [Google Scholar]
- (124).Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. Nat Immunol. 2004;5:1052. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- (125).Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. Nature. 2004;430:694. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- (126).Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS. Biochem J. 2004;378:727. doi: 10.1042/BJ20031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Komander D, Barford D. Biochem J. 2008;409:77. doi: 10.1042/BJ20071399. [DOI] [PubMed] [Google Scholar]
- (128).Lin SC, Chung JY, Lamothe B, Rajashankar K, Lu M, Lo YC, Lam AY, Darnay BG, Wu H. J Mol Biol. 2008;376:526. doi: 10.1016/j.jmb.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Iha H, Peloponese JM, Verstrepen L, Zapart G, Ikeda F, Smith CD, Starost MF, Yedavalli V, Heyninck K, Dikic I, Beyaert R, Jeang KT. Embo J. 2008;27:629. doi: 10.1038/emboj.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Coornaert B, Carpentier I, Beyaert R. J Biol Chem. 2008 doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Yen B, Woo T, Malynn BA, Ma A. Nature. 2008 doi: 10.1038/nature07575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Lang V, Symons A, Watton SJ, Janzen J, Soneji Y, Beinke S, Howell S, Ley SC. Mol Cell Biol. 2004;24:5235. doi: 10.1128/MCB.24.12.5235-5248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Papoutsopoulou S, Symons A, Tharmalingham T, Belich MP, Kaiser F, Kioussis D, O'Garra A, Tybulewicz V, Ley SC. Nat Immunol. 2006;7:606. doi: 10.1038/ni1334. [DOI] [PubMed] [Google Scholar]
- (134).Verstrepen L, Adib-Conquy M, Kreike M, Carpentier I, Adrie C, Cavaillon JM, Beyaert R. J Cell Mol Med. 2008;12:316. doi: 10.1111/j.1582-4934.2007.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O'Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, Zhang Z, Arnott D, Dixit VM. Science. 2007;318:1628. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- (136).Hurley JH, Lee S, Prag G. Biochem J. 2006;399:361. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Hofmann RM, Pickart CM. J Biol Chem. 2001;276:27936. doi: 10.1074/jbc.M103378200. [DOI] [PubMed] [Google Scholar]
- (138).Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, King RW, Gygi SP. Nat Cell Biol. 2006;8:700. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- (139).Kaiser P, Flick K, Wittenberg C, Reed SI. Cell. 2000;102:303. doi: 10.1016/s0092-8674(00)00036-2. [DOI] [PubMed] [Google Scholar]
- (140).Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. Science. 1989;243:1576. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- (141).Spence J, Sadis S, Haas AL, Finley D. Mol Cell Biol. 1995;15:1265–73. doi: 10.1128/mcb.15.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Sun L, Chen ZJ. Curr Opin Cell Biol. 2004;16:119. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- (143).Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, Komuves L, French DM, Ferrando RE, Lam C, Compaan D, Yu C, Bosanac I, Hymowitz SG, Kelley RF, Dixit VM. Cell. 2008;134:668. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- (144).Huang TT, D'Andrea AD. Nat Rev Mol Cell Biol. 2006;7:323. doi: 10.1038/nrm1908. [DOI] [PubMed] [Google Scholar]
- (145).Harper JW, Elledge SJ. Mol Cell. 2007;28:739. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]