Abstract
Protection by mild hypothermia has previously been associated with better mitochondrial preservation and suppression of the intrinsic apoptotic pathway. It is also known that the brain may undergo apoptotic death via extrinsic, or receptor mediated pathways, such as that triggered by Fas/FasL. Male Sprague Dawley rats subjected to 2h middle cerebral artery occlusion with 2h intraischemic mild hypothermia (33C) were assayed for Fas, FasL and caspase-8 expression. Ischemia increased Fas, but decreased FasL by ~50–60% at 6 and 24h post insult. Mild hypothermia significantly reduced expression of Fas and processed caspase-8 both by ~50%, but prevented ischemia-induced FasL decreases. Fractionation revealed that soluble/shed FasL (sFasL) was decreased by hypothermia, while membrane-bound FasL (mFasL) increased. To more directly assess the significance of the Fas/FasL pathway in ischemic stroke, primary neuron cultures were exposed to oxygen glucose deprivation. Since FasL is cleaved by matrix metalloproteinases (MMPs), and mild hypothermia decreases MMP expression, treatment with a pan-MMP inhibitor also decreased sFasL. Thus, mild hypothermia is associated with reduced Fas expression and caspase-8 activation. Hypothermia prevented total FasL decreases, and most of it remained membrane bound. These findings reveal new observations regarding the effect of mild hypothermia on the Fas/FasL and MMP systems.
Keywords: apoptosis, hypothermia, cerebral ischemia, matrix metalloproteinases, Fas/FasL, stroke
Introduction
Hypothermia has long been known to be a potent neuroprotectant. Experimental studies (Krieger and Yenari 2004) and now clinical trials (Bernard et al. 2002; Hypothermia after Cardiac Arrest Study Group 2002) have shown that hypothermia protects the brain from cerebral ischemic injury. Lowering body temperature is known to slow metabolism, prevent high-energy phosphate depletion and prevent accumulation of excitotoxins such as glutamate. However, even small decreases in body temperature are associated with a marked reduction in ischemic injury, and do not correspond to the expected Q10 for this protective effect (Yenari et al. 2004). This would suggest that other processes may be involved in the observed phenomenon. Work from our lab and others has shown that protection by mild hypothermia is associated with the inhibition of apoptosis via the intrinsic or mitochondria dependent pathway (Inamasu et al. 2000; Prakasa Babu et al. 2000; Zhang et al. 2001; Xu et al. 2002; Yenari et al. 2002; Zhao et al. 2005).
Recent work has also shown that extrinsic, or receptor mediated apoptosis pathways are involved in stroke pathogenesis (Rosenbaum et al. 2000). Death receptors are cell surface transmembrane proteins belonging to the tumor necrosis factor receptor superfamily, which includes Fas (also referred to as CD95 or APO-1), one of the most studied death receptors. Fas is a member of the tumor necrosis factor receptor superfamily, acting as a trigger for apoptosis (Nagata and Golstein 1995). Its ligand, FasL, is a 40 kD type II transmembrane protein. Death receptor-mediated apoptosis is a critical component of neuronal death in the developing and adult mammalian nervous system, and Fas activation is a recurrent feature of acute and chronic neuropathologies, including traumatic brain injury, multiple sclerosis, and Alzheimer’s disease (D’Souza et al. 1996; Morishima et al. 2001; Qiu et al. 2002). Binding of Fas ligand (FasL) to Fas initiates intracellular signaling cascades that ultimately terminate in caspase-dependent cell death.
Fas also appears to play an important role in brain damage following focal cerebral ischemia. Expression of Fas and its ligand have been documented in the brain following ischemia (Padosch et al. 2003), and neutralizing FasL with antibody treatment is associated with neuroprotection in experimental stroke models (Martin-Villalba et al. 2001; Blankenberg et al. 2006). Furthermore, Lpr mice containing dysfunctional Fas, have smaller infarcts than wildtype mice (Martin-Villalba et al. 1999; Rosenbaum et al. 2000).
Matrix metalloproteinases (MMPs) can modulate Fas activation by causing proteolytic shedding of FasL from the cell surface (Powell et al. 1999; Matsuno et al. 2001; Mitsiades et al. 2001). Thus, shed or soluble Fas ligand (sFasL) is then liberated to bind to its receptor. Prior work has shown that therapeutic hypothermia decreases MMP expression in stroke (Wagner et al. 2003; Hamann et al. 2004; Lee et al. 2005).
In this study, we explored the effects of mild hypothermia on the extrinsic Fas-mediated apoptosis pathway. We found that mild hypothermia suppressed expression of Fas and activation of caspase-8, but increased FasL expression. However, FasL was largely membrane bound under hypothermic conditions, probably due to decreased cleavage by MMPs.
Materials and Methods
Animal model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Brain ischemia was induced using an intraluminal suture to occlude the middle cerebral artery. Briefly, a total of 46 male Sprague-Dawley rats (22 maintained at normothermia, 22 exposed to hypothermia and 2 used as shams) weighing between 250–300g were anesthetized with 3–4% halothane in 30% oxygen and 70% nitrous oxide using a face mask for induction, and maintained at 1–1.5%. The right femoral artery was cannulated to record blood pressure and to obtain arterial blood samples. After a midline skin incision was made, the right external carotid artery was exposed, and its branches were electrocoagulated. A 3-0 nylon monofilament coated with silicon was introduced into the right internal carotid artery through the external carotid artery to occlude the origin of the middle cerebral artery. After 2 h of arterial occlusion, blood flow was restored by withdrawal of the nylon suture. Sham control animals were subjected to similar operations to expose the carotid arteries without occlusion of the middle cerebral artery. Physiological parameters were monitored and kept in the normal range. During surgery, each rat’s rectal temperature was kept between 37 and 38°C, which corresponds to a brain temperature between 38 and 39°C (Yenari et al. 2000). Mild hypothermia, defined as a rectal temperature of 32°C, which corresponds to a brain temperature of 33°C, was induced and maintained in a manner previously described (Yenari et al. 2000). This paradigm has consistently shown consistent neuroprotection in our hands (Yenari et al. 2000; Maier et al. 2001). The animals were cooled by placing the animal on a cooling blanket and spraying ethanol onto the body at the time of ischemia onset (intraischemic hypothermia), and hypothermia was maintained for 2 h. Rewarming took place by placing the animal under a lamp and on a heating blanket.
Preparation of tissue extracts
At 2, 6, and 24 h after the onset of ischemia, rats were deeply anesthetized with halothane and then transcardially perfused with ice-cold phosphate buffered solution (PBS), pH 7.4. The brains were removed quickly and divided into ipsilateral and contralateral hemispheres. Based on methods outlined in our prior studies, 1–3 mg samples of ischemic tissue from the ipsilateral striata (SI), adjacent cortex in the middle of the infarction (CI) and the periphery of the infarct at its upper medial border (PI), plus corresponding tissue from the contralateral hemisphere was dissected, frozen immediately on dry ice, and stored at −80C (Yenari et al. 2002). Brain samples were homogenized in lysis buffer with protease inhibitors on ice. Brain samples from non-ischemic animals were also prepared. After centrifugation, supernatant was collected, and total protein concentrations were determined using the Bradford assay (Bio-Rad, Hercules, CA).
To detect soluble/shed and membrane-bound Fas ligand, brain samples were prepared using previously published protocols (Bright et al. 2004). Tissue was homogenized in lysis buffer containing 0.5 M EDTA, 1M Tris pH7.5, 0.1M EGTA, 855 mg sucrose plus protease inhibitors. The homogenate was then centrifuged at 100,000 G for 30 min at 4C. The supernatants (soluble fraction) were collected and the pellets were resuspended in lysis buffer plus 0.1% triton and spun again after sitting on ice for 20 min. The second supernatant was collected and used as the particulate (membrane containing) fraction. 10 μg protein were loaded per lane, and subjected to sodium dodecyl sulfate polyacrylamidegel electrophoresis and Western blot analysis.
Western blot analysis
Fas, FasL and caspase-8 protein expression was estimated in hypothermic and normothermic brains after ischemic injury using Western blots. Total tissue extracts were prepared at various times after ischemic injury using methods familiar to us (Yenari et al. 2002; Lee et al. 2005). In all cases, equal amounts of protein (30 μg/lane) were subjected to electrophoresis in 12.5% polyacrylamide gels, and transferred to nitrocellulose membranes (Millipore, Bedford, Massachusetts, U.S.A.). After 2 h of blocking, membranes were incubated in primary antibodies against Fas (1:500, rabbit polyclonal antibody, C-20, Santa Cruz Biotechnology, Inc., CA, USA), FasL (1:500, rabbit polyclonal antibody, N-29, Santa Cruz) or caspase-8 (1:1000, rabbit polyclonal antibody, AAP-103, StressGen, Victoria, BC Canada) followed by appropriate secondary antibodies. Finally, antigen was detected by using the standard chemical luminescence method (ECL; Amersham Pharmacia Biotech). The membranes were stripped, then reprobed with monoclonal anti-β-actin antibody (1:1000, A-5441, Sigma) or G protein alpha inhibiting 3 (Gαi3, 1:1000, SC-262, Santa Cruz)and developed as above. Densitometric measurements were made from the protein bands using Gelscope (Imageline, Gardena, CA).
Histology and Immunostaining
2, 6 and 24 h after the onset of ischemia, brains were perfused in normal saline followed by 3% paraformaldehyde, then sunk in sucrose. Tissue sections were cryosectioned in the coronal plane into 25 μm thick slices, rinsed, blocked, and incubated overnight at 4C with the primary antibodies (1:200 dilution, Fas, FasL and caspase-8 as above) followed by the secondary antibody (Vector Labs, Burlingame, CA). Antibodies were detected using the Elite Vectastain ABC kit (Vector Labs) and colorized with diaminobenzidine (DAB, DAKO Corporation, Carpenteria, CA). Sections were counter stained with hematoxylin and eosin (H & E), cleared and mounted in permount. H & E stained sections from brains of animals survived 24 h were also analyzed for infarct size using methods previously published, including correction for the presence of edema (Maier et al. 1998; Yenari et al. 2000).
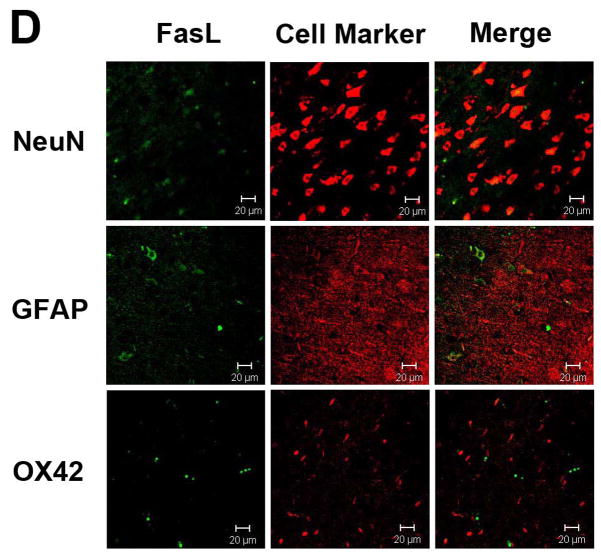
To determine the source of Fas and FasL production, double labeling for cell type markers was performed. Tissue sections were treated with proteinase K (1:2 dilution, Dako Corp.) for 15 minutes then washed in PBS. The sections were blocked in PBS containing 5% normal serum, 1% bovine serum albumin, and 0.3% Triton X-100 for 1 h at room temperature, after which they were incubated in primary antibodies diluted in blocking solution at 4°C overnight. Tissue sections were washed with PBS and incubated for 2 h at room temperature (light shielded) in secondary antibodies diluted in blocking solution. Slides were covered and the tissue was examined with the aid of a fluorescence microscope (Carl Zeiss, Switzerland). For double labeling, primary murine antibodies used to identify neurons (NeuN; 1:200, cat. # MAB377, dilution; Chemicon Corp, Billerica, MA), astrocytes (GFAP, 1:100, cat. # 556330, Pharmingen, San Diego, CA), or macrophages/microglia (OX42, 1:1000 dilution; AbCAM, Cambridge, MA) were combined with the above described primary antibodies against Fas or FasL. The secondary antibodies were FITC (fluorescein isothiocyanate)-conjugated goat anti rabbit IgG (1:400 dilution, Jackson ImmunoResearch, West Grove, PA), and Cy3-conjugated anti–mouse IgG (1:100 dilution; Jackson ImmunoResearch).
Primary neuronal cultures
To further determine the effects of hypothermia on FasL and its regulation, primary neuronal cultures were established from cerebral cortices of C57BL/6 embryos at gestation day 15 (Harlan, Indianapolis, IN), using enzymatic dissociation with trypsin as previously described (Xu et al. 2002). Timed pregnant females were sacrificed by halothane overdose and the embryos were removed by cesarean section. 4.8 × 105 cells were plated on poly-L-lysine-coated coverslips in 24 well plates. Cultures were maintained in Neurobasal media (Invitrogen Corp., Carlsbad, CA) supplemented with 2% B-27, 0.5 mM glutamine (Sigma, St. Louis, MO), 25 μM glutamate (Sigma), 100 units/ml penicillin, and 100μg/ml streptomycin. At 4 d in vitro (DIV) half of the media was removed and replaced with fresh medium without glutamate. Cultures were maintained in a humidified incubator at 37C with 5% CO2. At 7 DIV cultures were used for experimentation. The cortical cultures were primarily comprised of >95% neurons as identified by NeuN immunostaining.
Oxygen and glucose deprivation (OGD)
Cultures were subjected to OGD in balanced salt solution containing no glucose (BSS0) in an anoxic chamber (Coy Laboratories, MI) with O2 tension <0.02%. Cultures were subjected to OGD for 3 h. Upon removal from the anoxia chamber, 5.5 mM glucose was added and the cultures returned to the normoxic incubator (i.e., reperfusion) for 24 h. Hypothermic cultures were exposed to both OGD and reperfusion at 33C, while normothermic cultures were exposed at 37C. Cell death was quantified by assay of released lactate dehydrogenase (LDH) expressed as a percent of the total LDH released after freeze-thaw (=100%). Control cultures were incubated at normoxia in balanced salt solution containing 5.5 mM glucose (BSS5.5). To some cultures, GM6001, an MMP inhibitor (50 nM, Sigma) was added to the media at the onset of injury.
Statistical analysis
All data are presented as mean ± SD. Standard statistical tests were used to determine differences between groups using SigmaStat (Jandel Corp., San Rafael, CA). The Student’s t-test or ANOVA were used in the case of continuous data (e.g., infarct size), and Mann-Whitney for non-continuous data (semi-quantitative scores). A level of P < 0.05 was considered statistically significant.
Results
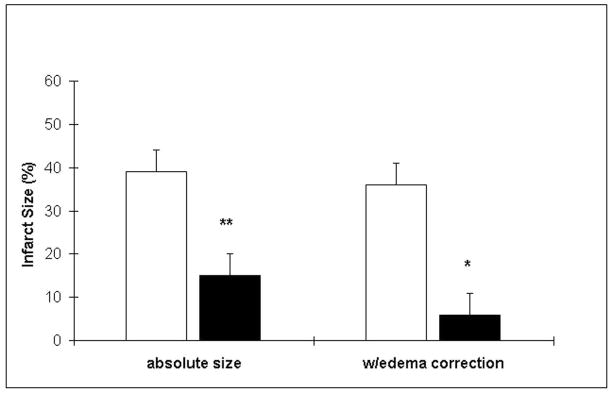
Mild hypothermia decreased infarct size by ~60% (Fig. 1). This was not associated with any significant differences in physiological parameters except temperature. Parameters monitored included (normothermia, then hypothermia data given): rectal temperature (37±0.1C, 32.5±0.1C), mean arterial blood pressure (92±2, 94±4 mmHg), heart rate (335± 5, 315±5), respiratory rate (54±6, 45±7), pH (7.32±0.03, 7.32±0.01), pO2 (100±5, 114±4), and pCO2 (47±2, 49±2).
Figure 1.
Mild hypothermia reduces infarct size 24h after ischemia onset. Infarct size, measured from H & E stained sections, was reduced among animals exposed to mild hypothermia (expressed as the % injury of the ipsilateral hemisphere). Data are shown with and without correction for edema. (*P<0.05, **P<0.01, white bars=normothermia, black bars=mild hypothermia, n=6/group).
Mild hypothermia reduces caspase-8 and Fas expression, but increases FasL
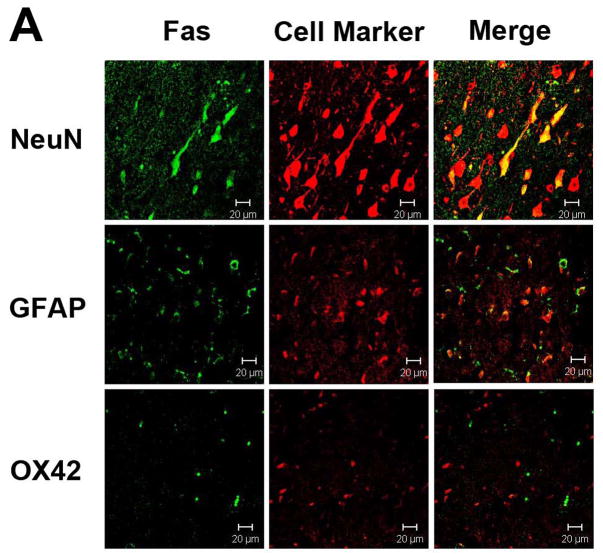
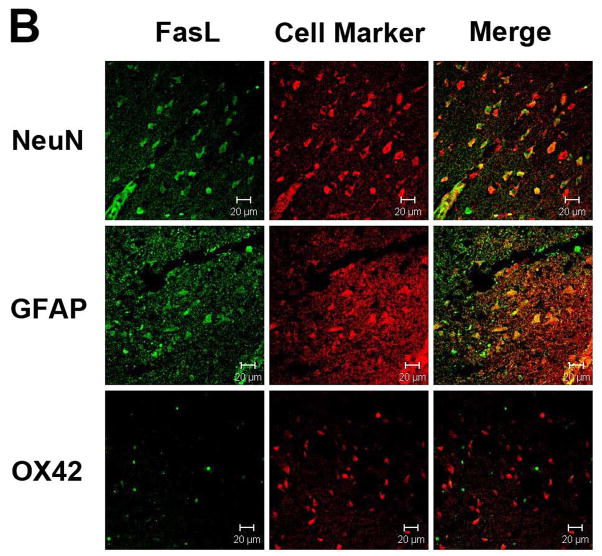
Immunostaining for Fas and FasL revealed that both proteins were expressed largely within neurons and astrocytes, and were rarely seen in microglia (Figs. 2A & B). Ischemia led to increases in neuronal and astrocytic Fas, and decreases in FasL (Figs. 2C & D). Fas and FasL was primarily seen within the ischemic cortex, with some staining observed in the ischemic striatum. Hypothermia decreased Fas and increased FasL staining compared to normothermia (Fig. 3). However, the types cell which expressed these respective proteins were similar. Since the antibody used to identify caspase-8 does not differentiate between pro- and processed caspase-8, immunostains did not show significant differences in staining patterns following ischemia, or following treatment with mild hypothermia (not shown).
Figure 2.
Fas and FasL expression in the brain. Double immunolabeling of the cortex of uninjured rats shows that both Fas (A) and FasL (B) are present within neurons and astrocytes, but not microglia. Following 24 h ischemia, Fas (C) expression increases in both neurons and astrocytes, with rare expression in microglia, whereas FasL (D) decreases in neurons and astrocytes. Brain regions within the ipsilateral ischemic cortex are shown. (Neun=neuronal marker, GFAP=glial fibrillary acid protein to identify astrocytes, OX42=binds to CD11b to identify microglial/monocytes; scale bar = 20 μm)
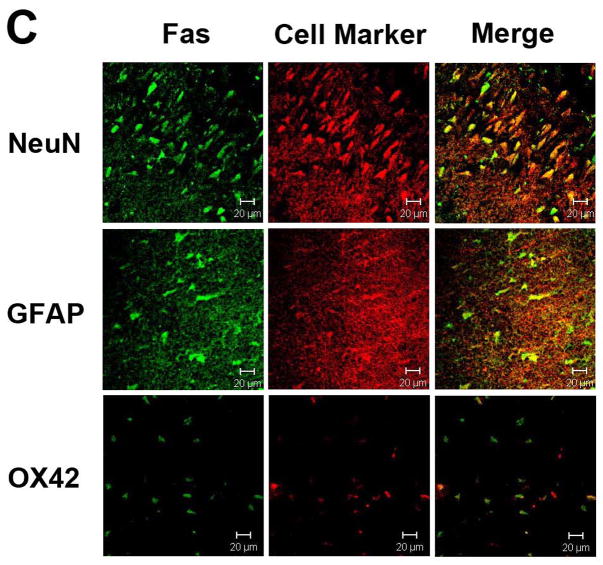
Figure 3.
Immunohistochemical stains show that within brain exposed to 2 h ischemia and 22 h reperfusion, mild hypothermia reduces Fas expression (A), but increases expression of FasL (B). Representative images are taken from within the ischemic striatum (A) and cortex (B). (scale bar = 50 μm)
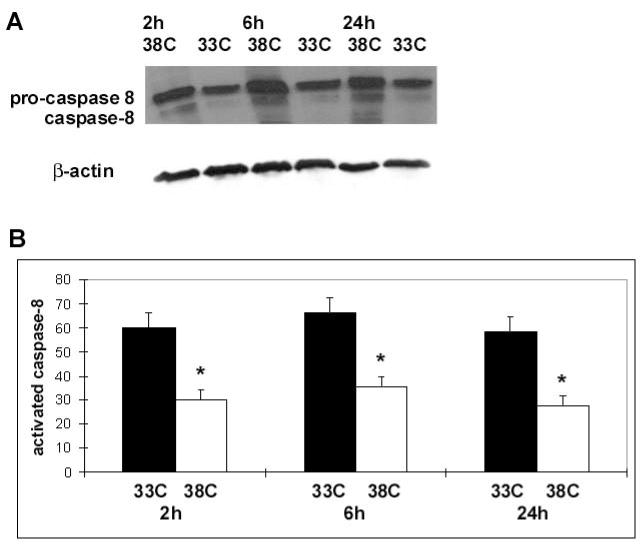
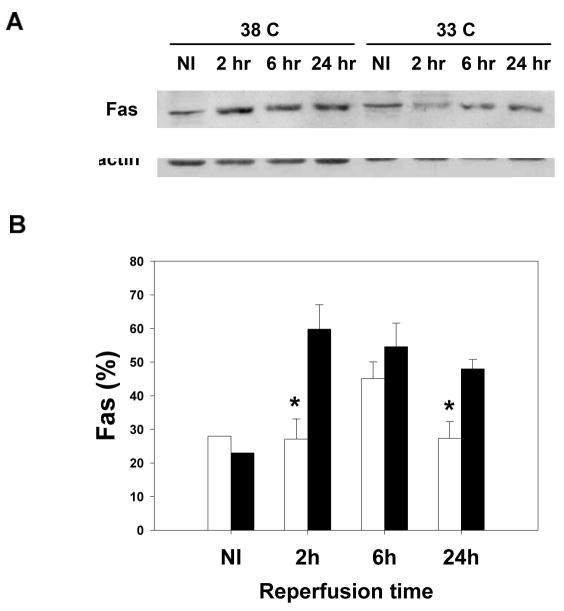
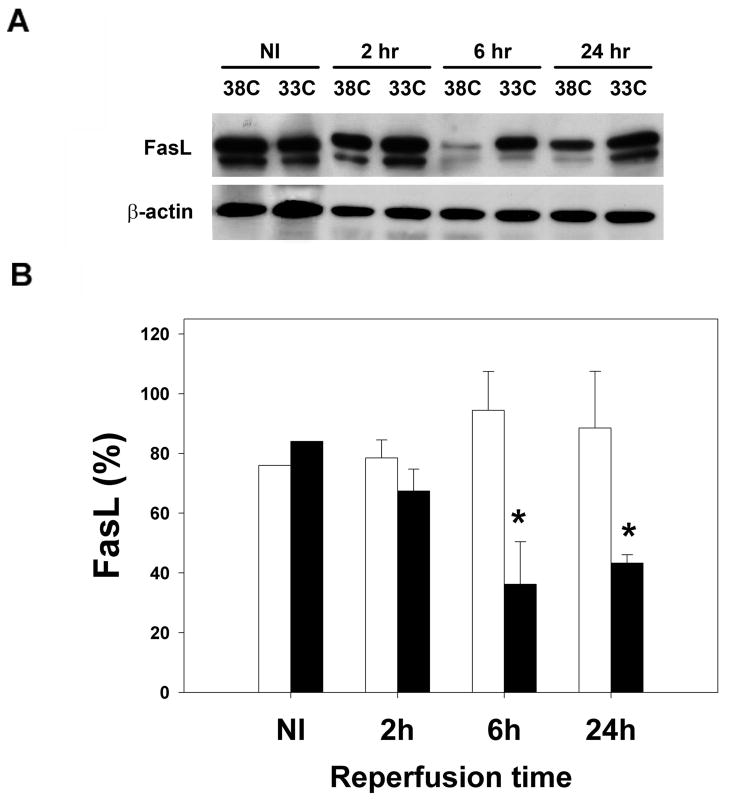
Protein levels of caspase-8, Fas, and FasL taken at various times from the ipsilateral ischemic cortex (CI) were quantified using Western blots. The total amount of caspase-8 protein was no different between hypothermic brains and normothermic brains after ischemic injury, but the amount of processed caspase-8 was lower in hypothermic brains (Fig. 4). Ischemia at normal temperature increased Fas, and hypothermia decreased Fas 2 and 24 h post ischemia, with trends observed at 6 h (Fig 5). In contrast, ischemia at normal temperature led to decreased FasL, while hypothermia prevented this FasL decrease, especially 6 and 24h post ischemia (Fig 6).
Figure 4.
Mild hypothermia reduces expression of processed caspase-8: A representative Western blot (A) of the ipsilateral ischemic cortex (CI, defined in the text) shows decreased processed caspase-8 (lower band) in brains of animals treated with mild hypothermia (33C) compared to normothermia (38C). Pro-caspase-8 expression (upper band) was not changed by mild hypothermia. Relative optical densities (ROD) of processed caspase-8 were lower in ischemic brains of animals exposed to mild hypothermia (white bars) compared to normothermia (black bars). Data are shown as the ROD of processed (activated) caspase-8 normalized to the actin ROD, used as a loading control (*P<0.05, n=3–4/ bar) (B).
Figure 5.
Mild hypothermia reduces Fas expression. A Western blot (A) of non ischemic brain (NI) and brain from the ipsilateral ischemic cortex (CI, defined in the text) shows increased Fas expression following ischemia at normothermia (38C). In animals exposed to mild hypothermia (33C), Fas expression decreased compared to normothermia (38C). Relative optical densities of Fas were lower in ischemic brains of animals exposed to mild hypothermia. Data are shown as the relative optical density (ROD) of Fas normalized to the β-actin ROD, used as a loading control (*P<0.05, white bars=hypothermia, black bars=normothermia, n=3–4/ bar) (B).
Figure 6.
Mild hypothermia prevents decreased expression of FasL. A representative Western blot (A) of non ischemic brain (NI) and brain from the ipsilateral ischemic cortex (CI, defined in the text) at normothermia (38C) shows decreased FasL (lower band) in ischemic brains. FasL in brains of animals treated with mild hypothermia (33C) does not decrease following ischemia. Relative optical densities (ROD) of FasL were lower in ischemic brains of animals exposed to mild hypothermia compared to normothermic ischemic brains. Data are shown as the ROD of FasL normalized to the actin ROD, used as a loading control (*P<0.05, white bars=hypothermia, black bars=normothermia, n=3–4/ bar) (B).
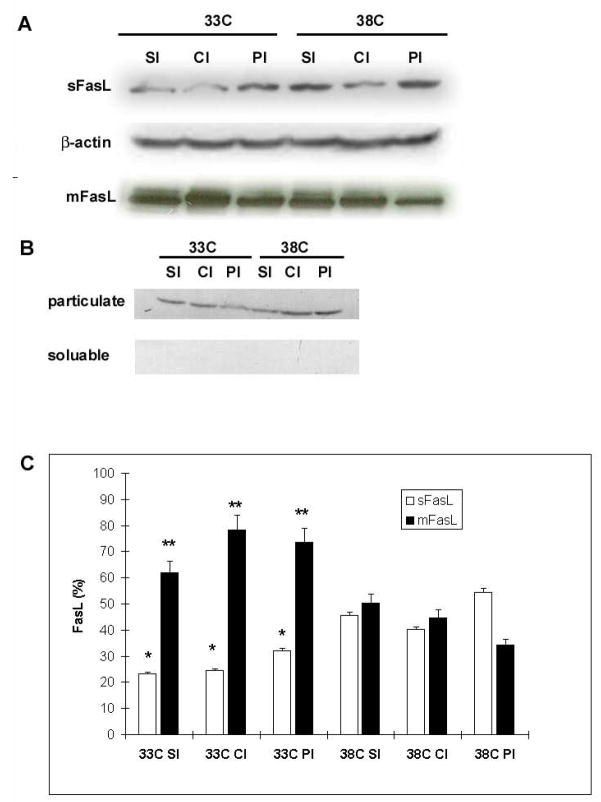
FasL remains largely membrane bound in hypothermic brains after ischemic injury compared to normothermia
FasL is normally bound to membrane surfaces, but is shed through cleavage by proteases such as the MMPs, thus producing soluble/shed FasL (sFasL). To explore the effect of hypothermia on sFasL versus membrane bound FasL (mFasL), we performed Western blots of particulate (membrane) and soluble fractions from ischemic brain samples. Samples were taken from the ipsilateral ischemic striatum (SI), ischemic cortex (CI) and infarct borderzone (PI). Not only did mild hypothermia lead to higher total levels of FasL, but most of the FasL was membrane bound, and mFasL levels were greater in hypothermic brains than in normothermic brains (Fig. 7). Soluble/shed FasL levels, in contrast, were lower in hypothermic brains.
Figure 7.
FasL remains mostly membrane bound under conditions of mild hypothermia. Western blots of particulate (mFasL) and soluble (sFasL) ischemic brain tissue fractions (24 h after ischemia onset) revealed less soluble and more membrane bound FasL among hypothermic brains compared to normothermic brains (A). A Western blot of housekeeping protein Gαi3, found only in cell membranes, shows successful separation of the two fractions (B). Mild hypothermia (33C) reduced the amount of sFasL, but increased the amount of m FasL (*P<0.05 vs. corresponding normothermic samples, **P<0.01 vs. corresponding normothermic samples, n-3-4 samples/bar). SI=ischemic striatum, CI=ischemic cortex, PI=peri-infarct cortex (C)
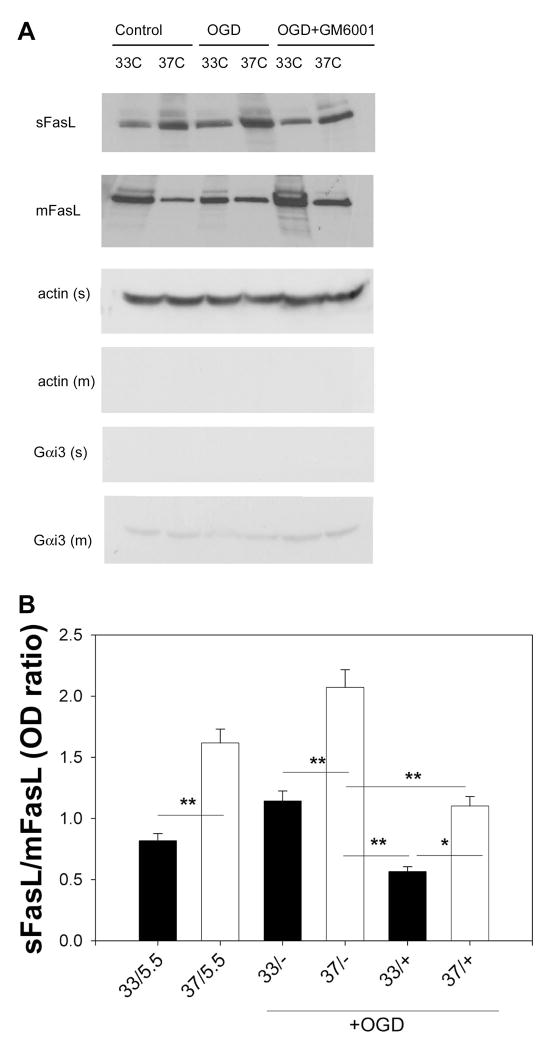
FasL remains membrane bound due to hypothermic suppression of MMPs
Because we found that FasL was expressed in neurons, we studied primary neuronal cultures to explore the mechanism of reduced FasL shedding by hypothermia. Since we previously showed that mild hypothermia was associated with suppression of MMP expression and function, we determined whether the decreased sFasL observed in hypothermic brains was due to decreased shedding of membrane bounded FasL by MMPs. Primary cultured neurons were exposed to OGD under hypothermic conditions. Some cultures were treated with GM6001, a pan-MMP inhibitor. Hypothermia led to increased mFasL levels, while decreasing sFasL compared to normothermia, consistent with our in vivo observations (Figure 8). This was the case even in cultures not exposed to OGD. Normothermic neuron cultures treated with GM6001 and subjected to OGD had less sFasL, and slightly more mFasL compared to untreated normothermic cultures exposed to OGD. The combination of hypothermia and GM6001 led to further increases in mFasL and decreases in sFasL. This would be consistent with the notion that hypothermia prevents cleavage of FasL, in part, by suppression of MMPs.
Figure 8.
Mild hypothermia prevents FasL cleavage by inhibiting MMPs. Cultured neurons were exposed to oxygen glucose deprivation (OGD) under normothermic (37C) or mild hypothermic (33C) conditions. Some cultures were treated with the pan-MMP inhibitor GM6001. Control cultures were kept in balanced salt solution containing 5.5 mM glucose (5.5). A. Western blots of control or OGD exposed cultures of the soluble fraction (sFasL) showed less sFasL under conditions of mild hypothermia, but more membrane bound FasL (mFasL). Cultures treated with GM6001 at 37C were no different from cultures treated at 33C. Cultures exposed to OGD at 33C and treated with inhibitor had further increases in mFasL and decreases in sFasL. B. Western blot data are shown as the ratio of relative optical densities of the soluble to membrane fractions. Results are shown from 3 experiments performed in triplicate from cells prepared from 3 separate dissections. (33, 37=culture temperature; 5.5=control wash; +=GM6001; +=no GM6001; *P<0.05, **P<0.001)
Discussion
Mild hypothermia has been shown to be an effective treatment against cerebral ischemic injury by numerous groups. Lowered body temperatures are thought to preserve metabolic stores and are associated with decreased accumulation of excitatory amino acids, but this does not fully explain the remarkably robust effects of cooling (Liu and Yenari 2007). A few studies have already shown that mild hypothermia suppresses ischemia-induced apoptosis as evidenced by fewer apoptotic/apoptotic-like cells and biochemical events largely associated with the intrinsic, or mitochondrial pathway (Maier et al. 1998; Inamasu et al. 2000; Prakasa Babu et al. 2000; Zhang et al. 2001; Zhao et al. 2005). Hypothermia can also directly reduce apoptotic death, as a study of serum deprivation in cultured neurons led to reduced cell death by hypothermia through the mitochondrial pathway (Xu et al. 2002). Hypothermia also renders cells more resistant to apoptotic stimuli including Fas ligation (Fu et al. 2004; Sakurai et al. 2005). In one study of hepatocytes, decreasing culture temperature from 37C to 32C suppressed Fas-mediated apoptosis and led to less cytochrome c release and activation of caspases-7 and 9. We show here that protection by hypothermia is associated with decreased Fas and processed caspase-8, and the prevention of FasL decreases.
Immunostaining showed that both Fas and FasL colocalized primarily to neurons and astrocytes rather than microglia. This is in line with our previous observations that caspase-8 is also observed primarily in neurons (Blankenberg et al. 2006). Ischemia led to increased Fas, and decreased FasL expression within the same types of cells. A prior study documented increases in FasL staining following focal cerebral ischemia, which is in contrast to the findings here (Martin-Villalba et al. 1999). The reason for this difference is not clear, but we should point out that the duration of occlusion in our study was somewhat longer than that of the prior study (2 h compared to 90 min), and might be consistent with some reports that some apoptosis related proteins are not present in some models of brain ischemia (Gill et al. 2002). Furthermore, we sampled brain tissue from the dorsal aspect of the ischemic lesion, whereas the prior studied sampled tissue from the ventral aspect in the piriform cortex.
We also found that hypothermia prevented decreases in overall FasL expression, and FasL was mostly membrane bound. Soluble/shed FasL was still lower in the hypothermia group. This observation is probably due to hypothermia-induced suppression of MMPs, which has previously been demonstrated by our group and others (Wagner et al. 2003; Hamann et al. 2004; Lee et al. 2005). Thus, we show that hypothermia prevents MMP-induced FasL shedding and reduces Fas expression and caspase-8 activation. Since neurons and astrocytes expressed both Fas and FasL, it is possible that FasL binds neuronal and glial Fas in an autocrine and/or paracrine fashion in the models studied here. Although rare microglia also expressed FasL, it seems less likely that microglia-generated sFasL contributed to Fas stimulation. We also observed overall decreases in Fas by mild hypothermia which would provide fewer receptors for sFasL to bind to. The reduction in Fas is probably not due to decreased sFasL, but decreased activation of nuclear factor kappa B (NFkB) by hypothermia (Han et al. 2003; Yenari and Han 2006) since NFkB is known to regulate FasL expression (Chan et al. 1999).
At present, few data are available regarding the role of Fas and FasL after cerebral ischemia. In models of cardiac arrest, Fas and FasL were expressed in the cortex and hippocampus after 6 h and 12 h after ischemia, with peak expression at 24–48 h (Padosch et al. 2003). Although Fas/FasL were originally characterized in the immune system, we found that non-immune cells express Fas and FasL. This is also consistent with prior reports (Rosenbaum et al. 2000; Qiu et al. 2002). Prior studies have suggested a detrimental effect of the Fas signaling pathway in experimental stroke, as treatment with Fas antibody or studying mice deficient in Fas led to less ischemic injury (Martin-Villalba et al. 1999; Martin-Villalba et al. 2001; Blankenberg et al. 2006), while overexpression of FasL led to increased apoptosis in a model of traumatic brain injury (Qiu et al. 2002).
Consistent with our findings presented here, a prior study in a similar stroke model showed that mild hypothermia was associated with decreased Fas and caspase-3 immunostaining (Phanithi et al. 2000). However, this latter study did not assess the influence of hypothermia on FasL or caspase-8. The present study further demonstrates that while hypothermia prevents the loss of FasL, most FasL remained membrane-bound. These observations could be linked to hypothermia’s ability to inhibit MMPs (Wagner et al. 2003; Hamann et al. 2004; Lee et al. 2005), proteases involved not only in disrupting the extracellular matrix, but also involved in cleaving and thus solubilizing FasL (Mitsiades et al. 2001). We show that inhibiting MMPs in our in vitro model led to decreased sFasL and increased mFasL, which was similar to that seen in cultures exposed to hypothermia. Interestingly, combining MMP inhibition with hypothermia led to even further decreases in sFasL. The reasons for this latter observation are not clear, but indicate synergy between the two interventions and may have translational relevance. Our findings also demonstrate the broad impact hypothermia has on various cell signaling systems.
The finding that mild hypothermia reduces sFasL is curious, as this might be consistent with a pro-apoptotic, pro-death role of sFasL. There are conflicting data as to whether FasL shedding leads to an active protein or not. FasL shedding, mediated by MMP-7 or -3 (Cunningham et al. 2005), has been thought to actually inactivate FasL thus preventing effective binding of Fas. Work in the cancer and immunology literature indicates that cleavage of FasL leads to increased resistance of tumor cells to chemotherapeutic agents (Mitsiades et al. 2001) and apoptosis (Knox et al. 2003). Increased levels of the MMP-3 inhibitor TIMP (tissue inhibitor of metalloproteinase)-3 were detected in brain regions with the most severe ischemia (Wallace et al. 2002). Inhibition of TIMP-3 has also been shown to prevent doxorubicin-induced apoptosis of neurons, presumably by allowing MMP-3 mediated FasL cleavage (Wetzel et al. 2003), and TIMP-3 deficiency led to decreased apoptosis due to brain ischemia in mice (Wetzel et al. 2008). Yet, in the work shown here, less apoptosis/apoptosis-like cell death was seen under hypothermic conditions, and this was associated with decreased FasL shedding. Active sFasL has been detected in some systems. Powell and colleagues (Powell et al. 1999) showed that MMP-7-generated sFasL caused apoptosis in prostate epithelial cells. The differences in the interpretation and significance of soluble FasL are not clear, but could due to inherent differences in the models and organ systems studied. It is also possible that different MMPs may cleave FasL at different sites so as to produce biologically different proteins.
In summary, we show that mild hypothermia alters expression and processing of proteins of the extrinsic Fas/FasL apoptotic pathway, and builds on prior observations that hypothermia decreases MMP activation. While largely thought to affect down regulation of cellular metabolism, we demonstrate the impact of hypothermia on other signaling pathways as well.
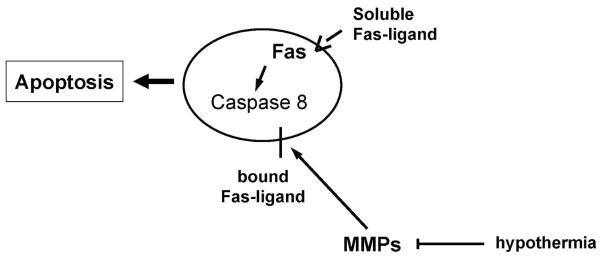
Figure 9.
Proposed FasL/ MMP interactions in ischemia/ischemia-like insults and modulation by mild hypothermia. Activated matrix metalloproteinases (MMPs) are capable of cleaving membrane bound FasL, and generating soluble FasL. Soluble FasL can then bind its receptor (Fas) and trigger apoptosis through the extrinsic, or receptor mediated pathway by activating caspase-8. Mild hypothermia appears to inhibit this pathway by inhibiting MMPs.
Acknowledgments
The authors would like to thank Dr. Guo Hua Sun for expert technical assistance. This work was supported by NIH grants R01 NS40516 (MAY), R01 NS27292 (GKS), P01 NS37520 (GKS, MAY), AHA Established Investigator Award #0540066N (MAY) and KOSEF grant (R01-2004-000-10671-0) funded by MOST (JEL).
Abbreviations
- MMP
matrix metalloproteinase
- sFasL
soluable Fas ligand
- mFasL
membrane bound Fas ligand
- PBS
phosphate buffered solution
- FITC
fluorescein isothiocyanate
- DIV
days in vitro
- OGD
oxygen glucose deprivation
- Bss0
balanced salt solution
- BSS5.5
balanced salt solution containing 5.5 mM glucose
- LDH
lactate dehydrogenase
- NKkB
nuclear factor kappa B
- TIMP
tissue inhibitor of metalloproteinase
- ROD
relative optical density
- NI
non ischemic brain
- SI
ischemic striatum
- CI
ischemic cortex
- PI
ischemic peri-infarct region
References
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Blankenberg FG, Kalinyak J, Liu L, Koike M, Cheng D, Goris ML, Green A, Vanderheyden JL, Tong DC, Yenari MA. 99mTc-HYNIC-annexin V SPECT imaging of acute stroke and its response to neuroprotective therapy with anti-Fas ligand antibody. Eur J Nucl Med Mol Imaging. 2006;33:566–574. doi: 10.1007/s00259-005-0046-6. [DOI] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H, Bartos DP, Owen-Schaub LB. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50-p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Blei AT, Takamura N, Lin T, Guo D, Li H, O’Gorman MR, Soriano HE. Hypothermia inhibits Fas-mediated apoptosis of primary mouse hepatocytes in culture. Cell Transplant. 2004;13:667–676. doi: 10.3727/000000004783983495. [DOI] [PubMed] [Google Scholar]
- Gill R, Soriano M, Blomgren K, Hagberg H, Wybrecht R, Miss MT, Hoefer S, Adam G, Niederhauser O, Kemp JA, Loetscher H. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J Cereb Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, DeGeorgia M, Krieger DW. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. 2004;35:764–769. doi: 10.1161/01.STR.0000116866.60794.21. [DOI] [PubMed] [Google Scholar]
- Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T. Postischemic hypothermia attenuates apoptotic cell death in transient focal ischemia in rats. Acta Neurochir Suppl. 2000;76:525–527. doi: 10.1007/978-3-7091-6346-7_110. [DOI] [PubMed] [Google Scholar]
- Knox PG, Milner AE, Green NK, Eliopoulos AG, Young LS. Inhibition of metalloproteinase cleavage enhances the cytotoxicity of Fas ligand. J Immunol. 2003;170:677–685. doi: 10.4049/jimmunol.170.2.677. [DOI] [PubMed] [Google Scholar]
- Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35:1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005;103:289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:679–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, Schenkel J, Herdegen T, Debatin KM. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–3817. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22–28. [PubMed] [Google Scholar]
- Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Padosch SA, Popp E, Vogel P, Bottiger BW. Altered protein expression levels of Fas/CD95 and Fas ligand in differentially vulnerable brain areas in rats after global cerebral ischemia. Neurosci Lett. 2003;338:247–251. doi: 10.1016/s0304-3940(02)01408-8. [DOI] [PubMed] [Google Scholar]
- Phanithi PB, Yoshida Y, Santana A, Su M, Kawamura S, Yasui N. Mild hypothermia mitigates post-ischemic neuronal death following focal cerebral ischemia in rat brain: immunohistochemical study of Fas, caspase-3 and TUNEL. Neuropathology. 2000;20:273–282. doi: 10.1046/j.1440-1789.2000.00346.x. [DOI] [PubMed] [Google Scholar]
- Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- Prakasa Babu P, Yoshida Y, Su M, Segura M, Kawamura S, Yasui N. Immunohistochemical expression of Bcl-2, Bax and cytochrome c following focal cerebral ischemia and effect of hypothermia in rat. Neurosci Lett. 2000;291:196–200. doi: 10.1016/s0304-3940(00)01404-x. [DOI] [PubMed] [Google Scholar]
- Qiu J, Whalen MJ, Lowenstein P, Fiskum G, Fahy B, Darwish R, Aarabi B, Yuan J, Moskowitz MA. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J Neurosci. 2002;22:3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Gupta G, D’Amore J, Singh M, Weidenheim K, Zhang H, Kessler JA. Fas (CD95/APO-1) plays a role in the pathophysiology of focal cerebral ischemia. J Neurosci Res. 2000;61:686–692. doi: 10.1002/1097-4547(20000915)61:6<686::AID-JNR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Itoh K, Liu Y, Higashitsuji H, Sumitomo Y, Sakamaki K, Fujita J. Low temperature protects mammalian cells from apoptosis initiated by various stimuli in vitro. Exp Cell Res. 2005;309:264–272. doi: 10.1016/j.yexcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wagner S, Nagel S, Kluge B, Schwab S, Heiland S, Koziol J, Gardner H, Hacke W. Topographically graded postischemic presence of metalloproteinases is inhibited by hypothermia. Brain Res. 2003;984:63–75. doi: 10.1016/s0006-8993(03)03088-9. [DOI] [PubMed] [Google Scholar]
- Wallace JA, Alexander S, Estrada EY, Hines C, Cunningham LA, Rosenberg GA. Tissue inhibitor of metalloproteinase-3 is associated with neuronal death in reperfusion injury. J Cereb Blood Flow Metab. 2002;22:1303–1310. doi: 10.1097/01.WCB.0000040943.89393.c1. [DOI] [PubMed] [Google Scholar]
- Wetzel M, Rosenberg GA, Cunningham LA. Tissue inhibitor of metalloproteinases-3 and matrix metalloproteinase-3 regulate neuronal sensitivity to doxorubicin-induced apoptosis. Eur J Neurosci. 2003;18:1050–1060. doi: 10.1046/j.1460-9568.2003.02838.x. [DOI] [PubMed] [Google Scholar]
- Wetzel M, Li L, Harms KM, Roitbak T, Ventura PB, Rosenberg GA, Khokha R, Cunningham LA. Tissue inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ. 2008;15:143–151. doi: 10.1038/sj.cdd.4402246. [DOI] [PubMed] [Google Scholar]
- Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22:21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Yenari M, Wijman C, Steinberg G. Effects of hypothermia on cerebral metabolism, blood flow and autoregulation. In: Mayer S, Sessler D, editors. Therapeutic hypothermia. Marcel Dekker, Inc; New York: 2004. pp. 141–178. [Google Scholar]
- Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Onley D, Hedehus M, deCrespigny A, Sun GH, Moseley ME, Steinberg GK. Diffusion- and perfusion-weighted magnetic resonance imaging of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res. 2000;885:208–219. doi: 10.1016/s0006-8993(00)02942-5. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Iwayama S, Cheng D, Sun GH, Fujimura M, Morita-Fujimura Y, Chan PH, Steinberg GK. Mild hypothermia attenuates cytochrome c release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab. 2002;22:29–38. doi: 10.1097/00004647-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sobel RA, Cheng D, Steinberg GK, Yenari MA. Mild hypothermia increases Bcl-2 protein expression following global cerebral ischemia. Brain Res Mol Brain Res. 2001;95:75–85. doi: 10.1016/s0169-328x(01)00247-9. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Biphasic cytochrome c release after transient global ischemia and its inhibition by hypothermia. J Cereb Blood Flow Metab. 2005;25:1119–1129. doi: 10.1038/sj.jcbfm.9600111. [DOI] [PubMed] [Google Scholar]