Abstract
Ventricular fibrillation (VF), despite its declining incidence as a cause of sudden cardiac death, is still a major health problem. The underlying mechanisms for the maintenance of VF are still disputed. Studies suggest that VF is unlikely one static mechanism but a dynamic process of electrical derangement that changes with duration. The two principal proposed mechanisms of VF are multiple wavelets and mother rotors. Most studies of these proposed mechanisms for VF maintenance have been during the first minute of VF. Yet, the time to external defibrillation in the community and pre-hospital setting where the majority of sudden cardiac death occurs ranges from 4 to 10 minutes and the “time to defibrillation” appears crucial as the odds of survival worsen with delay. Recent studies during the first 10 minutes of VF suggest that Purkinje fibers are important in maintaining VF after the first 1 to 2 minutes, either as a part of a reentrant circuit or as a source of focal activations.
Keywords: Ventricular fibrillation, Defibrillation, Purkinje Fibers, Sudden Cardiac Death
Although decreasing in incidence, ventricular fibrillation (VF) remains a significant cause of sudden cardiac death (SCD) in the United States.1 In recent years, the use of the implantable cardioverter defibrillator (ICD) has decreased the incidence of death among those at highest risk. This means of therapy however, remains a crude method for defending the patient against the poorly understood electrophysiologic state of VF. The large majority of individuals with SCD do not have an ICD. The time to external defibrillation in the community and pre-hospital setting where the majority of SCD occurs ranges from 4 to 10 minutes.2, 3 The time to defibrillation appears crucial as the odds of survival to hospital discharge decrease approximately 10% for every minute of lack of perfusion.2
VF changes as it progresses with time. Wiggers first proposed four distinct phases of VF based on epicardial motion visualized by high speed cinematography in canine hearts.4 His description of the four stages included 1.) Initial tachysystolic stage (<1s of VF), 2.) Stage of convulsive incoordination (1–40s of VF), 3.) Stage of tremulous incoordination (40s-3min of VF) and 4.) Stage of progressive atonic incoordination5 (after 3 min of VF).4 The overwhelming majority of studies attempting to map cardiac activation sequences during VF have been performed only during the first minute of VF. Therefore, although mapping of VF during long duration VF (>1min) remains rarely evaluated, it is during this period that the majority of patients with SCD receive initial therapy.
Huang et al. 6 performed a cluster analysis of the quantitative measurements of the epicardial activation sequence during the first 10 minutes of VF in canines, including the number of wavefronts, reentry episodes, wavefront propagation velocity, wavefront breakthroughs onto the epicardium, wavefront conduction block, wavefront fractionations, wavefront collisions, mean area activated by wavefronts, number of activation sequences, number of wavefronts that follow each activation sequence, negative peak rate of voltage change, and peak frequency of activation. They found that the data temporally clustered into 5 stages. Stage i, lasting the first 11 s after the onset of VF, exhibited many wavefronts, a rapid frequency of activation, reentry, and a rapid propagation velocity. During stage ii (12–62 s) these measures decreased albeit with rapid dynamic fluctuations. During a brief stage iii (63–86 s) many of these descriptors again increased consistent with a higher degree of organization, perhaps caused by a temporary decrease in extracellular potassium. Stage iv (87–310 s) demonstrated a steady decrease in most quantitative measures of VF organization, while during stage v (311–600 s) these measures all decreased gradually towards zero, which is indicative of cell death and cessation of electrical activity.6
Based on local VF dynamics recorded optically in isolated pig hearts, such as the action potential amplitude and duration, the incidence of wavebreak, and the repeatability of propagation direction, Huizar et al7 divided the first 10 min of non-perfused VF into 3 stages. The first stage, which they called “relatively periodic”, lasted about 1 min. The second stage, which they called “highly periodic”, was present between the first and second minute of VF and corresponds to the highly organized stage iii of Huang et al.6 Huizar and coworkers called the third stage lasting until the end of the 10 min recording period of VF “aperiodic”.7
From the above classifications, it is clear that VF passes through different stages as it progresses, whether the number of stages is 3, 4, or 5. These distinct phases raise the possibility that the relative importance of different arrhythmogenic mechanisms changes as VF is initiated and then progresses through different stages. It has also been proposed that different therapies are needed during different stages of VF.8
A controversy in cardiac electrophysiology exists as to whether VF is maintained by wandering wavelets with constantly changing, evanescent, reentrant circuits or by a “mother rotor” consisting of a sustained, stationary reentrant circuit in one region of the ventricles that gives rise to variable daughter wavelets that spread through the remainder of the ventricular myocardium. As reviewed below, the likely answer is that both can occur during different stages of VF. Recent data will also be reviewed suggesting that there are two types of VF and that Purkinje fibers appear to be active throughout long duration VF and may be either a reentrant or a focal source of wavefronts during VF.
Multiple Wavelet Hypothesis
Based on the results of computer simulations published in 1964, Moe first proposed the multiple wavelet hypothesis to explain the activation patterns maintaining atrial fibrillation.9 According to the multiple wavelet hypothesis, the conditions required for fibrillation are a premature stimulus to initiate the arrhythmia and inhomogeneity in the refractory periods of the tissue to cause conduction block and reentry that sustain it. Later simulations demonstrated that wandering wavelet type reentry can be sustained in completely homogenous tissue.10 Although inhomogeneity of refractoriness may occur in normal myocardial tissue, diseased myocardium as well as normal anatomic structures can exaggerate this heterogeneity and act as a substrate for conduction block and wavebreak. According to the wandering wavelet hypothesis, wavelets follow constantly changing pathways and are short lived. Wandering wavelets terminate in two ways: 1) they are extinguished when they collide with another wavelet, strike a non-conducting boundary, or completely block, or 2) they partially block and fractionate into two or more “daughter wavelets”. If the amount of tissue exceeds a critical mass, enough daughter wavelets that block and reenter are constantly being formed so that reentry circuits are constantly present to sustain fibrillation. According to the wandering wavelet hypothesis, because wandering wavelets are present throughout the tissue, all parts of the tissue help maintain fibrillation.
Mother Rotor Hypothesis
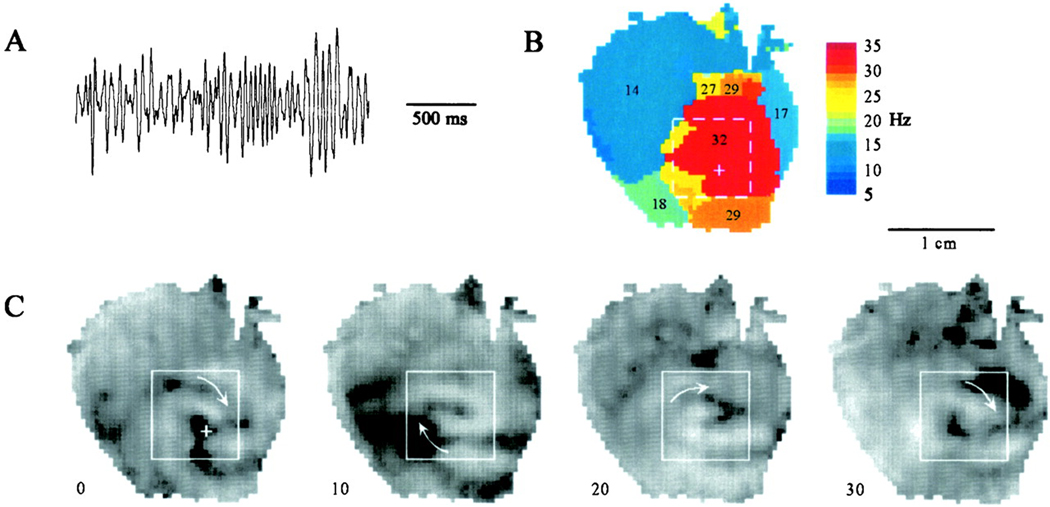
The mother rotor hypothesis for the maintenance of fibrillation was first proposed by Lewis in 1925,11 followed by Gurvich in 1975.12 This concept has recently been reemphasized by Jalife’s and Pertsov’s laboratories.13, 14 These groups have suggested that short duration VF (VF lasting less that 1 min, SDVF) is maintained by a stable reentrant circuit called the mother rotor localized in a single region.13 These investigations determined that the peak in the power spectrum of optical recordings varied across the surface of isolated slabs of sheep ventricles and of isolated guinea pig hearts. They also provided evidence that the peak in the power spectrum corresponded to the activation rate in the optical recordings during SDVF and that areas with a higher peak frequency had faster activation rates and shorter refractory periods than regions with lower peak frequencies (Figure 1).14
Figure 1.
Example of a high frequency mother rotor from an optically mapped guinea pig heart. A) ECG trace of VF. B) Dominant frequency map showing high frequency activation from a single region on the LV. C) Snapshots of a long-lasting rotor that rotates clockwise on the anterior LV wall. Numbers in Panel C are in ms. Reprinted with permission.14
Zaitsev et al. used the term “domain” to define a region in which all of the tissue has the same peak frequency in the VF power spectrum and reported the mean area of domains to be 1.1cm2 in isolated, perfused sheep right ventricles (RVs).13 Optical recordings during VF indicated that a single domain of highest peak frequency was present, called the dominant domain, that was surrounded by domains of lower peak frequencies that in turn were bordered by domains of even lower peak frequencies. While some wavefronts that arose in the dominant domain propagated into domains with lower peak frequencies, others blocked at the boundary between domains in a Wenckebach pattern.14 These findings suggest that all myocardial regions are not equally responsible for VF maintenance, but rather that VF is maintained by a single, stationary, stable reentrant circuit, i.e., the mother rotor, in the dominant domain, which has the shortest refractory period from which activations propagate into the more slowly activating domains with longer refractory periods.
While reentry is common in guinea pigs and rabbits during SDVF,15, 16 electrical and optical mapping of a small portion of the left ventricle in dogs and sheep has indicated that few wavefronts exhibit reentry during SDVF and this reentry is short-lived, usually lasting less than 2 cycles.17, 18 More recently, panoramic optical mapping performed on nearly the entire epicardial surface of swine hearts revealed that approximately 12 reentrant circuits are present at any one time during SDVF. 18 Most wavefronts are annihilated by block or collision without reentering. 18 In addition, the reentrant circuits are unstable, short lived, and do not fit the description of a mother rotor.
Epicardial mapping studies have demonstrated that SDVF wavefronts in pigs tend to propagate from the posterior basal LV to the anterior LV and on to the anterior RV, raising the possibility of a mother rotor in the posterior LV.15 However, no sustained reentry consistent with a mother rotor was found on the posterior LV epicardium, even though an intramural mapping study showed that the fastest activating transmural layer was near the epicardium.19 Many wavefronts in the posterior LV entered the mapped region from its posterior boundary, adjacent to the posterior descending coronary artery, raising the possibility that a mother rotor is located in the RV or septum. Because a previous study has shown that the RV activates more slowly than the LV during SDVF,20 the more likely site for a mother rotor was the septum. However, a study which recorded from the right side of the septum found that reentry was uncommon there also, and that the activation rate was slower than the posterobasal LV.21 Many of the VF wavefronts in the septum passed from the posterior septum toward the anterior septum. This fact coupled with the fact that many wavefronts passed from the posterior LV free wall toward the anterior LV free wall point to the region where the posterior free wall intersects with the septum, the region where the posterior papillary muscle is located, as the possible site of a mother rotor.21, 22 This possibility is also supported by Pak et al who found that ablation in the region of the posterior papillary muscle by a transmural incision between the posterior papillary muscle and the septum markedly reduced the inducibility of VF.23, 24
Computer simulations indicate that the central filament of a reentrant scroll tends to be parallel to the long axis of the myocardial fibers so that the reentrant circuit always travels across fibers.25 Because the long axes of the myofibers within both ventricular free walls are primarily parallel to the epicardium and endocardium, the reentrant pathways should be intramural, passing from the subepicardium to the subendocardium and then back to the subepicardium again. Thus, these reentrant pathways should appear as a focal activation pattern in epicardial maps where the reentrant wavefront breaks through to the heart surface. At the insertion of the papillary muscles, where the fibers are parallel to the long axis of the papillary muscles, reentry would be expected to rotate around this long axis. Depending on the angle of the filament of the reentrant rotor, either reentry or epicardial breakthrough could be observed in epicardial maps overlying the papillary muscle insertion. Thus, three-dimensional mapping with electrodes close enough together to map the complex activation pathways of VF is needed to determine if reentry is present.
Li and colleagues recently recorded 10 minutes of VF with a 3-D transmural unipolar 9 × 9 electrode array of plunge needles with 2-mm spacing over the anterior papillary muscle.26 Each plunge needle contained 6 electrodes 2 mm apart. The number of wavefronts, foci and reentrant circuits per second were counted. A focus was identified if a wavefront appeared de novo within the central mapped region indicating that it had not arisen from another wavefront or propagated into the mapped regions from outside. Intramural reentry was present during the first 3 minutes of VF but not later during VF when 27% of wavefronts arose focally.26 Thus, during early but not late VF, these findings are consistent with the wandering wavelet hypothesis that VF is maintained by a few, evanescent, constantly changing, reentrant circuits in the working myocardium. However, these results cannot rule out the possibility that a mother rotor is present in a region of the ventricles that was not mapped, and that the region mapped contained only daughter wavelets that arose from the mother rotor.
Heterogeneity of Tissue in VF (APD and Restitution)
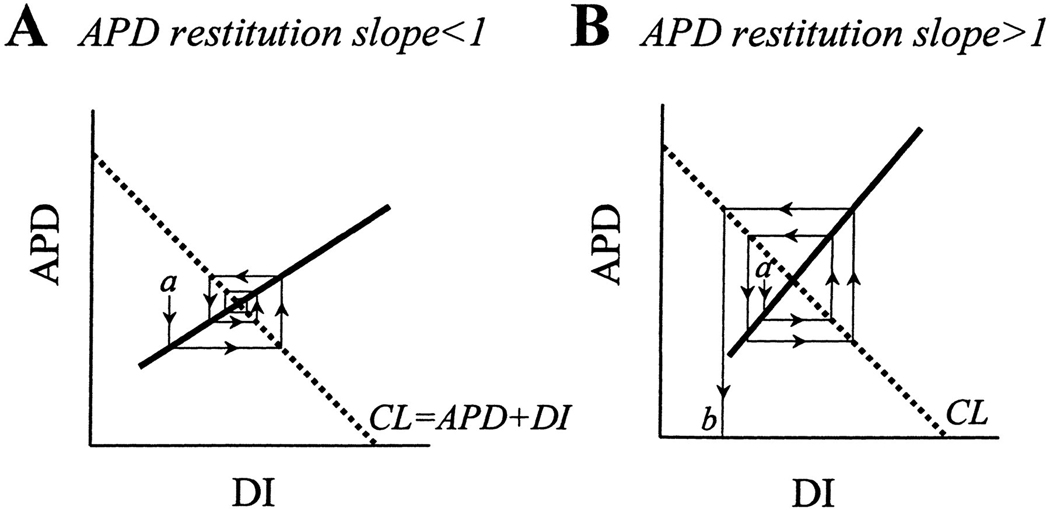
The classic mechanism for block leading to reentry is the non-uniform dispersion of refractoriness.9 This mechanism is supported by the findings that an increase in the non-uniformity of dispersion of refractoriness increases VF inducibility and duration and that a non-uniform dispersion of refractoriness is present during VF.27 A mechanism for block has been suggested that can occur even in totally homogenous tissue by causing an alternans in APD and/or cycle length that has been shown to be a precursor to block.28 Simulations have shown that a reentrant rotor becomes unstable and breaks down into multiple rotors when the slope of the restitution curve for APD versus the diastolic interval (DI) is >1.29 When a premature activation occurs (a in Figure 2A) and the slope is <1, oscillations in APDs and DIs decrease until the stable point is again reached. When the slope is >1, oscillations in APDs and DIs increase with each cycle until block occurs that can lead to reentry and VF (Figure 2B). It has also been shown in a small piece of isolated heart tissue that the slope of the APD restitution curve is >1 during VF and that drugs that decrease this slope to <1 terminate VF.30 Another type of restitution relationship based on the change in conduction velocity (CV) with DI has also been shown in simulations to be capable of causing conduction block.31 This relationship is closely related to the APD resititution relationship because CV affects the DI. For example, a short DI decreases CV which lengthens the next DI. Therefore, although CV does not affect APD directly, it does so indirectly through its effect on the DI. In addition, block and reentry have been reported to occur at sites where intracellular calcium (Cai) changes are divorced from the action potential (AP)32 and where differences in the density of ion channels such as the background potassium current are present.14 With some experimental conditions, conduction block has been reported more likely when wavefronts travel parallel than when they travel perpendicular to the long axis of the myocardial fibers.33 Under other experimental conditions, the opposite condition occurs in which block is more likely when wavefronts travel perpendicular than when they travel parallel to the long axis of the fibers.34
Figure 2.
Relationship between steepness of the action potential duration (APD) restitution slope and spiral wave stability. Cycle length (CL, dotted line) is the sum of the APD and the diastolic interval (DI). A spiral wave rotating at a constant CL reaches a steady state where the CL and the APD restitution lines cross. A) If the restitution slope is < 1, a small perturbation (a) will cause oscillating variations in APD and DI that converge back to a steady state. B) If the restitution slope is >1, a small perturbation (a) causes an increasing oscillation in APD and DI that eventually causes the DI to be too short to generate an action potential, causing conduction block and wave break. Reprinted with permission.53
Two types of VF
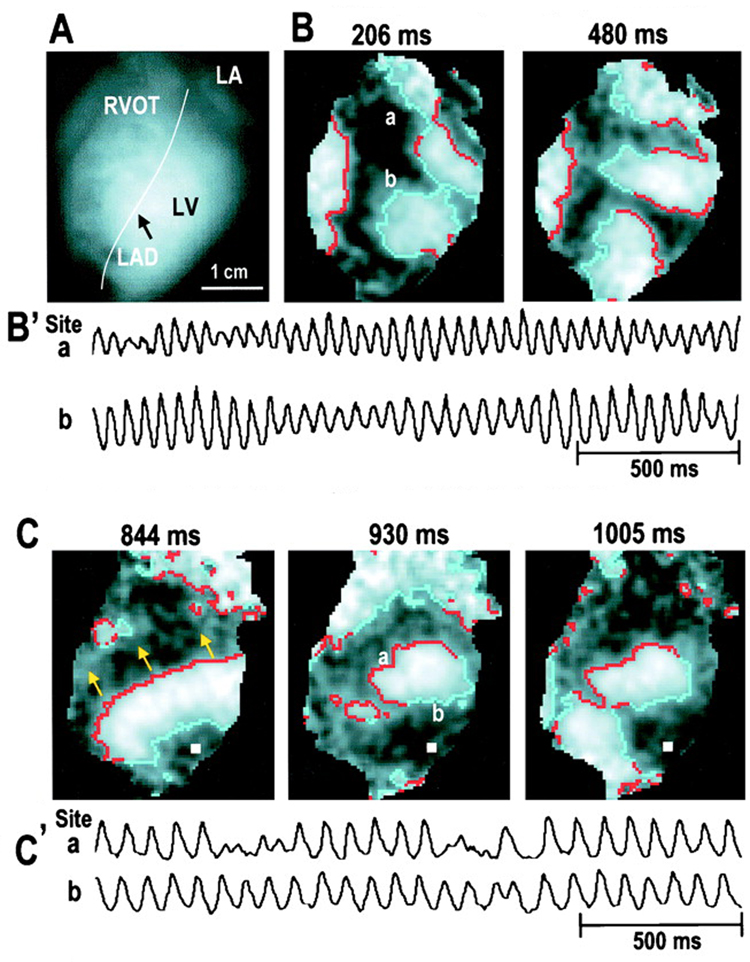
Chen’s group has reported that there are two types of VF (Figure 3) and that these two types resolve the controversy of whether VF is maintained by wandering wavelets or a mother rotor. 35 Type I VF is characterized by a rapid activation rate, uncommon and short-lived reentry, and low repeatability in wavefront direction and pathway (Figure 3B). Thus, VF is maintained by wandering wavelets during Type I VF. Chen’s laboratory has proposed that the steep APD restitution curve is responsible for conduction block during Type I VF. Type II VF is characterized by a slower activation rate with large wavefronts with many wavefronts following similar pathways (Figure 3C). Thus, VF may be maintained by mother rotors during type II VF. During this type of VF, Chen and coworkers have proposed that the APD restitution curve is flatter, but that excitability is reduced and the CV restitution curve is broad. The broad CV restitution may be responsible for conduction block in Type II VF. Evidence exists that the type of VF is influenced by drugs, heart disease, and VF duration with SDVF being type I and long duration VF (VF lasting more than 1 min, LDVF) being type II VF.35
Figure 3.
Optical maps of perfused rabbit hearts shows two types of VF. A) Mapped area with the right ventricular outflow tract (RVOT), left atrium (LA), left ventricle (LV), and the lateral anterior descending (LAD) artery shown. B) Type I or fast VF with multiple wavelets is shown. Red indicates sites of activation and light colored region denotes depolarized tissue. The tracings from sites ‘a’ and ‘b’ are shown below. C) Type II or slow VF. Large wavefronts (shown with arrows) activate much of the ventricular mass. Reprinted with permission.54
Anatomical Anchors of Reentry
Both wandering wavelets and mother rotors are affected by cardiac anatomy. In the case of wandering wavelets, different anatomical structures may cause an increase in tissue heterogeneity and thereby help maintain reentry.29 A rotor may become attached to a particular anatomical structure and remain there as a stable reentrant circuit, i.e, a mother rotor.13 Chen’s group has reported that these anatomic sites can be papillary muscles, blood vessels, and Purkinje fibers,29 or locations near the septum. They performed epicardial optical mapping of the isolated pig RV during SDVF and found that phase singularities, which indicate block and possibly reentry, cluster in the myocardium adjacent to blood vessels.36 They also showed that a stationary, long-lived rotor is centered on the epicardium directly over the endocardial insertion of the antero-lateral LV papillary muscle in isolated, perfused rabbit hearts during Type II but not Type I VF. With propranolol, reentry was present on the endocardium of pigs and dogs anchored around the posterior papillary muscle while breakthrough was simultaneously present on the epicardium overlying the posterior papillary muscle during VF.23 In addition, ablation lesions and surgical incisions extending away from the posterior papillary muscle made VF much more difficult to induce.23, 24 Mapping of explanted hearts from patients with dilated cardiomyopathy supported with Langendorff perfusion demonstrated lines of block in regions that later histologic inspection showed to contain areas of fibrosis separating epicardial muscle fibers,37 suggesting that the conduction block created by fibrosis also formed a substrate for reentry.
Purkinje Fiber Involvement in VF
Numerous reports now exist of ablative procedures which may cure VF that occurs in structurally normal hearts. The occurrence of VF in a normal heart suggests a triggered activity mechanism that has the potential to arise from the specialized conduction system.38, 39 Reports from Haissaguerre et al first reported that ablation at sites where the earliest triggers were recorded as Purkinje spikes prior to ventricular activation in the RV outflow tract, RV, or LV could prevent VF.38 The Purkinje signals were defined as an initial sharp potential <10ms in duration prior to a ventricular activation complex.
In addition to the role of Purkinje fibers in the initiation of VF, other studies have suggested involvement by Purkinje fibers in the maintenance of VF.40, 41 Two considerations support the hypothesis that activation during LDVF arises from Purkinje fibers. One is that Purkinje fibers are less sensitive to ischemia than is working myocardium so they may be more electrically active during the global ischemia of LDVF.42 Two is that accommodation is greater in Purkinje fibers than in working myocardium so that at the very rapid activation rates of VF, the APD and refractory period of Purkinje fibers is as short as or shorter than working myocardium.43 Although the porcine model is often used routinely in the investigation of VF, the distribution of Purkinje fibers is different in pigs than in humans, dogs, and rabbits. In all of these species, a Purkinje fiber network is present on the endocardium. However, while in humans, dogs, and rabbits, the Purkinje-myocardial junctions are confined to the subendocardium, in pigs the Purkinje fibers traverse almost the entire ventricular free wall so that Purkinje-myocardial junctions are present almost transmurally.44
The endocardium activates more rapidly than the epicardium in dogs after 1 to 2 minutes of VF.45 Possible causes for this phenomenon are (1) oxygen in the blood in the ventricular cavities prevents the endocardium from becoming ischemic as VF continues so that the endocardium activates more rapidly than the ischemic remainder of the ventricular walls, or (2) the rapid activations arise from Purkinje fibers or Purkinje-myocardial junctions in the subendocardium. Chen’s group substituted air for cavitary blood and showed that the transmural gradient of activation rate during LDVF was still present but that it disappeared after the Purkinje fibers were ablated by applying Lugol’s solution to the endocardium.46 These findings suggest that Purkinje fibers or some other tissue near the endocardium and not oxygenated cavitary blood is responsible for the difference in epicardial and endocardial activation rate during LDVF. If so, LDVF activation fronts should arise from the subendocardium and propagate toward the epicardium in dogs but not pigs, because the Purkinje-muscle junctions are near the endocardium in dogs but extend from the endocardium almost to the epicardium in pigs.
Two studies have tested this prediction and found it to be true.19, 47 Newton and coworkers recorded from 75 to 100 plunge needles in the RV and LV freewalls and septum of 6 pigs and 5 dogs during SDVF and LDVF. They used a previously developed and tested method to estimate the VF activation rate from the dominant frequency and to estimate conduction block from double peaks in the fast Fourier transform (FFT) power spectrum of the unipolar electrode recordings.19 For the first 70 s of VF in both pigs and dogs, the dominant frequency was highest at the epicardium and lowest at the endocardium, whereas the incidence of double peaks was highest at the endocardium and lowest at the epicardium for the entire LV and RV base. The distribution changed little for the first 3 min of VF in pigs, but reversed by 2 min in dogs so that the dominant frequency was highest at the endocardium and lowest at the epicardium as reported earlier by Worley et al.48 Thus, estimated activation rates and conduction block incidence are not uniformly distributed transmurally during VF in dogs. During the first 1–2 min of VF, the faster activation rate near the epicardium rather than near the endocardium raises the possibility that, at least in dogs, VF is not being driven during this period by activation fronts arising from the Purkinje fibers. The consistency over time of this transmural distribution of activation rates in pigs but its reversal by 2 min of VF in dogs lends support to the hypothesis that activation during LDVF is driven by Purkinje fibers.
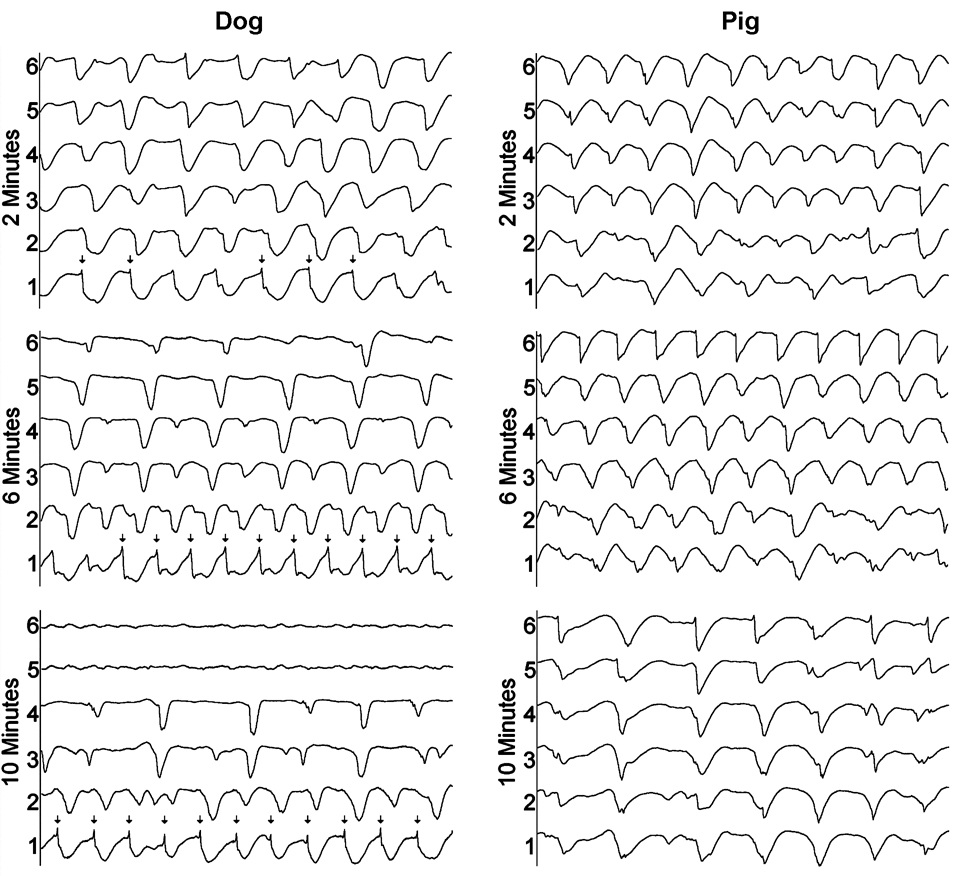
Newton and coworkers analyzed only the first 3 min of VF because the FFT method to estimate activation rate and conduction block was developed for SDVF so it might not be valid during later LDVF.19 Therefore, Allison et al. analyzed the first 10 min of LDVF with this same distribution of intramural electrodes by identifying individual activation times in the recordings.47 They found that activation remains rapid transmurally in the pig throughout the first 10 min of LDVF, consistent with activation wavefronts arising from the almost transmural Purkinje fibers in this species (Figure 4). In the dog, however, rapid activation was only present near the endocardium where Purkinje spikes could sometimes be observed, and the activation sequences along each individual plunge needle suggested that LDVF wavefronts pass from the endocardium towards the epicardium with many of them blocking en route (Figure 4). These findings in dogs are consistent with the epicardial mapping study of Huang and coworkers which reported that during stage v of LDVF, most wavefronts break through to the epicardium and terminate by conduction block.6
Figure 4.
Example of transmural activation during LDVF in a dog and a pig. Electrode 1 is near the endocardium while electrode 6 is near the epicardium. The endocardium continues to activate rapidly in the dog while conduction block occurs progressively closer to the endocardium as LDVF progresses. Purkinje activations (arrows) precede working myocardial activations at the most endocardial electrodes in the dog recordings. The activation rate slows more uniformly throughout the wall in the pig as LDVF progresses with no large transmural gradient of activation rate. Reprinted with permission.47
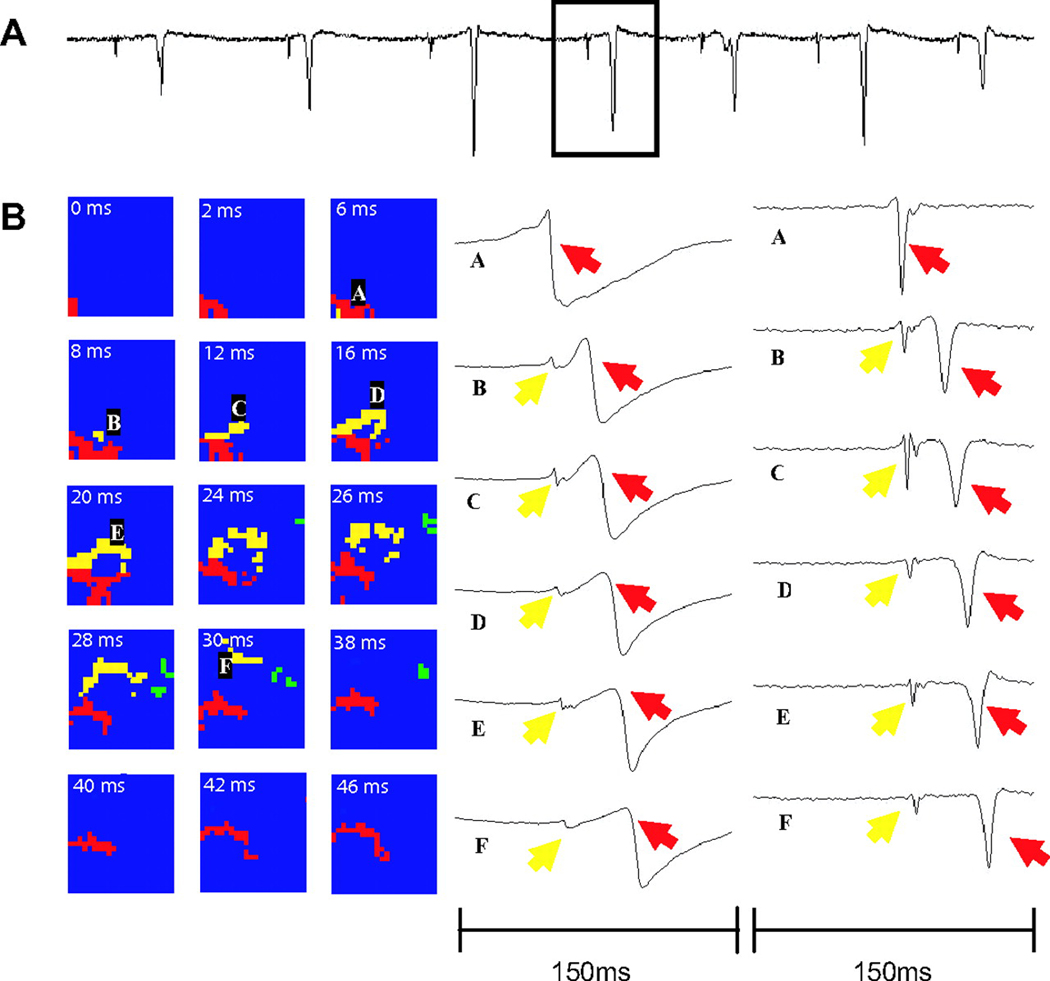
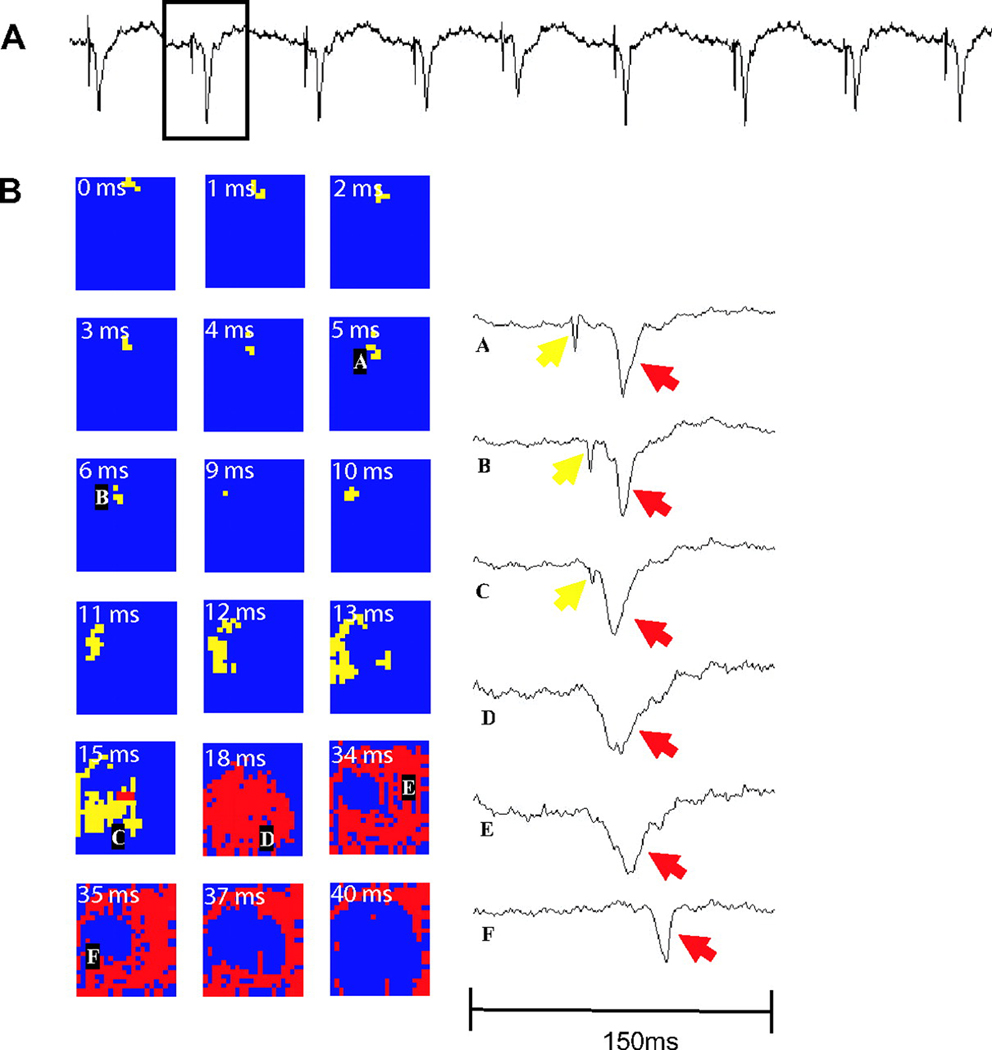
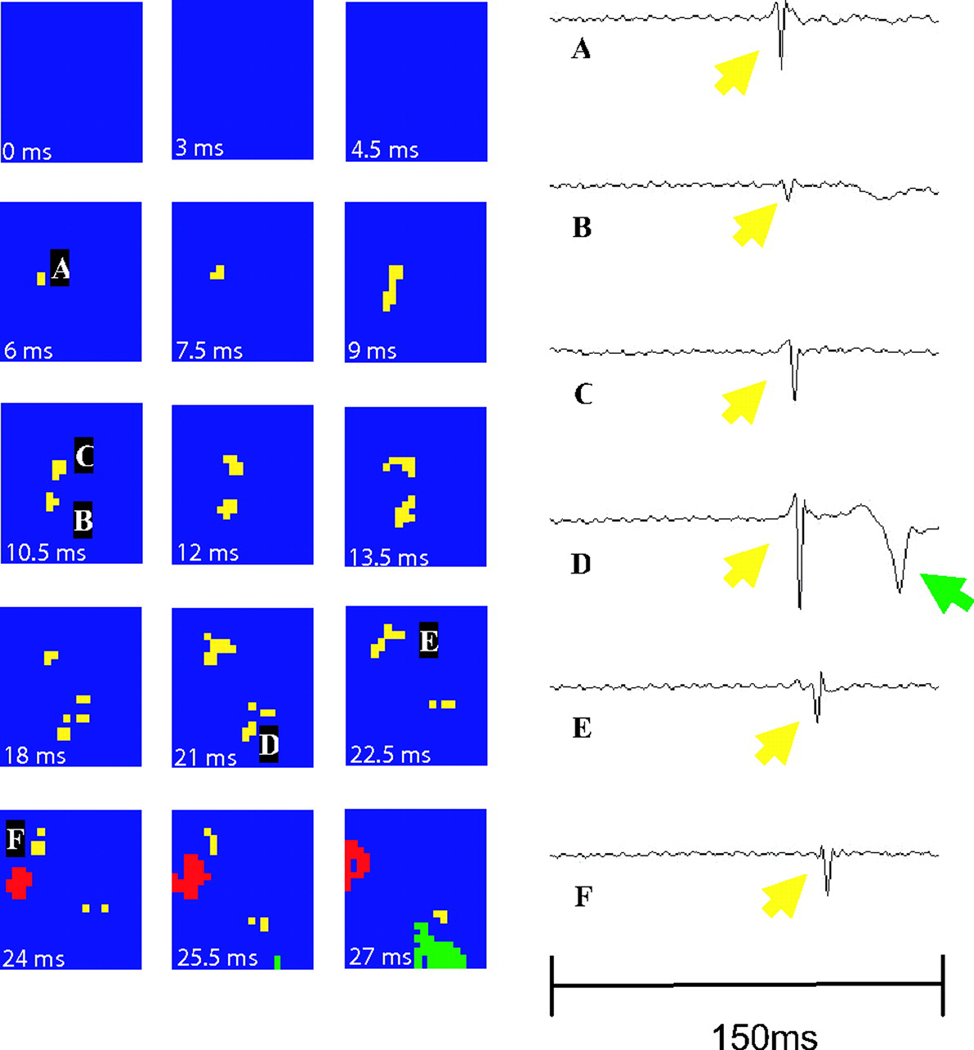
Tabereaux and coworkers have recently mapped activation within the Purkinje network directly from the endocardium in isolated dog hearts during the first 10 min of electrically induced VF.40 Six canine hearts were isolated, reperfused and the LV cavity opened via incisions in the RV free wall and the septum between the areas supplied by the posterior and anterior septal perforators to expose the endocardium and papillary muscles of the LV. Unipolar recordings were made from a 504-electrode (24 columns × 21 rows) array with 1mm spacing between electrodes that was seated over the endocardial insertion of the anterior papillary muscle. VF was then induced using a 9V battery applied to the RV and mapping was performed for 10 consecutive min. Six epochs of data each lasting 5 s during this time period were examined to identify Purkinje fiber (PF) activations as well as working ventricular myocardial (WVM) activations. PF activations were identified as rapid deflections 1–2 ms in duration preceding WVM activations. PF activations occurred in four types of patterns: 1) depolarization propagating from WVM to PF presumably through retrograde conduction at a PF-WVM junction (Figure 5), 2) PF arising from the leading edge of a WVM activation also presumably through retrograde conduction at a PF-WVM junction, 3) propagation of PF in from the border of the mapped region activating WVM in the mapped region presumably through orthograde conduction at a PF-WVM junction (Figure 6), and 4) PF or WVM activation arising de-novo in the mapped region (Figure 7).40
Figure 5.
A) 1.5-second example of dV/dt, with box highlighting the individual beat displayed in B) B, A wavefront in the working ventricular myocardium (WVM) activates the Purkinje fiber (PF) activation pattern is shown in which PF activation (yellow) appears to arise from the leading edge of the WVM wave front (red) as it propagates across the array (24×21 mm). The selected panels represent frames from a dynamic display of the activation patterns. The frames are separated at various time intervals, with the time for each frame shown in white. Green represents an unrelated wave front. In B, letters A through F indicate the locations of electrodes whose potentials (left) and temporal derivatives of the potentials (right) are shown during the wave-front propagation. Reprinted with permission.40
Figure 6.
A) 1.5-second example of dV/dt, with box highlighting the individual beat displayed in B) B, PF (yellow) to WVM (red) pattern. A rapidly propagating Purkinje wave front enters the top aspect of the array and propagates to the bottom left, stimulating a PF-WVM junction, after which an expanding Purkinje ring of activation appears, followed by a more slowly activating ring of ventricular myocardium. Temporal derivatives of the labeled electrode recordings are shown. Reprinted with permission.40
Figure 7.
A focal activation arising from the center of the array. The focal appearing PF activation (yellow) is followed by a WVM (red) activation wavefront. The temporal derivative of individual electrode recordings shows the wavefront initiating at electrode A and spreading outward toward electrodes D and E. The green represents a non-related wavefront. Reprinted with permission.40
Further evidence to support the role of Purkinje fibers in VF comes from canine heart studies in which ablation of the subendocardium was performed by painting the endocardium with either Lugol’s solution or phenol.41, 49, 50 Chemical ablation of the PF rich subendocardial layer in canine hearts dramatically elevated the VF threshold making inducibility difficult if not impossible.50 To the contrary, Chen and coworker’s reported that following subendocardial ablation by flushing Lugol’s solution in situ canine hearts VF thresholds remained relatively unchanged.49 However, some Purkinje fibers may have not been ablated by this procedure since PF potentials can be recorded at a depth of 2mm in the LV free wall51 while application of Lugol’s solution produces necrosis to a depth of only approximately 0.5mm.49, 50
Dosdall and coworkers41 evaluated the effects of Lugol’s solution on the same experimental model used by Tabereaux et al.40 They found that the rapid deflection called Purkinje activation disappeared after Lugol’s solution was applied to the endocardium, suggesting that those deflections did represent Purkinje activations. They also found that the time from the onset of VF until its spontaneous termination, which averaged 9.2 min in dog hearts not treated with Lugol’s solution, significantly decreased to 4.9 min in hearts with Lugol’s solution applied to the endocardium.41 This finding provides evidence that the specialized conduction system is important in the mechanism of maintenance of LDVF. These findings also show that the Purkinje fiber network is highly active during both SDVF and LDVF. The results raise the question of whether the Purkinje activation actively contributes to the maintenance of VF or whether it is merely a bystander. If activations had only been seen to pass from WVM to PF, the specialized conduction system could have been only a bystander, not contributing to VF maintenance. Because activations were also detected conducting antegrade from PF to WVM, it is likely that the Purkinje fibers are an integral part of activation sequences responsible for VF maintenance. However, it is not known if the Purkinje activations arise from reentry confined to the specialized conduction system or from reentry circuits involving both Purkinje fibers and working myocardium.
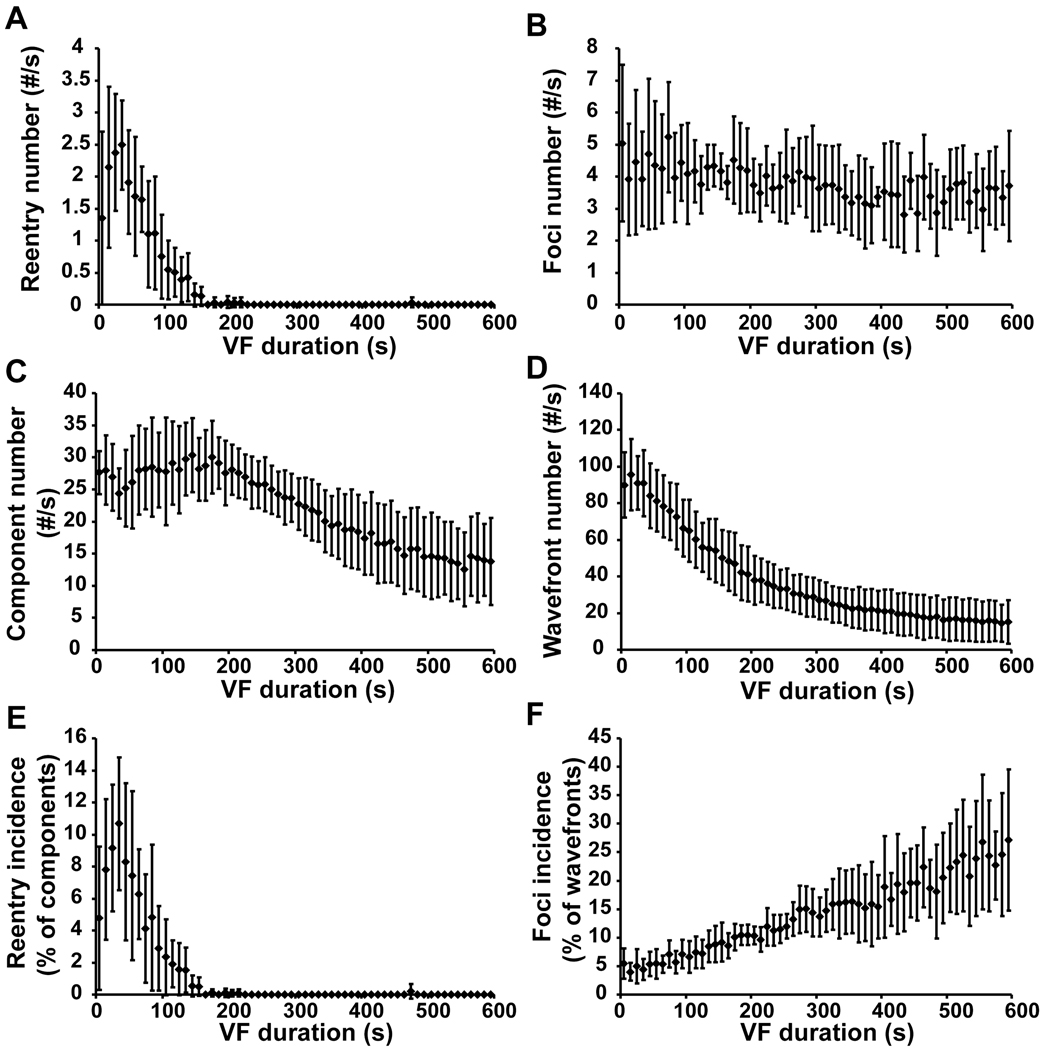
The finding of frequent focal activations arising from the Purkinje fibers or the working myocardium is intriguing, yet the possibility of intramural wavefronts propagating toward the endocardium with breakthrough to the endocardial surface cannot be ruled out. However, the plunge needle recordings of Newton et al. and Allison et al. indicate that during LDVF in the dog most wavefronts propagate from the endocardium towards the epicardium.19, 47 Therefore, frequent endocardial breakthrough of wavefronts traveling in a direction from the epicardium towards the endocardium would not be expected. In addition, a recent three-dimensional mapping study in pigs by Li et al. found numerous intramural foci in pigs, consistent with the intramural distribution of Purkinje myocardial junctions in that species (Figure 8).26 Therefore, it is possible that many of the focal activation patterns on the endocardium represent true foci. If so, it remains to be determined if these foci arise from abnormal automaticity, early afterdepolarizations, or late afterdepolarizations, instead of reentry.
Figure 8.
Incidence of intramural reentry and foci at 10 s intervals during VF. Panels A, C and E show the number of reentry occurrences per s, the number of components per s, and the reentry incidence (the number of occurrences of reentry divided by the number of components). Panels B, D and F show the number of foci per s, the number of wavefronts per s, and the foci incidence (the number of foci divided by the number of wavefronts). The mean values and the standard deviations from all 6 animals are displayed. A component is a family of wavefronts that interact with each other through collisions and fractionations. Reprinted with permission.26
Conclusions
While the mechanisms of VF have been studied extensively during SDVF, the mechanisms responsible for the maintenance of LDVF remain less understood. The importance of LDVF studies are amplified by the fact that the majority of clinically relevant episodes of VF do not receive defibrillation shocks in the field for 4–10 minutes.3 It is likely that multiple mechanisms including a mother rotor and multiple wavelets play a role in the initial stages of VF, while Purkinje fibers, either as part of reentrant circuit or as a source of focal activations, are important during the later stages of VF. Further clarification of the roles of these different mechanisms requires extensive 3-D intramural mapping , ideally with optrodes that can record Cai as well as the transmembrane potential.52
Acknowledgments
GRANT SUPPORT
This work was supported in part by National Institutes of Health grants HL28429, HL66256, and HL85370
Literature Cited
- 1.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. Jama. 2002;288(23):3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela TD, Roe DJ, Cretin S, Spaite DW, Larsen MP. Estimating effectiveness of cardiac arrest intervention: a logistic regression survival model. CIRC. 1997;96(10):3308–3313. doi: 10.1161/01.cir.96.10.3308. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343(17):1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 4.Wiggers CJ. Studies of ventricular fibrillation caused by electric shock: Cinematographic and electrocardiographic observations of the natural process in the dog's heart: Its inhibition by potassium and the revival of coordinated beats by calcium. AHJ. 1930;5:351–365. doi: 10.1046/j.1542-474X.2003.08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallstrom AP, Ornato JP, Weisfeldt M, Travers A, Christenson J, McBurnie MA, Zalenski R, Becker LB, Schron EB, Proschan M. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351(7):637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Rogers JM, Killingsworth CR, Singh KP, Smith WM, Ideker RE. Evolution of activation patterns during long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol. 2004;286(3):H1193–H1200. doi: 10.1152/ajpheart.00773.2003. [DOI] [PubMed] [Google Scholar]
- 7.Huizar JF, Warren MD, Shvedko AG, Kalifa J, Moreno J, Mironov S, Jalife J, Zaitsev AV. Three distinct phases of VF during global ischemia in the isolated blood-perfused pig heart. Am J Physiol Heart Circ Physiol. 2007;293(3):H1617–H1628. doi: 10.1152/ajpheart.00130.2007. [DOI] [PubMed] [Google Scholar]
- 8.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. Jama. 2002;288(23):3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 9.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. AHJ. 1964;67(2):200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 10.Fenton FH, Cherry EM, Hastings HM, Evans SJ. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos. 2002;12(3):852–892. doi: 10.1063/1.1504242. [DOI] [PubMed] [Google Scholar]
- 11.Lewis T. The Mechanism and Graphic Registration of the Heart Beat. 3rd ed. London: Shaw and Sons, Ltd.; 1925. [Google Scholar]
- 12.Gurvich NL. The Main Principles of Cardiac Defibrillation. Moscow, Russia: Medicine; 1975. [Google Scholar]
- 13.Zaitsev AV, Berenfeld O, Mironov SF, Jalife J, Pertsov AM. Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circulation Research. 2000;86(4):408–417. doi: 10.1161/01.res.86.4.408. [DOI] [PubMed] [Google Scholar]
- 14.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89(12):1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 15.Nanthakumar K, Huang J, Rogers JM, Johnson PL, Newton JC, Walcott GP, Justice RK, Rollins DL, Smith WM, Ideker RE. Regional differences in ventricular fibrillation in the open-chest porcine left ventricle. Circ Res. 2002;91(8):733–740. doi: 10.1161/01.res.0000038945.66661.21. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Mandapati R, Berenfeld O, Skanes AC, Jalife J. High-frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. CircRes. 2000;86:86–93. doi: 10.1161/01.res.86.1.86. [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Kamjoo K, Hough D, Hwang C, Fan W, Fishbein MC, Bonometti C, Ikeda T, Karagueuzian HS, Chen P-S. Reentrant wavefronts in Wiggers' stage II ventricular fibrillation. CIRCRES. 1996;78(4):660–675. doi: 10.1161/01.res.78.4.660. [DOI] [PubMed] [Google Scholar]
- 18.Rogers JM, Walcott GP, Gladden JD, Melnick SB, Kay MW. Panoramic optical mapping reveals continuous epicardial reentry during ventricular fibrillation in the isolated swine heart. Biophys J. 2007;92(3):1090–1095. doi: 10.1529/biophysj.106.092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton JC, Smith WM, Ideker RE. Estimated global transmural distribution of activation rate and conduction block during porcine and canine ventricular fibrillation. Circulation Research. 2004;94(6):836–842. doi: 10.1161/01.RES.0000120860.01645.17. [DOI] [PubMed] [Google Scholar]
- 20.Newton JC, Johnson PL, Justice RK, Smith WM, Ideker RE. Estimated global epicardial distribution of activation rate and conduction block during porcine ventricular fibrillation. J Cardiovasc Electrophysiol. 2002;13(10):1035–1041. doi: 10.1046/j.1540-8167.2002.01035.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Walcott GP, Killingsworth CR, Melnick SB, Rogers JM, Ideker RE. Quantification of activation patterns during ventricular fibrillation in open-chest porcine left ventricle and septum. Heart Rhythm. 2005;2(7):720–728. doi: 10.1016/j.hrthm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Ideker RE, Huang J. Our search for the porcine mother rotor. Ann Noninvasive Electrocardiol. 2005;10(4 Suppl):7–15. doi: 10.1111/j.1542-474X.2005.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pak HN, Kim YH, Lim HE, Chou CC, Miyauchi Y, Fang YH, Sun K, Hwang C, Chen PS. Role of the posterior papillary muscle and purkinje potentials in the mechanism of ventricular fibrillation in open chest dogs and Swine: effects of catheter ablation. Journal of Cardiovascular Electrophysiology. 2006;17(7):777–783. doi: 10.1111/j.1540-8167.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 24.Pak HN, Kim GI, Lim HE, Fang YH, Choi JI, Kim JS, Hwang C, Kim YH. Both purkinje cells and left ventricular posteroseptal reentry contribute to the maintenance of ventricular fibrillation in open-chest dogs and Swine. Circ J. 2008;72(7):1185–1192. doi: 10.1253/circj.72.1185. [DOI] [PubMed] [Google Scholar]
- 25.Berenfeld O, Pertsov AM. Dynamics of intramural scroll waves in three-dimensional continuous myocardium with rotational anisotropy. Journal of Theoretical Biology. 1999;199(4):383–394. doi: 10.1006/jtbi.1999.0965. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural Foci During Long Duration Fibrillation in the Pig Ventricle. Circ Res. 2008;102(10):1256–1264. doi: 10.1161/CIRCRESAHA.107.170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janse MJ. Vulnerability to ventricular fibrillation. Chaos. 1998;8(1):149–156. doi: 10.1063/1.166295. [DOI] [PubMed] [Google Scholar]
- 28.Vinet A, Chialvo DR, Jalife J. Irregular dynamics of excitation in biologic and mathematical models of cardiac cells. Ann N Y Acad Sci. 1990;601:281–298. doi: 10.1111/j.1749-6632.1990.tb37307.x. [DOI] [PubMed] [Google Scholar]
- 29.Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, Garfinkel A, Karma A. The dynamics of cardiac fibrillation. Circulation. 2005;112(8):1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 30.Gilmour RF., Jr. A novel approach to identifying antiarrhythmic drug targets. Drug Discov Today. 2003;8(4):162–167. doi: 10.1016/s1359-6446(02)02567-9. [DOI] [PubMed] [Google Scholar]
- 31.Laurita KR, Girouard SD, Rosenbaum DS. Modulation of ventricular repolarization by a premature stimulus. Role of epicardial dispersion of repolarization kinetics demonstrated by optical mapping of the intact guinea pig heart. Circ Res. 1996;79(3):493–503. doi: 10.1161/01.res.79.3.493. [DOI] [PubMed] [Google Scholar]
- 32.Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, Attin M, Lee MH, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen PS, Weiss JN. Intracellular Ca dynamics in ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2004;286(5):H1836–H1844. doi: 10.1152/ajpheart.00123.2003. [DOI] [PubMed] [Google Scholar]
- 33.Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle: A model of reentry based on anisotropic discontinuous propagation. CIRCRES. 1988;62(4):811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- 34.Delgado C, Steinhaus B, Delmar M, Chialvo DR, Jalife J. Directional differences in excitability and margin of safety for propagation in sheep ventricular epicardial muscle. CIRCRES. 1990;67:97–110. doi: 10.1161/01.res.67.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Chen PS, Wu TJ, Ting CT, Karagueuzian HS, Garfinkel A, Lin SF, Weiss JN. A tale of two fibrillations. Circulation. 2003;108(19):2298–2303. doi: 10.1161/01.CIR.0000094404.26004.07. [DOI] [PubMed] [Google Scholar]
- 36.Valderrábano M, Chen PS, Lin SF. Spatial distribution of phase singularities in ventricular fibrillation. Circulation. 2003;108(3):354–359. doi: 10.1161/01.CIR.0000080322.67408.B4. [DOI] [PubMed] [Google Scholar]
- 37.Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. Journal of the American College of Cardiology. 1998;32(1):187–196. doi: 10.1016/s0735-1097(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 38.Haïssaguerre M, Shoda M, Jaïs P, Nogami A, Shah DC, Kautzner J, Arentz T, Kalushe D, Lamaison D, Griffith M, Cruz F, de Paola A, Gaïta F, Hocini M, Garrigue S, Macle L, Weerasooriya R, Clémenty J. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106(8):962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 39.Nogami A, Sugiyasu A, Kubota S, Kato K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm. 2005;2(6):646–649. doi: 10.1016/j.hrthm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Tabereaux PB, Walcott GP, Rogers JM, Kim J, Dosdall DJ, Robertson PG, Killingsworth CR, Smith WM, Ideker RE. Activation patterns of Purkinje fibers during long-duration ventricular fibrillation in an isolated canine heart model. Circulation. 2007;116(10):1113–1119. doi: 10.1161/CIRCULATIONAHA.107.699264. [DOI] [PubMed] [Google Scholar]
- 41.Dosdall DJ, Tabereaux PB, Kim JJ, Walcott GP, Rogers JM, Killingsworth CR, Huang J, Robertson PG, Smith WM, Ideker RE. Chemical Ablation of the Purkinje System Causes Early Termination and Activation Rate Slowing of Long Duration Ventricular Fibrillation in Dogs. Am J Physiol Heart Circ Physiol. 2008;295:H883–H889. doi: 10.1152/ajpheart.00466.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmour RF, Jr., Zipes DP. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res. 1980;46(6):814–825. doi: 10.1161/01.res.46.6.814. [DOI] [PubMed] [Google Scholar]
- 43.Robinson RB, Boyden PA, Hoffman BF, Hewett KW. Electrical restitution process in dispersed canine cardiac Purkinje and ventricular cells. Am J Physiol. 1987;253(5 Pt 2):H1018–H1025. doi: 10.1152/ajpheart.1987.253.5.H1018. [DOI] [PubMed] [Google Scholar]
- 44.Holland RP, Brooks H. The QRS complex during myocardial ischemia. An experimental analysis in the porcine heart. J Clin Invest. 1976;57(3):541–550. doi: 10.1172/JCI108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worley SJ, Swain JL, Colavita PG, Smith WM, Ideker RE. Development of an endocardial-epicardial gradient of activation rate during electrically induced, sustained ventricular fibrillation in dogs. Am J Cardiol. 1985;55(6):813–820. doi: 10.1016/0002-9149(85)90162-6. [DOI] [PubMed] [Google Scholar]
- 46.Cha YM, Uchida T, Wolf PL, Peters BB, Fishbein MC, Karagueuzian HS, Chen PS. Effects of chemical subendocardial ablation on activation rate gradient during ventricular fibrillation. Am J Physiol. 1995;269(6 Pt 2):H1998–H2009. doi: 10.1152/ajpheart.1995.269.6.H1998. [DOI] [PubMed] [Google Scholar]
- 47.Allison JS, Qin H, Dosdall DJ, Huang J, Newton JC, Allred JD, Smith WM, Ideker RE. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18(12):1306–1312. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 48.Worley SJ, Ideker RE, Mastrototaro J, Smith WM, Vidaillet H, Jr., Chen PS, Lowe JE. A new sock electrode for recording epicardial activation from the human heart: one size fits all. Pacing Clin Electrophysiol. 1987;10(1 Pt 1):21–31. doi: 10.1111/j.1540-8159.1987.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen PS, Wolf PL, Cha YM, Peters BB, Topham SL. Effects of subendocardial ablation on anodal supernormal excitation and ventricular vulnerability in open-chest dogs. Circulation. 1993;87(1):216–229. doi: 10.1161/01.cir.87.1.216. [DOI] [PubMed] [Google Scholar]
- 50.Damiano RJ, Jr., Smith PK, Tripp HF, Jr., Asano T, Small KW, Lowe JE, Ideker RE, Cox JL. The effect of chemical ablation of the endocardium on ventricular fibrillation threshold. Circulation. 1986;74(3):645–652. doi: 10.1161/01.cir.74.3.645. [DOI] [PubMed] [Google Scholar]
- 51.Spach MS, Huang SN, Ayers CR. Electrical and anatomic study of the Purkinje system of the canine heart. Am Heart J. 1963;65:664–673. doi: 10.1016/0002-8703(63)90129-7. [DOI] [PubMed] [Google Scholar]
- 52.Kong W, Fakhari N, Sharifov OF, Ideker RE, Smith WM, Fast VG. Optical measurements of intramural action potentials in isolated porcine hearts using optrodes. Heart Rhythm. 2007;4(11):1430–1436. doi: 10.1016/j.hrthm.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen PS. Chaos and the transition to ventricular fibrillation: a new approach to antiarrhythmic drug evaluation. Circulation. 1999;99(21):2819–2826. doi: 10.1161/01.cir.99.21.2819. [DOI] [PubMed] [Google Scholar]
- 54.Wu TJ, Lin SF, Weiss JN, Ting CT, Chen PS. Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation. 2002;106(14):1859–1866. doi: 10.1161/01.cir.0000031334.49170.fb. [DOI] [PubMed] [Google Scholar]