Abstract
The Ca2+-activated K+ channel KCa3.1 is required for Ca2+ influx and the subsequent activation of T-cells. We previously showed that nucleoside diphosphate kinase beta (NDPK-B), a mammalian histidine kinase, directly phosphorylates and activates KCa3.1 and is required for the activation of human CD4 T lymphocytes. We now show that the class II phosphatidylinositol 3 kinase C2β (PI3K-C2β) is activated by the T-cell receptor (TCR) and functions upstream of NDPK-B to activate KCa3.1 channel activity. Decreased expression of PI3K-C2β by siRNA in human CD4 T-cells resulted in inhibition of KCa3.1 channel activity. The inhibition was due to decreased phosphatidylinositol 3-phosphate [PI(3)P] because dialyzing PI3K-C2β siRNA-treated T-cells with PI(3)P rescued KCa3.1 channel activity. Moreover, overexpression of PI3K-C2β in KCa3.1-transfected Jurkat T-cells led to increased TCR-stimulated activation of KCa3.1 and Ca2+ influx, whereas silencing of PI3K-C2β inhibited both responses. Using total internal reflection fluorescence microscopy and planar lipid bilayers, we found that PI3K-C2β colocalized with Zap70 and the TCR in peripheral microclusters in the immunological synapse. This is the first demonstration that a class II PI3K plays a critical role in T-cell activation.
INTRODUCTION
The Ca2+-activated K+ channel KCa3.1 and the voltage-activated K+ channel Kv1.3 play a critical role in the activation of a number of immune cells including T- and B-lymphocytes and mast cells. By mediating the efflux of K+, these channels function to maintain a negative membrane potential, which is critical for sustained calcium entry into these cells via calcium release-activated Ca2+ channels (CRAC) after antigen receptor activation (Cahalan et al., 2001; Wulff et al., 2003a; Srivastava et al., 2006b). Sustained Ca2+ entry then leads to the activation of calcineurin, which by dephosphorylating the transcription factor NFAT (nuclear factor of activated T-cells) induces the production of NFAT-dependent cytokines and the subsequent proliferation of these cells (Crabtree and Olson, 2002; Cahalan et al., 2007; Feske, 2007).
Although both channels are expressed in human T- and B-cells, Kv1.3 and KCa3.1 have been reported to play quite different roles in the activation of different T- and B-cell subsets. For example, in resting naive T-cells Kv1.3 is the dominating channel and is required for maximal Ca2+ influx into these cells. In contrast, KCa3.1 channels are expressed at low levels in resting naive T-cells and are not required for activation of these cells. However, KCa3.1 channels are rapidly up-regulated after T-cell activation through AP-1–dependent transcription and are required for maximal Ca2+ influx and proliferation during the reactivation of naive T-cells (Ghanshani et al., 2000). KCa3.1 channels are also expressed in central memory T-cells, whereas Kv1.3 channels are expressed in effector memory T-cells, where they play pivotal roles in Ca2+ influx and the activation of these cells (Wulff et al., 2003b; Beeton et al., 2006).
Over the past several years, it has become increasingly clear that KCa3.1 activity is regulated by various mechanisms. It has been appreciated for some time that the carboxy-terminus of KCa3.1 is constitutively bound to calmodulin, and channel opening occurs only after binding of Ca2+ to calmodulin (Xia et al., 1998; Keen et al., 1999; Fanger et al., 2001; Maylie et al., 2004). This finding made physiological sense as it would provide a mechanism whereby the initial influx in Ca2+could feed forward to stimulate sustained calcium entry by activating KCa3.1. We recently found that, in addition to Ca2+, phosphatidylinositol 3-phosphate [PI(3)P] and the histidine kinase nucleoside diphosphate kinase B (NDPK-B, also known as nm23 H2) are also required for KCa3.1 activation (Srivastava et al., 2005, 2006a,b). These studies demonstrated that NDPK functions downstream of PI(3)P and activates KCa3.1 by phosphorylating histidine (H) 358 in KCa3.1's carboxy-terminal (CT) tail (Srivastava et al., 2006b). In addition, we identified two new negative regulators of KCa3.1, the PI(3)P phosphatase myotubularin-related protein 6 (MTMR6) and the histidine phosphatase, phosphohistidine phosphatase-1 (PHPT-1), which inhibit KCa3.1 by dephosphorylating PI(3)P and KCa3.1, respectively (Srivastava et al., 2006a, 2008). These molecules also play a critical role in the reactivation of human CD4 T-cell; NDPK-B is required for T-cell receptor (TCR)-stimulated Ca2+ flux and proliferation, whereas both MTMR6 and PHPT-1 inhibit these responses.
One of the unanswered questions has been the identification of the phosphatidylinositol 3 kinase (PI3K) responsible for generating the pool of PI(3)P that mediates activation of KCa3.1 in CD4 T-cells. PI3Ks are composed of a family of lipid kinases that phosphorylate the 3′ position of the inositol head group of d-myo-phosphatidylinositol (Cantley, 2002; Foster et al., 2003). Members of this family have been divided into three classes (I, II, and III) based on sequence homology and substrate specificity. Most of the previous work on PI3Ks in lymphocyte activation have focused on the class I PI3Ks (p110 α, β, γ, and δ), which are responsible for the acute rise in phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] after antigen receptor activation, which then mediates the recruitment and activation of a number of pleckstrin homology (PH)-containing proteins to cell membranes (Traer et al., 2006; Fruman, 2007; Patton et al., 2007). Previous studies of knockout mice have demonstrated diminished TCR signaling and PI3K activation in peripheral T-cells from p110δ and P110γ single knockout mice (Sasaki et al., 2000; Okkenhaug et al., 2002; Rodriguez-Borlado et al., 2003). In addition, mice lacking both p110δ and P110γ have a profound defect in T-cell development and survival, indicating that class I PI3Ks have partially redundant functions (Webb et al., 2005; Swat et al., 2006). Surprisingly, we found that the class II PI3K-C2β, and not the class I PI3Ks, is required for the activation of KCa3.1 in T-cells. This is the first demonstration for a role of a class II PI3K in lymphocyte activation.
MATERIALS AND METHODS
Reagents
Cells.
Jurkat T-cells were cultured in RPMI + 10% FBS. Jurkat T-cells were purchased from the ATCC (Manassas, VA) and then transfected with a flag-tagged KCa3.1 and neo-resistant cell lines overexpressing KCa3.1 (Jurkat-KCa3.1) were obtained. Green fluorescent protein (GFP)-tagged PI3K-C2α and PI3K-C2β were kindly provided by J. Domin (Imperial College, London). Jurkat-KCa3.1 cells overexpressing PI3K-C2α and PI3K-C2β were obtained by transfection using AMAXA reagents (Amaxa Biosystems, Gaithersburg, MD). CD4 T-cells were isolated from peripheral adult blood buffy coats (NY Blood Center) using the CD4 isolation kit from Miltenyi Biotec (Auburn, CA) according to manufacturers protocol. We routinely obtained >95% CD4 T-cells as assessed by FACS using this procedure.
For small interfering RNA (siRNA) transfection, unstimulated human CD4 T-cells or Jurkat T-cells were electroporated using Amaxa reagents (Amaxa Biosystems) according to manufacturer's protocol as previously described (Srivastava et al., 2006a). After resting overnight to allow recovery, human CD4 T-cells were stimulated for 2 d with anti-CD3 and anti-CD28 antibodies, and whole-cell patch-clamp was performed. A pool of siRNAs to human PI3K-C2α and PI3K-C2β was purchased from Dharmacon Research (Boulder, CO) and used together or individually in experiments. The following target sequences of siRNA oligos against PI3K-C2α were used in the pool: GAUGAUUCCUUCAGGGUUA, GCACAAACCCAGGCUAUUU, GCUCAUGGAAUUUCAAGUA, or GGAUUUCAGCUACCAGUUA, and for PI3K-C2β: GUUCGACACUUACCACAAU, GCUACCAGCUAUGAAGAUU, CAACUGUUCCUCCACUGUA, or GAGCUAAACGGUUACAUCU.
To silence PI3K-C2α and PI3K-C2β, Jurkat T-cells were electroporated using the same siRNAs to PI3K-C2α or PI3K-C2β using Amaxa reagents and were studied 2 d after transfection. Silencing of PI3K-C2α and PI3K-C2β in the various experiments was confirmed by RT-PCR as previously described (Srivastava et al., 2008). PI3K-C2β mutant (PI3K-C2β mt) was generated by a point mutation and cloned into the vector pEGFP (Clontech, Palo Alto, CA).
Whole-Cell Patch-Clamp
CD4 T-Cells.
Whole-cell patch-clamping was performed on activated CD4 T-cells 48 h after stimulation with anti-CD3 and anti-CD28 antibodies as described (Wulff et al., 2003b) with some modification (Srivastava et al., 2006a). Free intracellular calcium at 1 μM was used in the internal solution. Current–voltage (IV) relationships were measured using ramp voltage-clamp protocols (at 10-s intervals) from a holding potential of −80 to −120 mV, followed by ramp depolarization to +60 mV of 20-ms duration. The IV relationship was obtained by plotting the current during the depolarizing ramp phase as a function of the corresponding voltage.
Jurkat T-Cells.
Whole-cell patch-clamping on Jurkat T-cells was performed as described above. To assess whether TCR stimulation leads to an increase in KCa3.1 channel activity, Jurkat T-cells were cultured together with Raji B-cells as an antigen presenting cells (at ration of 5:1) that had been preincubated with (activated) or without (control) the superantigen staphylococcal enterotoxin E (SEE) for 30 min at 37°C as previously described (Bueno et al., 2006). Activated KCa3.1 channel activity was assessed on Jurkat T-cells 15 min after forming a stable synapse with SEE-pulsed Raji B-cells. To assess whether overexpression of GFP-PI3K-C2α or PI3K-C2β affected KCa3.1 channel, whole-cell patch-clamp was performed on GFP-positive Jurkat cells conjugated to Raji B-cells.
To verify that silencing of PI3K-C2β led to decreased KCa3.1 channel activity by inhibiting the production of PI(3)P, we determined whether the addition of PI(3)P (100 nM) into the pipette solution during patch-clamping restored channel activity as previously described (Srivastava et al., 2005). PI(3)P diC16 [C41H45Na3O16P2 (C6)]was purchased from Echelon Biosciences (Salt Lake City, UT) and used according to specifications. PI(3)P diC16 was resuspended in water and flash frozen in liquid nitrogen and used at a concentration of 100 nM in the pipette solution.
Intracellular Ca2+ Activity.
Cells were loaded at 1 × 106 cells/ml with 5 μM Fura-2 AM ester (Molecular Probes, Eugene, OR) in RPMI medium for 30 min at room temperature, washed, and then resuspended in RPMI. Cells were attached to poly-l-lysine–coated coverslips for 20 min in a RC-20 bath flow chamber (Warner Instrument, Hamden, CT) and fura-2 fluorescence was recorded (Delta Ram; PTI, South Brunswick, NJ) at excitation wavelengths of 340 and 380 nm. Background fluorescence was obtained by treating the cells with 100 mM MnCl2 at the end of the experiment. Data are represented as the ratio of 340 to 380 after background subtraction. Cells were perfused with the bath solution (composition described before) in the presence or absence of extracellular calcium and stimulated with 5 μg/ml anti-CD3 cross-linked with 5 μg/ml rat anti-mouse IgG.
Quantitative RT-PCR.
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and then reverse-transcribed using random hexamer primers. Quantitative PCR was then assessed using SYBR Green 1 by iCycler iQ (Bio-Rad, Richmond, CA) using gene-specific primers purchased from Qiagen (Chatsworth, CA).
Planar Lipid Bilayers.
Glass-supported lipid bilayers were generated as described previously (Campi et al., 2005). Briefly, glass-supported dioleoylphosphatidylcholine bilayers incorporating ICAM-1 (intercellullar adhesion molecule 1; 300 molecules/μm2) and 0.1% cap-biotin were prepared in a Bioptechs (Butler, PA) flow cell. Unlabeled streptavidin (8 μg/ml) and monobiotinylated anti-human CD3, OKT3 clone (10 μg/ml) without a fluorophore (to assess localization of Cherry-Zap-70) or conjugated to Cy3, were loaded sequentially in HBS/HSA buffer.
Imaging of TCR, Zap-70, PI3K, and Phosphoprotein Immunofluorescence.
To assess whether GFP-PI3K-C2α or PI3K-C2β localize with the TCR or Zap-70 at the immunological synapse (IS), Jurkat cells that were transfected with either GFP-PI3K-C2α or GFP-PI3K-C2β with or without ch-Zap-70 were suspended in HEPES-buffered saline supplemented with 5 mM glucose, 2 mM MgCl2, 1 mM CaCl2, and 1% human serum albumin (HBS/HSA) and floated onto the lipid bilayer. Total internal reflection fluorescence microscopy (TIRFM) was used to assess localization of the TCR and GFP-PI3K-C2α or PI3K-C2β as previously described (Varma et al., 2006).
PI3K Assay.
Jurkat T-cells, transfected with GFP-PI3K-C2β, were stimulated with anti-CD3 and anti-CD28 antibodies for various times. Cells were lysed, and PI3K assay was performed on anti-GFP immunoprecipitates as previously described (Yan et al., 2009).
RESULTS
PI3KC2β Is Required for KCa3.1 Channel Activity in Activated Human CD4 T-Cells
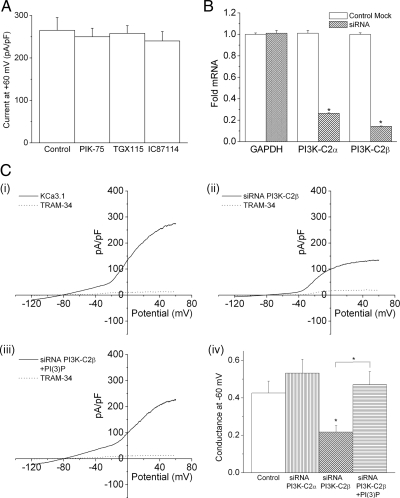
We have previously shown that PI(3)P is required for KCa3.1 channel activity in activated human CD4 T-cells (Srivastava et al., 2006a,b). To identify the specific PI3K isoform that is required to generate the PI(3)P pool in CD4 T-cells that mediates activation of KCa3.1, KCa3.1 channel activity was determined in cells treated with various class I isoform-specific p110 kinase inhibitors kindly provided by K. Shokat (UCSF Medical Center; Knight and Shokat, 2007). We found that none of these inhibited KCa3.1 channel activity (Figure 1A), suggesting that a class II or III PI3K is responsible for KCa3.1 activation in T-cells. Of the three class II PI3Ks that have been identified, PI3K-C2α and PI3K-C2β are expressed in lymphocytes (Cantley, 2002; Foster et al., 2003; Traer et al., 2006). To assess whether either of these two PI3Ks generate the PI(3)P pool that mediates activation of KCa3.1, naive human CD4 T-cells were transfected with a pool of siRNAs to PI3K-C2α or PI3K-C2β, and KCa3.1 channel activity was determined by whole-cell patch-clamp 48 h after stimulation with antibodies to CD3 and CD28. siRNA knockdown of PI3K-C2β, but not PI3K-C2α, markedly inhibited KCa3.1 channel activity (Figure 1C). The decrease in KCa3.1 channel activity was due to decreased levels of PI(3)P because dialyzing PI3K-C2β siRNA-transfected cells with PI(3)P, but not other phosphorylated phosphoinositide (PIs), rescued KCa3.1 channel activity (Figure 1C, iii and iv, and data not shown).
Figure 1.
Silencing of PI3KC2β in CD4 T cells by siRNA leads to a decrease in KCa3.1 channel activity. (A) Purified human CD4 T lymphocytes were stimulated with antibodies to CD3 and CD28 for 48 h. Whole cell patch clamping was performed 48 h after stimulation with or without 1 μM of the class 1 PI3K inhibitors shown (Knight and Shokat, 2007). (B, C) Purified CD4 T lymphocytes were transfected with a pool of siRNAs to PI3K-C2α or PI3K-C2β mRNA (Dharmacon) using AMAXA reagents and, after resting overnight, were stimulated with antibodies to CD3 and CD28 for 48 h. Whole cell patch-clamping was performed 48 h after stimulation. (B) Real time PCR showing >80% silencing of PI3K-C2α and PI3K-C2β mRNA. *p < 0.05 as compared to control cells. (C) Whole cell patch-clamping showing the I–V plot of (i) control cells (ii) siRNA PI3K-C2β cells and (iii) siRNA PI3K-C2β cells with 100 nM PI(3)P in the pipette solution. (iv) Bar graph summary of KCa3.l conductance (pS) measured at −60 mV (N = 12–l5 cells). Also shown is 1) decreased expression of PI3K-C2α by siRNA does not affect KCa3.1 conductance and 2) dialyzing PI3K-C2β siRNA transfected cells with 100 nM PI(3)P rescue KCa3.l conductance. *p < 0.05 as compared to control KCa3.1 conductance and as indicated in the graph.
TCR Stimulation Activates KCa3.1 Channel Activity via a Calcium-independent Mechanism
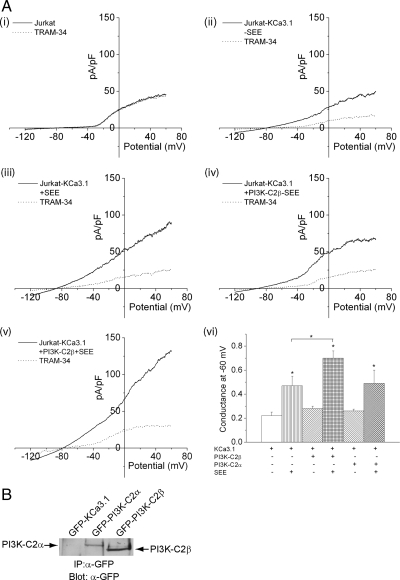
KCa3.1 channel activity is known to increase after TCR stimulation. The increase in KCa3.1 channel activity has been proposed to be mediated by the rise in intracellular calcium that occurs after TCR stimulation; binding of calcium to calmodulin, which is bound to the carboxy-terminus of KCa3.1, is critical for KCa3.1 channel activation (Fanger et al., 2001). Our finding that PI3K-C2β is also critical for KCa3.1 channel activity suggested that activation of PI3K-C2β by TCR activation could contribute to KCa3.1 activation via the generation of PI(3)P. In contrast to “normal” T-cells, Jurkat T-cells do not contain KCa3.1 channel activity (Figure 2Ai). Instead, they express apamin-sensitive KCa2.2 channels (Fanger et al., 2001). Therefore, to assess whether TCR stimulation contributes to KCa3.1 channel activity via activation of PI3K-C2β, Jurkat T-cells that overexpress KCa3.1 channels were generated (Jurkat-KCa.3.1) and found to contain KCa3.1 channel activity by whole-cell patch-clamp as determined by dependence on Ca2+ and inhibition with TRAM-34, a known inhibitor of KCa3.1 channel (Figure 2Aii). To assess whether TCR stimulation activates KCa3.1 via a Ca2+-independent mechanism, Jurkat-KCa3.1 cells were cocultured with Raji B-cells that were pulsed with the superantigen SEE and KCa3.1 channel activity was determined 5–15 min after establishing a productive synapse. These findings demonstrated that KCa3.1 channel activity in Jurkat-KCa3.1 cells was increased about twofold after TCR activation when compared with Jurkat-KCa3.1 cells cocultured with Raji B-cells in the absence of SEE (Figure 2A, compare ii and iii, summary vi). The increase in KCa3.1 channel activity was independent of TCR-stimulated increase in intracellular Ca2+ because whole-cell patch-clamp experiments were performed in the presence of 1 μM free calcium in the pipette solution, and therefore intracellular calcium concentrations are not rate-limiting in these experiments.
Figure 2.
TCR stimulation of Jurkat T-cells activates KCa3.1 channel activity. Jurkat T-cells overexpressing KCa3.1 (Jurkat-KCa3.1) were transfected with GFP, GFP-PI3K-C2β, or GFP-PI3K-C2α and incubated with Raji B-cells that were either untreated or treated with the superantigen SEE as described in Materials and Methods. (A, ii–v) Whole-cell patch- clamp was then performed on GFP-positive cells that were incubated with Raji B-cells that were either untreated or treated with the superantigen SEE as described in Materials and Methods. (vi) Bar graph summary of KCa3.1 conductance (pS) measured at −60 mV from 10 independent experiments. (A, i) shows control Jurkat T-cells do not have KCa3.1 channel activity. *p < 0.05 compared with KCa3.1 conductance measured at −60 mV without the addition of SEE in each group and as indicated in the figure. (B) α-GFP Western blot of GFP-sorted cells demonstrating equal protein expression of GFP-PI3K-C2α and PI3K-C2β.
PI3K-C2β Mediates KCa3.1 Channel Activation after TCR Stimulation
To assess whether the increase in KCa3.1 channel activity was due to the activation of PI3K-C2β, KCa3.1channel activity was performed on Jurkat-KCa3.1 cells that overexpress PI3K-C2β. Although overexpression of PI3K-C2β did not affect basal KCa3.1 channel activity (Figure 2A, compare ii and iv, summary vi), stimulation with Raji B-cells pulsed with SEE led to a further 1.5-fold increase in KCa3.1 channel activity when compared with Jurkat-KCa3.1 cells not overexpressing PI3K-C2β (Figure 2A, compare iii and v, with vi). In contrast, KCa3.1 channel activity was not increased in Jurkat-KCa3.1 cells that overexpressed PI3K-C2α (Figure 2Avi) despite similar levels of protein expression (Figure 2B).
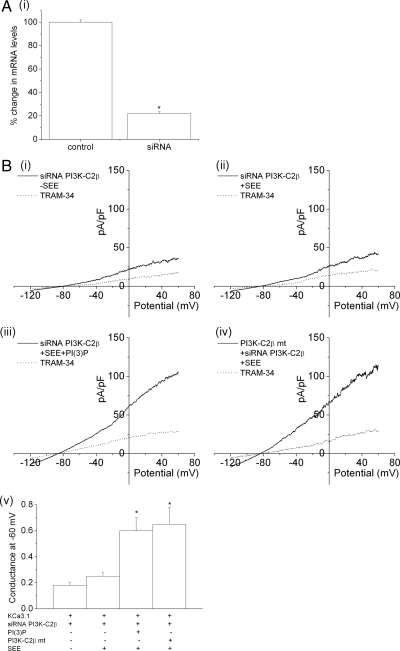
To directly assess whether endogenous PI3K-C2β mediates TCR-stimulated increase in KCa3.1 channel activity, KCa3.1 channel activity was assessed in Jurkat-KCa3.1 cells transfected with a siRNA to PI3K-C2β. In comparison to control Jurkat-KCa3.1 cells, TCR-stimulated increase in KCa3.1 channel activity was markedly impaired in siRNA PI3K-C2β–transfected cells (Figure 3B, compare i and ii, summary v). Moreover, the decrease in KCa3.1 channel activity in siRNA PI3K-C2β–transfected cells was due to decreased levels of PI(3)P because adding back PI(3)P into the pipette solution rescued KCa3.1 channel activity to levels comparable to cells transfected with PI3K-C2β (Figure 3, iii and v). Moreover, the inhibition was specific; channel activity was rescued in siRNA-transfected by transfecting a GFP-PI3K-C2β mutant that abrogated binding to the siRNA (Figure 3B, iv and v). These findings when taken together suggest that the calcium-independent increase in TCR-stimulated KCa3.1 channel activity is mediated via the activation of PI3K-C2β.
Figure 3.
siRNA knockdown of GFP-PI3K-C2β inhibits TCR-stimulated activation of KCa3.1 channel activity. (A) Real-time PCR showing >80% silencing of PI3K-C2β in siRNA PI3K-C2β siRNA-transfected cells. *p < 0.05 compared with control. (B) Jurkat-KCa3.1 cells were transfected with a pool of siRNAs to PI3K-C2β and stimulated 48 h after transfection with Raji B-cells that were either untreated (i) or treated with SEE (ii). Whole-cell patch clamp was then performed as described in Figure 2. (iii) To determine whether inhibition of KCa3.1 channel activity in siRNA PI3K-C2β–transfected cells was due to decreased levels of PI(3)P, siRNA PI3K-C2β–transfected cells were dialyzed with PI(3)P as described in Figure 1. (iv) In addition, KCa3.1 channel activity was rescued by transfecting a GFP-PI3K-C2β mutant (PI3K-C2β mt) that abrogated interaction with the siRNA. (v) Bar graph summary of KCa3.1 conductance (pS) measured at −60 mV (n = 10 cells). *p < 0.05 compared with siRNA PI3KC2β + SEE–transfected cells.
PI3K-C2β Also Plays a Critical Role in Augmented Calcium Influx after TCR Stimulation
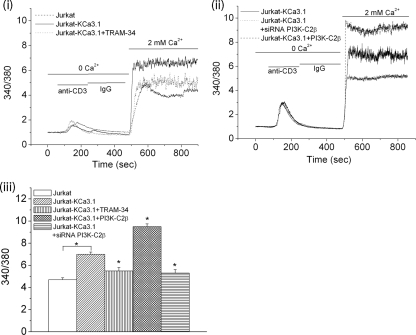
By mediating the efflux of K+, KCa3.1 functions to maintain a hyperpolarized membrane potential that provides the electrochemical gradient that drives Ca2+ entry into a subset of T- and B-lymphocytes (Cahalan et al., 2001; Wulff et al., 2003a). Although control Jurkat T-cells do not contain KCa3.1 channels (Figure 2A), they have other K+ channels such as KCa2.2, which are not regulated by PI(3)P and can substitute for KCa3.1 to promote anti-CD3 stimulated Ca2+ influx (Figure 4i). Overexpression of KCa3.1 in Jurkat T-cells (Jurkat-KCa3.1) led to marked increase in anti-CD3–stimulated Ca2+ influx that, upon treatment with TRAM-34, inhibited Ca2+ influx to levels seen in control Jurkat T-cells, confirming that the increase in Ca2+ influx was due to KCa3.1 (Figure 4i). To address whether PI3K-C2β plays a critical role in TCR-stimulated Ca2+ influx by KCa3.1, Ca2+ influx was assessed in TCR-stimulated Jurkat-KCa3.1 cells in which PI3K-C2β was overexpressed or silenced with a PI3K-C2β siRNA (Figure 4, ii and iii). Consistent with the changes seen in KCa3.1 channel activity shown in Figure 3, TCR-stimulated Ca2+ influx was increased in Jurkat-KCa3.1 cells overexpressing PI3K-C2β, whereas Ca2+ influx was inhibited in siRNA PI3K-C2β–transfected cells (Figure 4, ii and iii). The finding that both siRNA PI3K-C2β and TRAM-34 inhibited Ca2+ influx to a similar degree (Figure 4iii), coupled with the findings that TRAM-34 did not further inhibit Ca2+ influx in siRNA PI3K-C2β–transfected cells (data not shown), indicates that PI3K-C2β specifically regulates KCa3.1-dependent Ca2+ influx in these cells.
Figure 4.
PI3K-C2β is required for maximal Ca2+ flux in Jurkat-KCa3.1 cells. (i and ii) A representative trace of 340/380 fura-2 fluorescence ratio from Jurkat T-cells or Jurkat-KCa3.1 T-cells transfected with GFP, GFP-PI3K-C2β, or a siRNA to PI3K-C2β. Cells were loaded with Fura-2 AM (5 μM) for 30 min, and the fluorescence measurements were made at RT. Fura-2 fluorescence was recorded (Delta Ram; PTI) at excitation wavelengths of 340 and 380 nm after cross-linking with anti-CD3 antibodies as indicated. (iii) Bar graph summary of peak 340/380 fluorescence after perfusing the cells with 2 mM Ca2+. Average values from 50–70 cells are shown for each condition. *p < 0.05 compared with fluorescence values of Jurkat-KCa3.1 alone and as indicated.
PI3K-C2β Is Recruited to the IS Where it Colocalizes with the TCR and ZAP-70
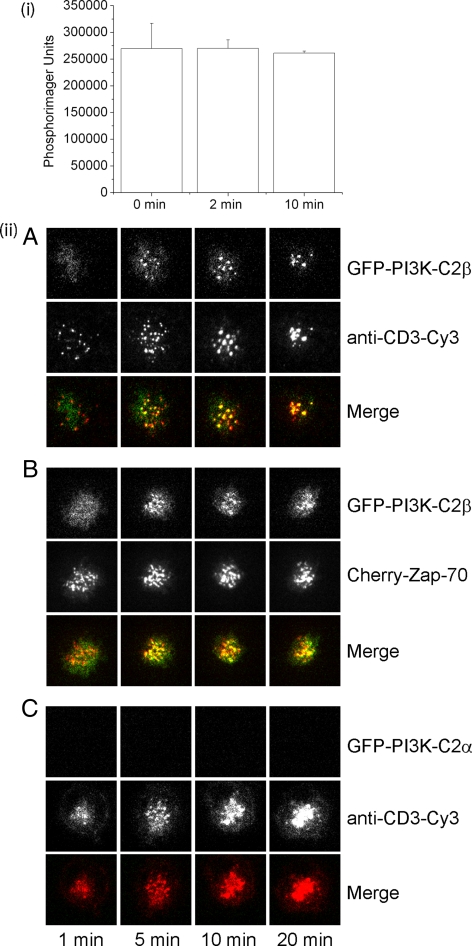
To explore the mechanism whereby PI3K-C2β is activated by TCR stimulation, we initially determined whether PI3K-C2β's enzymatic activity was increased after TCR stimulation. Jurkat-KCa3.1 cells were transfected with GFP- PI3K-C2β, and PI3K-C2β enzymatic activity was determined on anti-GFP immunoprecipitates at various times after treatment with anti-CD3 antibodies. These studies demonstrated that TCR cross-linking did not increase PI3K enzyme activity (Figure 5i). PI3K activity was not detected in untransfected cells, indicating that the PI3K activity detected was specific. In addition, anti-phosphotyrosine Western blots demonstrated an increase in tyrosine-phosphorylated proteins, indicating that the cells were stimulated (data not shown).
Figure 5.
PI3K-C2β colocalizes with the TCR and Zap70 in the immunological synapse (IS) after stimulation with anti-CD3 antibodies. (i) PI3K enzyme activity is not increased after TCR stimulation. Jurkat T-cells overexpression PI3K-C2β were stimulated with anti-CD3 and anti-CD28 antibodies. Cells were then lysed at various time points, and PI3K activity was determined on anti-GFP immunoprecipitates. Shown are phosphorimager units incorporated into PI. The data shown are ±SEM done in triplicate. (ii) Jurkat T-cells that were transfected with GFP-PI3K-C2β (A) or PI3K-C2α (C) were floated onto glass-supported dioleoylphosphatidylcholine bilayers incorporating GPI-linked ICAM-1– and Cy3-conjugated anti-human CD3 (10 μg/ml). Total internal reflection fluorescence microscopy (TIRFM) was used to assess localization of CD3 and PI3K-C2β at various times after adhering to the bilayer. (B) Jurkat T-cells transfected with GFP-PI3K-C2β together with Cherry-Zap70, were floated onto planar lipid bilayers containing unlabeled CD3, and cellular localization of PI3K-C2β and Zap70 was determined as described in A.
We next determined whether TCR stimulation affected the subcellular localization of PI3K-C2β; recruitment of GFP- PI3K-C2β to the plasma membrane (PM) could be an alternative mechanism whereby TCR stimulation could lead to an increase in PM PI(3)P. It is now known that organization of signaling molecules in T-cells at the IS is central to their regulation (Dustin, 2006; Varma et al., 2006). After activation, TCR clusters that are initially scattered throughout the interface are transported to the center of the contact area within 5–30 min to form a central supramolecular activation cluster (cSMAC) (Monks et al., 1998; Grakoui et al., 1999b; Dustin and Cooper, 2000; Freiberg et al., 2002a; Campi et al., 2005). Adhesion molecules such as LFA-1 and the cytoskeletal protein talin rapidly segregate from the TCR and form a ring referred to as the peripheral SMAC (pSMAC; Monks et al., 1998; Grakoui et al., 1999a). The formation of a stable IS with a ring of adhesion molecules is a common molecular pattern associated with T-cell activation and effector functions. A third structure in the IS distal to the pSMAC has been referred to as the distal SMAC (dSMAC), which is rich in CD45 (Freiberg et al., 2002b). In addition, TCR microclusters, which contain active signaling molecules such as Lck and Zap-70, form early after TCR stimulation and continually form during sustained signals (Krummel et al., 2000; Campi et al., 2005; Seminario and Bunnell, 2008). TCR microclusters eventually converge on the cSMAC where elements involved in sustained signaling and signal termination are segregated (Varma et al., 2006; Cemerski et al., 2008). The balance between T-cell receptor signaling and degradation at the center of the IS is determined by antigen quality and spatiotemporal regulation of T-cell costimulation by TCR-CD28 microclusters and protein kinase C translocation (Yokosuka et al., 2008).
To determine whether GFP-PI3K-C2β is recruited to the IS, Jurkat T-cells transfected with GFP-PI3K-C2β, were floated over planar lipid bilayers that were preloaded with ICAM-1 and Cy-3–labeled anti-CD3 antibodies. Cells were then visualized at various time points using TIRFM as previously described (Varma et al., 2006). We found that within 5 min after being exposed to the planar lipid bilayer PI3K-C2β colocalizes with CD3 in the microclusters, which over time converge in cSMAC (Figure 5ii, A). The recruitment was specific because under the same conditions, GFP-PI3K-C2α, which does not play a role in activation of KCa3.1, was not recruited (Figure 5ii, C). The tyrosine kinase Zap-70 is rapidly recruited to the microclusters where it also colocalizes with the TCR. To assess whether GFP-PI3K-C2β also colocalizes with proximal signaling molecules downstream of TCR, experiments were carried out in the Jurkat T-cells transfected with GFP-PI3K-C2β cherry-Zap-70. In similar experiments, we found that GFP-PI3K-C2β also colocalized with ZAP-70 (Figure 5ii, B). Thus, these findings suggests that recruitment of PI3K-C2β, to the IS may play a critical role in its regulation.
DISCUSSION
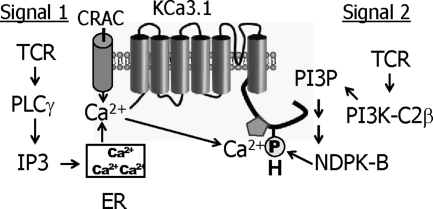
In contrast to the class I and III PI3K, little is still known about either the biological functions regulated by the class II PI3Ks or the mechanism whereby they are regulated. We now find that the class II PI3K-C2β is activated after TCR stimulation and plays a central role in generating the PI(3)P pool that mediates activation of the K+ channel KCa3.1. Moreover, although TCR stimulation does not increase PI3K-C2β catalytic activity, it does stimulate the recruitment of PI3K-C2β to the IS where it colocalizes with both the TCR and Zap-70. These findings suggest that calcium influx in some T-cell subsets requires the simultaneous activation of two parallel pathways after TCR stimulation (Figure 6). On the one hand, TCR stimulation leads to the activation of PLCγ leading to the generation of IP3 and diacylglycerol. Binding of IP3 to its receptor in the ER leads to release of Ca2+, which in turn results in opening of CRAC channels and the influx of calcium (Cahalan et al., 2007; Feske, 2007). Ca2+ influx, via binding to calmodulin bound to the CT of KCa3.1, also activates KCa3.1. TCR stimulation also activates PI3K-C2β, which generates the pool of PI(3)P required for KCa3.1 activation. The combined effect of increased intracellular calcium, together with PI(3)P-stimulated activation of NDPK-B, ensures that KCa3.1 channels are fully active to facilitate sufficient calcium entry via CRAC channels to activate NFAT-dependent signaling pathways.
Figure 6.
Schematic for TCR-stimulated activation of KCa3.1. Activation of two signaling pathways is required for TCR-stimulated activation of KCa3.1. Signal 1, TCR stimulation leads to the activation of PLCγ leading to the generation of IP3, stimulating release of Ca2+ from the ER, resulting in opening of CRAC channels and the influx of Ca2+; signal 2, TCR stimulation also leads to the activation of PI3K-C2β leading to the generation of PI(3)P at the plasma membrane, which is required for NDPK-B to phosphorylate histidine 358 in the carboxy-terminus of KCa3.1. Both binding of Ca2+ to the calmodulin bound to the CT of KCa3.1 and phosphorylation of H358 in CT of KCa3.1 by NPDK-B is required for KCa3.1 activation.
Previous work on PI3 kinase in T-cells has focused on the class I PI3Ks for which the biological function and regulation in multiple biological processes are well described. Previous studies using knockout mice have demonstrated important roles for both p110δ and p110γ in T-cell activation (Webb et al., 2005; Swat et al., 2006; Fruman, 2007; Patton et al., 2007). These studies demonstrated that TCR-stimulated activation of these class I PI3Ks generate predominantly PI(3,4)P2 and PI(3,4,5)P3, which function to bind and activate PH-domain–containing proteins such as AKT. Our demonstration that PI3K-C2β is activated after TCR stimulation and generates the PI(3)P pool in response to TCR stimulation which mediates KCa3.1 activation, adds to the increasing role for class II PI3Ks in regulating agonist-stimulated intracellular functions. This is consistent with other studies demonstrating that PI and to a lesser extent PI(4)P are the preferred substrate for class II PI3Ks, and that one function of class II PI3Ks is to increase the level of PI(3)P at the PM (Maffucci et al., 2005; Falasca et al., 2007). These studies demonstrated that generation of PM PI(3)P by PI3K-C2β is critical for lysophosphatidic acid (LPA)-stimulated cell migration (Maffucci et al., 2005) and that the generation of PM PI(3)P by PI3K-C2α is critical for insulin-stimulated GLUT 4 translocation (Falasca et al., 2007). PM PI(3)P is also critical for activation of KCa3.1 (Srivastava et al., 2005). Moreover, our finding that KCa3.1 channel activity in T-cells transfected with an siRNA to PI3K-C2β is restored by dialyzing these cells with PI(3)P, and not other phosphorylated PIs, confirms that PI(3)P is the in vivo product generated by PI3K-C2β in T-cells. The ability of agonist activation of the class II PI3K to increase PM PI(3)P levels is therefore distinct from the role for the class III PI3K Vps34, which generates the majority of the constitutive PI(3)P in the cell that is localized primarily to the endosomal compartment (Gillooly et al., 2001; Yan and Backer, 2007).
There are three mammalian class II PI3Ks: PI3K-C2α, PI3K-C2β, and PI3K-C2γ, of which PI3K-C2α and PI3K-C2β are widely expressed (Cantley, 2002; Foster et al., 2003; Traer et al., 2006). In contrast to class I PI3K, all the class II PI3Ks contain an extended C-terminus composed of tandem PX and C2 domains and lack regulatory domains. A number of studies have demonstrated activation of PI3K-C2α or PI3K-C2β by a number of agonists including epidermal growth factor (EGF), integrins, insulin, LPA, stem cell factor (SCF), and chemokines, as well as via the interaction with clatharin (Arcaro et al., 2000, 2002; Gaidarov et al., 2001; Maffucci et al., 2005; Traer et al., 2006; Falasca et al., 2007), and although insulin has been shown to activate both isoforms (Brown and Shepherd, 2001), for the most part either PI3K-C2α or PI3K-C2β is activated by these stimuli. Our finding that PI3K-C2β and not PI3K-C2α generates the pool of PI(3)P that is required for KCa3.1 channel activity and T-cell activation reinforces the idea that each class II PI3K isoform mediates distinct biological functions. This was further supported by the finding that only PI3K-C2β, and not PI3K-C2α, is recruited to the peripheral microclusters in the IS after TCR (activation). Although potential mechanisms whereby only one class II PI3K isoform couples to a specific upstream signal are still poorly defined, both the N- and C-terminal extension have been proposed to play critical role in their regulation (Traer et al., 2006). Recruitment of PI3K-C2β to peripheral microclusters, by localizing PI3K-C2β to the PM, is likely to play an important role in generating PM PI(3)P after TCR stimulation. In addition, the finding that PI3K-C2β colocalizes with the TCR and Zap70 in peripheral microclusters containing active tyrosine kinases, such as Zap-70 and Lck, suggests that TCR signaling may also directly activate PI3K-C2β. However, so far we have been unable to demonstrate that this recruitment functions to either stimulate the tyrosine phosphorylation of PI3K-C2β as has been described in insulin, EGF, and SCF-stimulated cells, or to increase PI3K-C2β's enzymatic activity as has been described for insulin (Brown and Shepherd, 2001).
Another hypothesis we are now testing is that spatial arrangement in the IS of the various molecules that have been shown to regulate KCa3.1 is critical to KCa3.1 regulation. For example, our previous work has demonstrated that generation of PI(3)P is required to enable NDPK-B to phosphorylate the CT of KCa3.1, leading to KCa3.1 activation (Srivastava et al., 2006b). Thus, one plausible hypothesis is that recruitment of NDPK-B, PI3K-C2β, and KCa3.1 to peripheral microclusters in the IS is critical for the activation of KCa3.1. This is at least partly supported by a previous report showing that KCa3.1 is recruited to the IS, although the exact location of in the IS was not identified (Nicolaou et al., 2007). On the flip side, segregation of the negative regulators MTMR6 and PHPT1 away from peripheral microclusters would be one way of ensuring continuous signaling in the context of sustained TCR activation. As discussed above, it is also now recognized that signaling molecules in peripheral microcluster stream into the cSMAC, where TCR signaling is terminated by dephosphorylation and incorporation into multivesicular bodies (Campi et al., 2005; Varma et al., 2006), while an active signaling compartment is maintained (Yokosuka et al., 2008). Localization of MTMR6 and PHPT1 to the cSMAC could provide one possible mechanism that would enable both these molecules to inhibit KCa3.1 activity once TCR stimulation abates, without interfering with channel activation in the context of ongoing TCR stimulation. In fact, such a model has been proposed for the tyrosine phosphatase CD45, which is both excluded from peripheral microclusters and concentrated in the cSMAC where it is positioned to contribute to the dephosphorylation of active signaling molecules associated with the TCR (Varma et al., 2006).
ACKNOWLEDGMENTS
We thank J. Domin (Imperial College, London) for the GFP-PI3K-C2α and PI3K-C2β constructs and K. Shokat (UCSF Medical Center) for the class I PI3K inhibitors. E.Y.S. is supported by National Institutes of Health Grants RO1GM084195 and RO1AI052459.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0390) on July 8, 2009.
REFERENCES
- Arcaro A., Khanzada U. K., Vanhaesebroeck B., Tetley T. D., Waterfield M. D., Seckl M. J. Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation. EMBO J. 2002;21:5097–5108. doi: 10.1093/emboj/cdf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A., Zvelebil M. J., Wallasch C., Ullrich A., Waterfield M. D., Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol. Cell. Biol. 2000;20:3817–3830. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C., et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A., Shepherd P. R. Growth factor regulation of the novel class II phosphoinositide 3-kinases. Biochem. Soc. Trans. 2001;29:535–537. doi: 10.1042/bst0290535. [DOI] [PubMed] [Google Scholar]
- Bueno C., Lemke C. D., Criado G., Baroja M. L., Ferguson S. S., Rahman A. K., Tsoukas C. D., McCormick J. K., Madrenas J. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Wulff H., Chandy K. G. Molecular properties and physiological roles of ion channels in the immune system. J. Clin. Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Zhang S. L., Yeromin A. V., Ohlsen K., Roos J., Stauderman K. A. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi G., Varma R., Dustin M. L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cemerski S., Das J., Giurisato E., Markiewicz M. A., Allen P. M., Chakraborty A. K., Shaw A. S. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Olson E. N. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Dustin M. L. Impact of the immunological synapse on T cell signaling. Results Probl. Cell Differ. 2006;43:175–198. doi: 10.1007/400_019. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Cooper J. A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- Falasca M., Hughes W. E., Dominguez V., Sala G., Fostira F., Fang M. Q., Cazzolli R., Shepherd P. R., James D. E., Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J. Biol. Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- Fanger C. M., Rauer H., Neben A. L., Miller M. J., Wulff H., Rosa J. C., Ganellin C. R., Chandy K. G., Cahalan M. D. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J. Biol. Chem. 2001;276:12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Foster F. M., Traer C. J., Abraham S. M., Fry M. J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- Freiberg B. A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D. M., Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002a;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- Freiberg B. A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D. M., Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002b;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- Fruman D. A. The role of class I phosphoinositide 3-kinase in T-cell function and autoimmunity. Biochem. Soc. Trans. 2007;35:177–180. doi: 10.1042/BST0350177. [DOI] [PubMed] [Google Scholar]
- Gaidarov I., Smith M. E., Domin J., Keen J. H. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell. 2001;7:443–449. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Ghanshani S., Wulff H., Miller M. J., Rohm H., Neben A., Gutman G. A., Cahalan M. D., Chandy K. G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Gillooly D. J., Simonsen A., Stenmark H. Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J. 2001;355:249–258. doi: 10.1042/0264-6021:3550249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999a;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999b;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Keen J. E., Khawaled R., Farrens D. L., Neelands T., Rivard A., Bond C. T., Janowsky A., Fakler B., Adelman J. P., Maylie J. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J. Neurosci. 1999;19:8830–8838. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z. A., Shokat K. M. Chemically targeting the PI3K family. Biochem. Soc. Trans. 2007;35:245–249. doi: 10.1042/BST0350245. [DOI] [PubMed] [Google Scholar]
- Krummel M. F., Sjaastad M. D., Wulfing C., Davis M. M. Differential clustering of CD4 and CD3zeta during T cell recognition. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- Maffucci T., Cooke F. T., Foster F. M., Traer C. J., Fry M. J., Falasca M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell Biol. 2005;169:789–799. doi: 10.1083/jcb.200408005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Bond C. T., Herson P. S., Lee W. S., Adelman J. P. Small conductance Ca2+-activated K+ channels and calmodulin. J. Physiol. 2004;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Nicolaou S. A., Neumeier L., Peng Y., Devor D. C., Conforti L. The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am J. Physiol. Cell Physiol. 2007;292:C1431–C1439. doi: 10.1152/ajpcell.00376.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K., et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Patton D. T., Garcon F., Okkenhaug K. The PI3K p110delta controls T-cell development, differentiation and regulation. Biochem. Soc. Trans. 2007;35:167–171. doi: 10.1042/BST0350167. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Borlado L., Barber D. F., Hernandez C., Rodriguez-Marcos M. A., Sanchez A., Hirsch E., Wymann M., Martinez A. C., Carrera A. C. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J. Immunol. 2003;170:4475–4482. doi: 10.4049/jimmunol.170.9.4475. [DOI] [PubMed] [Google Scholar]
- Sasaki T., et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Seminario M. C., Bunnell S. C. Signal initiation in T-cell receptor microclusters. Immunol. Rev. 2008;221:90–106. doi: 10.1111/j.1600-065X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Ko K., Choudhury P., Li Z., Johnson A. K., Nadkarni V., Unutmaz D., Coetzee W. A., Skolnik E. Y. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol. Cell. Biol. 2006a;26:5595–5602. doi: 10.1128/MCB.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol. Cell. 2006b;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Li Z., Lin L., Liu G., Ko K., Coetzee W. A., Skolnik E. Y. The phosphatidylinositol 3-phosphate phosphatase myotubularin- related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol. Cell. Biol. 2005;25:3630–3638. doi: 10.1128/MCB.25.9.3630-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Zhdanova O., Di L., Li Z., Albaqumi M., Wulff H., Skolnik E. Y. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc. Natl. Acad. Sci. USA. 2008;105:14442–14446. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swat W., Montgrain V., Doggett T. A., Douangpanya J., Puri K., Vermi W., Diacovo T. G. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traer C. J., Foster F. M., Abraham S. M., Fry M. J. Are class II phosphoinositide 3-kinases potential targets for anticancer therapies? Bull. Cancer. 2006;93:E53–58. [PubMed] [Google Scholar]
- Varma R., Campi G., Yokosuka T., Saito T., Dustin M. L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. M., Vigorito E., Wymann M. P., Hirsch E., Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J. Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- Wulff H., Beeton C., Chandy K. G. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Dev. 2003a;6:640–647. [PubMed] [Google Scholar]
- Wulff H., Calabresi P. A., Allie R., Yun S., Pennington M., Beeton C., Chandy K. G. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Invest. 2003b;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. M., et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Yan Y., Backer J. M. Regulation of class III (Vps34) PI3Ks. Biochem. Soc. Trans. 2007;35:239–241. doi: 10.1042/BST0350239. [DOI] [PubMed] [Google Scholar]
- Yan Y., Flinn R. J., Wu H., Schnur R. S., Backer J. M. hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem. J. 2009;417:747–755. doi: 10.1042/BJ20081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T., Kobayashi W., Sakata-Sogawa K., Takamatsu M., Hashimoto-Tane A., Dustin M. L., Tokunaga M., Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]