Abstract
Obscurin is a large (∼800-kDa), modular protein of striated muscle that concentrates around the M-bands and Z-disks of each sarcomere, where it is well positioned to sense contractile activity. Obscurin contains several signaling domains, including a rho-guanine nucleotide exchange factor (rhoGEF) domain and tandem pleckstrin homology domain, consistent with a role in rho signaling in muscle. We investigated the ability of obscurin's rhoGEF domain to interact with and activate small GTPases. Using a combination of in vitro and in vivo approaches, we found that the rhoGEF domain of obscurin binds selectively to rhoA, and that rhoA colocalizes with obscurin at the M-band in skeletal muscle. Other small GTPases, including rac1 and cdc42, neither associate with the rhoGEF domain of obscurin nor concentrate at the level of the M-bands. Furthermore, overexpression of the rhoGEF domain of obscurin in adult skeletal muscle selectively increases rhoA expression and activity in this tissue. Overexpression of obscurin's rhoGEF domain and its effects on rhoA alter the expression of rho kinase and citron kinase, both of which can be activated by rhoA in other tissues. Injuries to rodent hindlimb muscles caused by large-strain lengthening contractions increases rhoA activity and displaces it from the M-bands to Z-disks, similar to the effects of overexpression of obscurin's rhoGEF domain. Our results suggest that obscurin's rhoGEF domain signals at least in part by inducing rhoA expression and activation, and altering the expression of downstream kinases in vitro and in vivo.
INTRODUCTION
The three largest proteins of the sarcomere, titin, nebulin, and obscurin, are critical for the assembly of myofibrils and the structural integrity of the sarcomere. The organization of actin and myosin in the mature myofibril is regulated by nebulin and titin, respectively, but the functions of obscurin, the most recently discovered of the three, are still poorly understood. An orthologue of Unc89 (Benian et al., 1996; Sutter et al., 2004), obscurin was first identified in mammals as a ligand of titin. Like titin, it is a multidomain protein (Young et al., 2001) composed largely of immunoglobulin-like (Ig) domains, which, with two fibronectin-like III (FnIII) domains, comprise its N-terminal and central regions. In the A isoform of obscurin, the C-terminal region of obscurin contains a calmodulin-binding (IQ) motif, followed by several Ig domains, a Src homology (SH) 3 domain, a rho-guanine nucleotide exchange factor (rhoGEF) domain, and tandem pleckstrin homology (PH) domain, two more Ig domains, and some nonmodular sequence. The nonmodular region at the extreme carboxy terminus includes several putative phosphorylation sites for extracellular signal-regulated kinase (ERK) kinases, as well as a binding site for a small, integral membrane form of ankyrin that concentrates in the network sarcoplasmic reticulum (nSR) (Young et al., 2001; Russell et al., 2002; Bagnato et al., 2003; Kontrogianni-Konstantopoulos et al., 2003; Borzok et al., 2007). This isoform of obscurin, which is ∼800 kDa in mass, localizes predominantly to the M-band during sarcomerogenesis and in mature muscle (Young et al., 2001; Borisov et al., 2004; Kontrogianni-Konstantopoulos et al., 2006a). Striated muscle also expresses a larger (∼900-kDa) B isoform of obscurin, which instead of the C-terminal Ig domains and nonmodular sequence contains Ser/Thr kinase domains (Young et al., 2001; Russell et al., 2002). This isoform is likely to be present primarily at the A/I junction of skeletal muscle (Bowman et al., 2007). Other small, alternatively spliced forms of obscurin are also expressed in muscle and associate with distinct structures. In particular, smaller forms containing the rhoGEF domain of obscurin accumulate at the level of the Z-disk or Z/I junction as sarcomeres mature (Borisov et al., 2004).
Studies using small interfering RNAs (siRNAs) directed against the 5′ coding region of the large isoform of obscurin demonstrate that obscurin is critical for organization of the M-band, A-band, and nSR in developing myotubes (Borisov et al., 2004, 2006; Kontrogianni-Konstantopoulos et al., 2004, 2006b), consistent with the presence of obscurin or its splice variants at several locations around the sarcomere, including M-bands and Z-disks (Kontrogianni-Konstantopoulos et al., 2006a,b; Musa et al., 2006). Its interaction with the nSR is almost certainly facilitated by its presence at the periphery, rather than the interior, of myofibrils (Kontrogianni-Konstantopoulos and Bloch, 2005; Carlsson et al., 2008). The subcellular localization of obscurin and its interactions with key elements of the contractile apparatus and intracellular membranes underscore its importance to the architecture and structural integrity of muscle, but the fact that it is alternatively spliced has made it difficult to define the particular subcellular locations or functional roles of the various forms of obscurin in developing or mature cardiac and skeletal muscle (Bagnato et al., 2003; Kontrogianni-Konstantopoulos et al., 2003, 2004, 2006a,b; Kontrogianni-Konstantopoulos and Bloch, 2005; Armani et al., 2006; Raeker et al., 2006; Borisov et al., 2008; Borzok et al., 2007; Bowman et al., 2007; Carlsson et al., 2008).
The roles obscurin plays in signal transduction have been a topic of interest since its signaling motifs, including a calmodulin-binding motif, SH3 domain, rhoGEF domain, and tandem PH domain, as well as its phosphorylation consensus motifs for ERK kinases were first recognized (Young et al., 2001). Although each of these domains may also bind to other structural proteins—indeed, we recently reported that the rhoGEF domain binds to RanBP9 in muscle (Bowman et al., 2008)—the presence of these signaling regions suggests that obscurin may participate in multiple signal transduction pathways in muscle. This is consistent with obscurin's central roles in assembling the sarcomere and sarcoplasmic reticulum. Of particular interest is the role or roles that obscurin may play in regulating rho signaling in muscle.
The rho family of small GTPases, particularly rhoA, rac1, and cdc42, have been well characterized for their ability to modulate actin reorganization, regulate transcription, and participate in control of the cell cycle (Kjoller and Hall, 1999; Brown et al., 2006), and they play key roles in the development and maintenance of skeletal muscle. Rac1 and cdc42 are critical for myotube formation, and they are necessary for transcription of muscle-specific genes, including myogenin, troponin T, and the heavy chain of myosin (Luo et al., 1994; Takano et al., 1998; Meriane et al., 2000). RhoA activates myogenesis and promotes differentiation of skeletal muscle, in part by regulating serum response factor (SRF) (Hill et al., 1995; Takano et al., 1998; Wei et al., 1998; Charrasse et al., 2003, 2006; Castellani et al., 2006), which in turn is required for MyoD expression (Carnac et al., 1998). It also enhances survival of myoblasts and myoblast fusion, which promotes skeletal muscle cell differentiation (Reuveny et al., 2004; Castellani et al., 2006). RhoA elicits its effects through activation of kinases, including rho-kinase (ROCK) and citron kinase (CRIK), as well as through interaction with scaffolding proteins, including rhotekin and diaphanous, and through transcriptional activation of genes containing a serum response element (SRE) in their promoter (Sahai et al., 1998; Wei et al., 1998; Lin et al., 2002; Carson et al., 2003; Shandala et al., 2004; Ahuja et al., 2007). RhoA-CRIK signaling mediates cytokinesis and Golgi organization via changes in the actin cytoskeleton in both muscle and nerve (Camera et al., 2003; Shandala et al., 2004; Ahuja et al., 2007; Berto et al., 2007). RhoA-ROCK1 signaling regulates myoblast fusion as well as muscle hypertrophy, primarily by regulating the nuclear localization of SRF (Wei et al., 1998; Sotiropoulos et al., 1999; Lin et al., 2002; Liu et al., 2003; Li et al., 2005; Castellani et al., 2006; Charrasse et al., 2006).
Despite the central role that small GTPases play in skeletal muscle, we know little about their regulation, in particular their regulation by the guanine nucleotide exchange factors (GEFs) that typically activate them (O'Brien et al., 2000; Bryan et al., 2005a,b). The presence of a rhoGEF domain in obscurin and its potential to regulate small GTPases is particularly interesting, because obscurin localizes to the periphery of the sarcomere and is therefore well positioned to sense, transmit, and potentially respond to changes in the length and diameter of sarcomeres, related to contraction and stretch, as well as to changes in tension linked to the contractile cycle. In this study, we investigate the ability of obscurin's rhoGEF domain to interact with small GTPases of the rho family, and identify rhoA as a primary ligand. We show that rhoA colocalizes with obscurin at the level of the M-band in developing myotubes and in adult skeletal muscle. Furthermore, we demonstrate that obscurin's rhoGEF domain can activate rhoA in adult skeletal muscle and affect expression of kinases downstream of rhoA.
MATERIALS AND METHODS
Culture and Transfection of COS-7 Cells
COS-7 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and 0.1% amphotericin B (Fungizone, Invitrogen, Carlsbad, CA), at 37°C with 10% CO2 and 90% air. Cells were transfected with DNA constructs encoding green fluorescent protein (GFP), GFP-tagged rhoGEF, or rhoGEF-PH domains of obscurin (rhoGEF-pEGFP-C2 or rhoGEF-PH-pEGFP-C2) and hemagglutinin (HA)-tagged constructs of small GTPases of the rho family. The primers used to clone the rhoGEF domain of obscurin into pEGFP-C2 were 5′-gaattcgtcatccaggagttgctgagttc-3′ and 5′-ggatccctagcgctgtggcagggcaga-3′, and the primers used to clone the rhoGEF-PH domains were 5′-gaattcgtcatccaggagttgctgagttc-3′ and 5′-ggatccctagccacgatctgcttcac-3′. The DNA constructs for the small GTPases were obtained from University of Missouri cDNA Resource Center (Rolla, MO), and include the wildtype (wt) dominant-negative (DN), or constitutively active (CA) forms of rhoA, cdc42, rac1, or tc10. FuGENE 6 reagent was used to transfect cells, according to the manufacturer's protocol (Roche, Basel, Switzerland). Cells were collected for lysates or processed for fluorescent immunolabeling 24 h after transfection.
Immunoprecipitation and Western Blot Analysis
COS-7 cells were lysed in a buffer containing 50 mM Tris, 0.5% NP-40, 0.1% sodium deoxycholate, 150 mM NaCl, 4 mM EDTA, and Complete protease inhibitors (Roche), collected on ice, sheared with a 21-gauge needle and syringe, and then subjected to centrifugation at 10,000 × g for 10 min at 4°C. After quantification of protein with the Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA), 400 μg of total protein was incubated with 40 μl of protein A-Sepharose beads and 5 μl of rabbit polyclonal antibody to GFP (Invitrogen, Carlsbad, CA) for 2 h at 4°C with gentle rotation. The beads were then washed four times with lysis buffer to reduce nonspecific binding. An equal volume of 2× SDS-polyacrylamide gel electrophoresis (PAGE) buffer (Bio-Rad Laboratories) was added to the beads, and samples were heated at 90°C for 5 min before loading on a Bis-Tris SDS protein gel (Invitrogen). An aliquot of the cell homogenate (50 μg/well) was also analyzed.
After SDS-PAGE, proteins were transferred to nitrocellulose for Western blot analysis. Membranes were incubated in a 3% milk/Tris-buffered saline solution before incubating with primary antibodies in the same solution. Primary antibodies were rabbit anti-GFP (Invitrogen), mouse anti-HA (Sigma-Aldrich, St. Louis, MO), mouse anti-RhoA (catalog no. sc-418; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-cdc42 (catalog no. sc-87; Santa Cruz Biotechnology), or mouse anti-rac1 (catalog no. ARC03; Cytoskeleton, Denver, CO). We confirmed many of our results with rhoA with a second antibody specific for this protein, from Cytoskeleton (catalog no. ARH03). Species-specific secondary antibodies conjugated to horseradish peroxidase (HRP; GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom), followed by chemiluminescent detection (Pierce), were used to detect bound antibody.
Culture and Collection of Primary Skeletal Myotubes
Primary cultures of rat myotubes were prepared as described previously (Bloch, 1979; Kontrogianni-Konstantopoulos et al., 2006b). Cultures were maintained in DMEM with 10% FBS, 1% penicillin-streptomycin, and 0.1% amphotericin B until 96 h after initial plating, at which point the media were supplemented with 4 × 10−5 M cytosine arabinoside (Sigma-Aldrich). Cultures were collected 24–168 h after initial plating.
For immunolabeling, cultures were washed twice with phosphate-buffered saline (PBS) and fixed with 1% paraformaldehyde in PBS for 10 min at room temperature. Fixed cells were permeabilized with 70% ethanol in PBS for 10 min at room temperature, washed with PBS, and then immunolabeled and examined under confocal optics (see below).
For Western blotting, cultures were scraped with a rubber policeman and collected into a tube on ice. An equal volume of homogenate buffer was added to the cells (2% NP-40, 150 mM NaCl, 4 mM EDTA, and Complete protease inhibitors in PBS). After 30-min incubation on ice, the samples were homogenized and spun, as described above. Equal amounts of total protein (60 μg/well) were heated at 42°C for 45 min in 2× SDS-PAGE sample buffer before separation by SDS-PAGE. After transfer to nitrocellulose, proteins were incubated with primary antibodies to rhoA (Santa Cruz Biotechnology) or obscurin (Kontrogianni-Konstantopoulos et al., 2003; Bowman et al., 2007), and the appropriate secondary antibodies conjugated to HRP.
Culture of Flexor Digitorum Brevis Fibers
Sprague Dawley rats (Zivic-Miller Laboratories, Zelienople, PA), 28–32 d postnatal, were anesthetized with isoflurane and killed by cervical dislocation. The flexor digitorum brevis muscle was immediately isolated from each foot, and the fibers were dissociated for culture as described previously (Zuurveld et al., 1984). Fibers were maintained in DMEM supplemented with 0.2% bovine serum albumin (BSA), 0.1% gentamicin, and 0.1% amphotericin B, then plated on Matrigel-coated glass coverslips and incubated at 37°C with 5% CO2, 95% air. Cells were fixed 24 h after plating with 1% paraformaldehyde in PBS for 10 min at room temperature, permeabilized for 10 min in 0.1% Triton in PBS at room temperature, and immunolabeled.
Fluorescent Immunolabeling and Confocal Microscopy
All samples processed for immunolabeling were first incubated in 1 mg/ml BSA in PBS for 1 h. Primary antibodies used were rabbit anti-GFP (Invitrogen), mouse anti-HA (Sigma-Aldrich), mouse anti-rhoA (Santa Cruz Biotechnology), guinea pig anti-RhoGEF (Bowman et al., 2007), rabbit anti-TitinZ (antibodies to the first 2 Ig domains of titin, which are located adjacent to the Z-disk) (Kontrogianni-Konstantopoulos et al., 2006b), rabbit anti-TitinM (antibodies to Ig domains in titin, which are located at the level of the M-band) (Centner et al., 2001), mouse anti-obscurin N terminus (Nt, generated to the first 2 Ig domains of obscurin) (Kontrogianni-Konstantopoulos et al., 2006b), rabbit anti-obscurin C terminus (generated to the last 2 Ig-like domains and the nonmodular C-terminal region of obscurin) (Kontrogianni-Konstantopoulos et al., 2003), rabbit anti-cdc42 (Santa Cruz Biotechnology), mouse anti-rac1 (Cytoskeleton), rabbit anti-ROCK1 (Santa Cruz Biotechnology), and rabbit anti-CRIK (BioLegend, San Diego, CA). Samples were incubated in primary antibodies at a final concentration of 2 μg/ml in 1 mg/ml BSA in PBS, for 2 h at room temperature (cells) or overnight at 4°C (cryosections). Cells were washed twice with PBS before incubating for 1.5 h at room temperature with fluorescein-, rhodamine-, or Cy5-conjugated species-specific antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Samples were washed three times with PBS before mounting with Aqua-Poly/Mount solution (Polysciences, Warrington, PA). Samples were imaged with a 510 Meta confocal laser scanning microscope (Carl Zeiss, Tarrytown, NY).
In Vivo Gene Transfer via Electroporation
Male 6-wk-old Sprague-Dawley rats (Zivic-Miller Laboratories) were anesthetized with a continuous flow of isoflurane (2 l/min) during the entire procedure. While the animal was anesthetized and unresponsive to painful stimuli, the hindlimb was surgically incised to expose the tibialis anterior muscle. DNA encoding GFP, GFP-rhoGEF, or GFP-rhoGEF-PH was injected into the tibialis anterior (TA) muscle, with a 30-gauge insulin needle and syringe, at a concentration of 1 μg/μl in 0.9% sterile saline, in a total volume of 100 μl. After injection, the injected muscle was electroporated using 5 × 150 V/cm pulses of 20 ms each, with 200msec intervals between pulses. The hindlimb was sutured, and the animal was monitored during recovery. Animals were killed 7 d after in vivo gene transfer (IVGT), by anesthetization with isoflurane and perfusion with 2% paraformaldehyde in PBS (for cryosections) or PBS with protease inhibitors (for homogenates). The electroporated muscles were collected and snap-frozen in liquid nitrogen. Muscles fixed with paraformaldehyde were cryosectioned into 20-μm longitudinal or cross sections, and immunolabeled as described above. Muscles perfused with PBS were homogenized to yield whole muscle extracts, using homogenate buffer (see above, but without 4 mM EDTA) and a mortar and pestle to grind the tissue. Homogenized samples were processed as described above.
Rho Activity Assay
Muscle homogenates were assayed for rho activity by using agarose beads coupled to glutathione transferase (GST)-rhotekin binding domain (GST-RBD; Cytoskeleton). In brief, 500 μg of protein from muscle homogenates was incubated with 30 μg of GST-RBD agarose beads for 2 h at 4°C with gentle rotation. Beads were washed three times with PBS and then heated in SDS-PAGE sample buffer at 90°C for 5 min. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with mouse antibody to rhoA.
Antibodies for Western Blot Analysis of Adult Rat TA Muscle Homogenates
Rabbit anti-ROCK1, goat anti-citron kinase, rabbit anti-cdc42, and mouse anti-rhoA were from Santa Cruz Biotechnology, and were used at 200 ng/ml for Western blots. Mouse anti-rac1 was from Cytoskeleton and was also used at 200 ng/ml. Rabbit anti-TC10 was from Sigma-Aldrich and was used at 1 μg/ml for Western blots, as recommended by the manufacturer.
Injury Induced by Eccentric Lengthening Contractions
Male age-matched Sprague-Dawley rats (Zivic-Miller Laboratories), weighing 275–325 g, were anesthetized with a continuous flow of isoflurane (2 l/min) during the entire procedure. Rats were injected intraperitoneally with fluorescein dextran (FDX), a marker for sarcolemmal damage. Injury induced by eight lengthening contractions was performed as described previously (Lovering et al., 2005; Lovering et al., 2007). As injury occurs, FDX is taken up and retained by injured myofibers and serves as a marker for those fibers for many days (Roche et al., 2008). Within 1 h of the injury, rats were perfused with paraformaldehyde or PBS, and tissue was collected for cryosections or Western blot analysis, respectively, as described above.
Rats were used according to the guidelines set by the National Institutes of Health Guide for Care and Use of Laboratory Animals. The University of Maryland Institutional Animal Care and Use Committee approved our procedures.
RESULTS
The rhoGEF-PH Domains of Obscurin Bind and Activate RhoA In Vitro
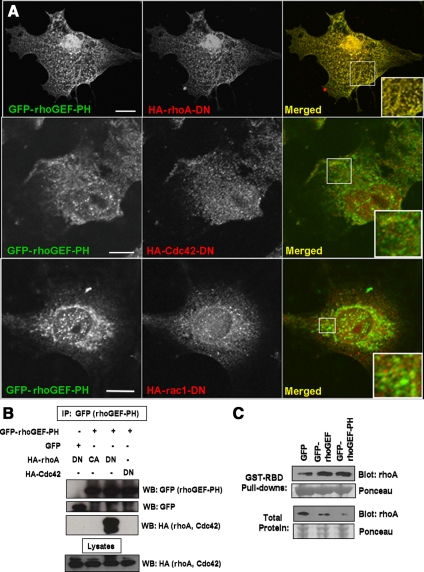
We first identified the small GTPases that interact with the rhoGEF domain of obscurin by coexpressing the rhoGEF-PH domains of obscurin as GFP-fusion proteins, together with the dominant-negative HA-tagged forms of small GTPases of the rho family. Immunofluorescent confocal microscopic imaging of cotransfected COS-7 cells showed that rhoA-DN extensively colocalizes with GFP-rhoGEF-PH, whereas cdc42-DN and rac1-DN do not (Figure 1A). Similar results were obtained with the rhoGEF domain alone (data not shown). In a parallel set of experiments, the GFP-rhoGEF-PH fusion protein was immunoprecipitated from lysates of transfected COS-7 cells, with an antibody to the GFP tag, and separated by SDS-PAGE. Western blots revealed that the dominant-negative form of rhoA interacts with GFP-rhoGEF-PH, whereas the constitutively active form of rhoA and the dominant-negative form of cdc42 do not (Figure 1B). We were unable to determine whether the dominant-negative form of rac1 binds the GFP-rhoGEF or GFP-rhoGEF-PH domains, due to low transfection efficiency or possibly cell toxicity (data not shown).
Figure 1.
The rhoGEF domain of obscurin interacts with the catalytically inactive form of rhoA and activates wt-rhoA in vitro. (A) COS-7 cells were cotransfected with plasmids encoding GFP-tagged rhoGEF-PH domains of obscurin (left, shown in green on the right) and HA-tagged, catalytically inactive forms of rhoA, cdc42 or rac1 (rhoA-DN, cdc42-DN or rac1-DN; center, shown in red on the right). HA-rhoA-DN colabels extensively with GFP-rhoGEF-PH, but HA-cdc42-DN and rac1-DN do not (right, shown in yellow, insets). Bars, 10 μm. (B) COS-7 cells were cotransfected as in A, or with plasmids encoding GFP and HA-rhoA-DN or GFP-rhoGEF-PH and HA-rhoA-CA. After transfection, rabbit anti-GFP antibody was used to immunoprecipitate GFP or GFP-rhoGEF-PH from cell lysates. Western blot analysis reveals that HA-rhoA-DN coimmunoprecipitates with the GFP-rhoGEF-PH domains of obscurin but that HA-rhoA-CA and HA-cdc42-DN do not. (C) COS-7 cells were cotransfected with plasmids encoding wt-rhoA and GFP, GFP-rhoGEF, or GFP-rhoGEF-PH. After transfection, GST-tagged rhotekin binding domain (GST-RBD) was used to adsorb active, GTP-bound rhoA from cell lysates. Western blot analysis shows that coexpression of GFP-rhoGEF or GFP-rhoGEF-PH increases the amount of active rhoA, compared with GFP alone.
To determine whether obscurin's rhoGEF domain can activate rhoA in COS-7 cells, we cotransfected cells with wt rhoA and GFP, GFP-rhoGEF, or GFP-rhoGEF-PH. We used GST-tagged rhotekin binding domain, a rhoA effector fragment that binds only the GTP-bound form of rhoA, to precipitate active rhoA from cell lysates. Western blot analysis with a rhoA-specific antibody showed that expression of either GFP-rhoGEF or GFP-rhoGEF-PH increases the amount of GTP-bound rhoA (Figure 1C). These results suggest that obscurin acts as a guanine exchange factor for rhoA.
RhoA Organizes with the rhoGEF Domain of Obscurin in Developing Myotubes and in Adult Skeletal Muscle
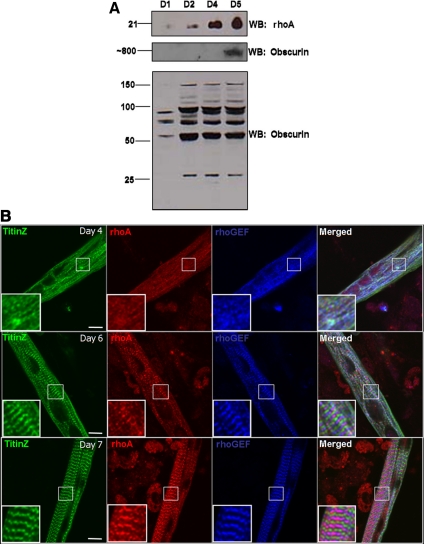
We next examined the expression and subcellular organization of endogenous rhoA in skeletal myotubes developing in culture. Western blots of homogenates of primary myotube cultures, prepared from skeletal muscles from the hindlimb of neonatal rats, revealed that rhoA is expressed early in myotube formation and quickly reaches steady-state levels (Figure 2A), consistent with its role in the early stages of sarcomerogenesis (Carnac et al., 1998; Takano et al., 1998; Wei et al., 1998). RhoA localizes to the M-band by day 7 after initial plating, where it colocalizes with obscurin, labeled with antibodies to its rhoGEF domain (Figure 2B). The C terminus of obscurin accumulates around the M-band ∼2 d earlier (Kontrogianni-Konstantopoulos et al., 2004), which, together with these results, suggest that obscurin and rhoA are appropriately placed to interact in vivo.
Figure 2.
RhoA is expressed and colocalizes with the rhoGEF domain of obscurin in primary myotubes differentiating in culture. (A) Total protein from homogenates of primary myotubes was analyzed as a function of time in culture by Western blotting for rhoA and obscurin. RhoA expression increases up to day 5 in culture (top), at which time the large, ∼800-kDa isoform of obscurin is clearly expressed (middle). Smaller bands that label with antibody to the rhoGEF domain of obscurin are expressed at apparently constant levels between D2 and D5 in culture (bottom). (B) Primary myotube cultures were labeled at 4, 6, or 7 d after initial plating with primary antibodies for the Z-disk region of titin (titinZ, green), rhoA (red), and the rhoGEF domain of obscurin (blue). RhoA organizes with obscurin at the M-band after the ∼800-kDa form of obscurin is expressed (overlay, purple). Bars, 10 μm.
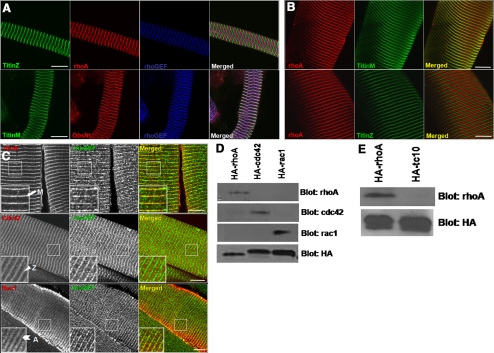
We also investigated the localization of endogenous rhoA in adult skeletal muscle. As with developing myotubes, rhoA colocalizes with the rhoGEF domain of obscurin at the M-band in cultures of fibers from the rat flexor digitorum brevis (FDB) muscle (Figure 3A), as well as in cryosections of mouse TA muscle (Figure 3, B and C). This localization is in contrast to that for cdc42, which predominantly localizes to the Z-disk, and for rac1, which localizes to the A-band (Figure 3C). Specificity of the rhoA antibody was confirmed by immunoprecipitation of wildtype HA-rhoA, HA-cdc42, HA-rac1, or HA-tc10 from transfected COS-7 cell lysates, and assay by immunoblotting with primary antibodies specific for each small GTPase (Figure 3, D and E). We obtained similar results for rhoA localization with other skeletal muscles (data not shown). Thus, the accumulation of rhoA at the M-band that occurs during development persists in adult muscle.
Figure 3.
RhoA colocalizes with the rhoGEF domain of obscurin at the M-band of adult skeletal muscle. (A) Enzymatically dissociated flexor digitorum brevis muscle fibers from a young adult rat were labeled for the carboxy terminus of titin (titinM) or the amino terminus of titin (titinZ; green), rhoA or the amino terminus of obscurin (ObsNt; red), and the rhoGEF domain of obscurin (blue). (B) Longitudinal cryosections (20 μm) of mouse TA muscle were labeled with primary antibodies to rhoA (shown in red) and the carboxy terminus of titin (titinM) or the amino terminus of titin (titinZ), shown in green. (C) Longitudinal cryosections (20 μm) of mouse TA muscle were labeled with primary antibodies to rhoA, cdc42, or rac1 (red), and the rhoGEF domain of obscurin (green). RhoA codistributes with obscurin's rhoGEF domain at the M-band in both preparations (shown in purple in A; yellow in B and C). Cdc42 is present predominantly at the Z-disk, and rac1 at the A-band. Bars, 10 μm. (D) COS-7 cells were cotransfected with HA-tagged, wild-type forms of rhoA, cdc42, or rac1. The HA-tagged proteins were extracted and immunoprecipitated with antibody to the HA tag. Western blot analysis shows that the rhoA antibody used in this study is specific for rhoA and does not recognize two closely related proteins, cdc42 or rac1 and that antibodies to the latter proteins do not recognize rhoA. (E) COS-7 cells were cotransfected with HA-tagged forms of rhoA or tc10. The HA tagged proteins were extracted, immunoprecipitated and analyzed by Western blot as described in D. The rhoA antibody used in these experiments does not recognize tc10.
Expression of the RhoGEF Domain of Obscurin in Adult Skeletal Muscle Alters RhoA Localization
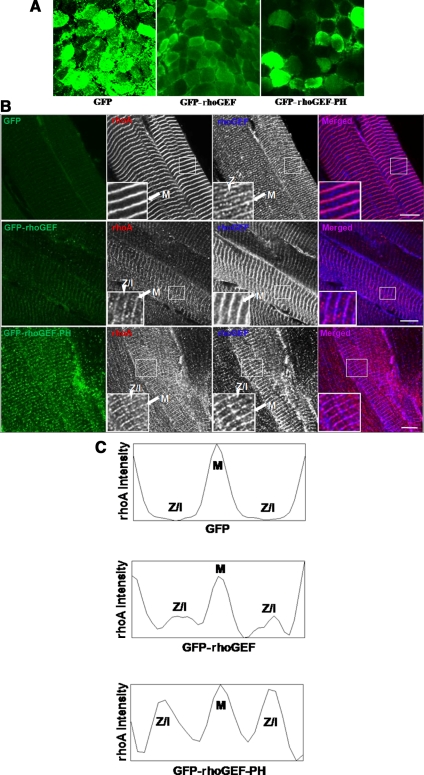
To determine the effects of exogenous expression of the rhoGEF and rhoGEF-PH domains of obscurin in adult skeletal muscle, we used IVGT to overexpress these domains as GFP-fusion proteins in muscles of 6-wk-old male rats. DNA encoding GFP-rhoGEF or GFP-rhoGEF-PH was injected into one TA muscle, and GFP alone was injected into the contralateral TA as a control. Subsequent electroporation of the injected muscle greatly increased transfection efficiency (Mir et al., 1998, 1999), with ∼60 or 30% of the muscle fibers showing a moderate to high levels of GFP-rhoGEF or GFP-rhoGEF-PH expression, respectively, 1 wk after transfection (Figure 4A). In longitudinal sections, we found that rhoA localizes to the M-band in GFP-transfected muscle (Figure 4B, top), as seen in nontransfected controls (Figure 3A). In muscle transfected with GFP-rhoGEF or GFP-rhoGEF-PH, rhoA still localizes to the M-band, but it is also found at locations between M-bands, especially at the level of the I-band and possibly the Z-disk and the Z/I junction (Figure 4B, middle and bottom). Quantification of the relative immunofluorescence intensity of labeling for rhoA across a sarcomere confirmed this altered distribution (Figure 4C).
Figure 4.
Overexpression of obscurin's rhoGEF domain alters rhoA localization in vivo. (A) Cross sections (20 μm) from 7-wk-old rat TA muscles were examined 1 wk after IVGT of GFP-rhoGEF or GFP-rhoGEF-PH, induced by electroporation. IVGT resulted in ∼60% of the fibers expressing GFP-rhoGEF, ∼30% expressing GFP-rhoGEF-PH, and ∼80% expressing GFP. (B) Longitudinal cryosections (20 μm) from rat TA were examined as described in A. The contralateral TA, transfected via IVGT with GFP alone, was used as a control. Top, after transfection of a vector encoding GFP, endogenous rhoA remains localized to the M-band (red; purple in merged image to right). Obscurin's rhoGEF localizes to both the M-band and the Z-disk or Z-I junction (blue; purple in merged image to right). Middle, transfection of GFP-rhoGEF results in rhoA localization at both the M-band and Z-disks or Z-I junctions (red). Bottom, GFP-rhoGEF-PH expression promotes more extensive association of rhoA with Z-disks and Z/I junction (red). The rhoGEF antibody recognizes both endogenous obscurin and the GFP-rhoGEF fusion proteins. Bars, 10 μm. (C) Quantification of rhoA intensity, measured with ImageJ software across one sarcomere length from the insets shown in B. Top, intensity of rhoA staining in GFP transfected muscle, from Z-disk to M-band to Z-disk. Middle, intensity of rhoA staining in GFP-rhoGEF transfected muscle. Bottom, intensity of rhoA staining in GFP-rhoGEF-PH transfected muscle. RhoA localizes to both the M-band and the Z-disk in the presence of GFP-rhoGEF or GFP-rhoGEF-PH.
Overexpression of Obscurin's RhoGEF Domain in Adult Skeletal Muscle Increases RhoA Expression and Activity and Leads to Increased ROCK1 and Decreased CRIK Expression
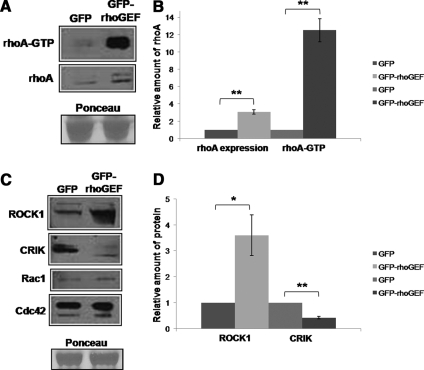
We next examined homogenates of transfected muscle to learn the effects of the expression of GFP-rhoGEF on the amount and activity of rhoA. Because ∼60% of the fibers expressed GFP-rhoGEF, compared with only ∼30% that expressed GFP-rhoGEF-PH, we focused on homogenates from muscle transfected with GFP-rhoGEF. Notably, expression of GFP-rhoGEF led to an increase in rhoA expression, which was approximately threefold higher than in muscle transfected with GFP alone (n = 3; p < 0.005). Similar results were seen with expression of the GFP-rhoGEF-PH domains, although lower transfection efficiency of this construct resulted in less dramatic effects on rhoA expression in total homogenates. The increased rhoA expression was accompanied by a 12.5-fold increase in active, GTP-bound rhoA in GFP-rhoGEF–transfected muscle, as determined by binding to GST-rhotekin binding domain (Figure 5, A and B; n = 3; p < 0.005). Equal loading was confirmed by staining with Ponceau Red.
Figure 5.
Overexpression of obscurin's rhoGEF domain increases rhoA expression and activity in vivo and alters expression of two downstream effectors of rhoA, ROCK1 and CRIK. (A) Homogenates from rat TA transfected by IVGT with plasmids encoding GFP or GFP-rhoGEF were analyzed for rhoA activity and expression. Top, RhoA activity was assayed with a GST fusion protein containing the rhotekin binding domain (GST-RBD), which binds specifically to the GTP-bound form of rhoA, followed by Western blotting. Middle, Western blotting was used to determine the relative levels of rhoA in the homogenates used for the GST-RBD pull-down assays. Bottom, labeling by Ponceau Red indicates equal protein loading in the two samples. (B) Quantification of the results shown in A (and data not shown), expressed as the ratio of samples transfected with GFP-rhoGEF compared with those transfected with GFP alone, shows an approximately threefold increase in rhoA levels and an ∼12-fold increase in active rhoA when the rhoGEF domain is overexpressed (n = 3). These differences were significant (**p < 0.005). (C) After IVGT, as described in A, homogenates of transfected muscles were analyzed by Western blotting for expression of ROCK1 and CRIK. Overexpression of the rhoGEF domain of obscurin results in increased levels of ROCK1 and decreased levels of CRIK (top). The levels of small GTPases rac1 and cdc42 do not change in samples that overexpress the rhoGEF domain of obscurin (bottom). As described above, Ponceau Red staining verifies equal loading of the two samples. (D) Quantification of the differences illustrated in C, determined by comparison of samples expressing GFP and GFP-rhoGEF, shows a 3.6-fold increase in ROCK1 and a 2.4-fold decrease in CRIK expression when GFP-rhoGEF is overexpressed in adult rat TA. The differences for both ROCK1 and CRIK are statistically significant (*p < 0.05 and **p < 0.005, respectively).
We investigated the levels of expression of ROCK1 and CRIK, to determine whether overexpression of the rhoGEF domain affected these downstream rhoA effectors. Both enzymes are activated by rhoA in other tissues (Zhang et al., 2003; Shandala et al., 2004; Berto et al., 2007; Del Re et al., 2007; Hilgers et al., 2007; Peters and Michel, 2007). Increased rhoA expression and activity consistently correlates with increased ROCK1 expression and decreased CRIK expression (Figure 5C, top). The measured changes in expression of ROCK1 and CRIK between GFP- and GFP-rhoGEF–transfected samples, averaged from three different experiments, indicate that the 12.5-fold increase in rhoA activity that occurred upon overexpression of the rhoGEF domain is associated with a 3.6-fold increase in ROCK1 expression and a 2.4-fold decrease in CRIK expression (Figure 5D; n = 3, p < 0.05 and p < 0.005, respectively). Ponceau staining confirmed equal loading for all experiments. Note that the expression of two related GTPases, cdc42 and rac1, does not change upon overexpression of obscurin's rhoGEF domain (Figure 5C, bottom; n = 3), consistent with the ability of obscurin's rhoGEF domain to discriminate among the small GTPases of the rho family.
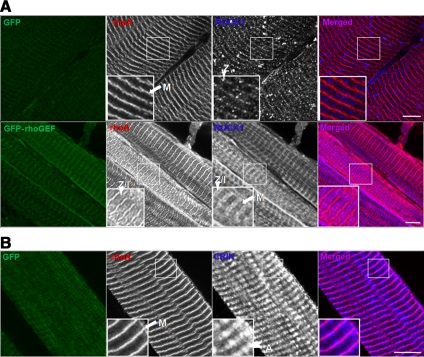
Overexpression of Obscurin's RhoGEF Domain Leads to Altered Localization of ROCK1 and CRIK In Vivo
To determine whether overexpression of the rhoGEF domain affects the localization of ROCK1 and CRIK, as well as their levels of expression, we examined endogenous ROCK1 and CRIK in longitudinal sections after IVGT. In GFP-transfected rat TA muscle, ROCK1 localizes primarily at the level of the Z-disk (Figure 6A), as it does in untransfected muscle (data not shown). In the contralateral TA muscle, transfected with plasmid encoding GFP-rhoGEF, ROCK1 immunofluorescence is not only visibly greater, consistent with its increased level of expression, but also is present at the I-band and Z/I junction, and to some extent at the M-band (Figure 6A). CRIK localizes primarily at the level of the M-band and A-band in GFP-transfected muscle (Figure 6B) and untransfected muscle (data not shown). In the contralateral GFP-rhoGEF–transfected muscle, CRIK immunofluorescence decreases to below the level of detection (data not shown). In conjunction with the data from Western blot analysis, these data suggest that GFP-rhoGEF-mediated increases in rhoA activity diminish CRIK protein expression. These results indicate that, in addition to increasing ROCK1 protein expression and decreasing CRIK expression, overexpression of obscurin's rhoGEF domain, and the corresponding increase in rhoA activity, can alter the sarcomeric localization of these downstream kinases.
Figure 6.
Obscurin's rhoGEF domain alters localization of ROCK1 and CRIK in vivo. Longitudinal cryosections (20 μm) from rat TA were examined as described in Figure 4B. The contralateral TA, transfected via IVGT with plasmid encoding GFP, was used as a control. (A) Top, after transfection of a vector encoding GFP, ROCK1 primarily localizes to the Z-disk (blue). RhoA localizes to the M-band (red). Bottom, transfection of GFP-rhoGEF results in increased labeling for ROCK1, and its localization to the Z-disk and Z/I junction, with some staining at the M-band. RhoA localizes to both the M-band and the I-band, and possibly the Z-disk (red). (B) After transfection of a vector encoding GFP, CRIK localizes to the M-band and A-band regions (blue). Bars, 10 μm.
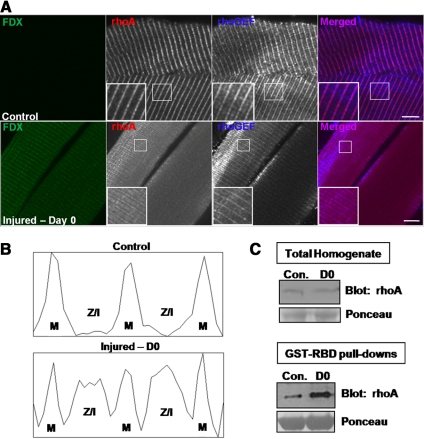
Contraction-induced Injury Activates RhoA and Displaces It from the M-Band
To determine whether rhoA can be activated in vivo, we analyzed tissue collected from rats after an injury induced by large-strain lengthening contractions (Lovering et al., 2007; Roche et al., 2008). Shortly after injury to the TA muscle, rhoA localized to the M-bands, Z-disks, and Z/I junctions of injured myofibers, labeled with FDX (Figure 7A; see Materials and Methods). The contralateral muscle, not subjected to injury, showed rhoA only at M-bands (Figure 7A), as in untreated controls (see above). Quantitative line-scans confirmed that in the control tissue rhoA localized to the M-bands, whereas in the injured tissue rhoA localization was altered (Figure 7B). Concomitant with the displacement of rhoA from the M-bands after injury, we measured an increase in the active, GTP-bound form of rhoA, compared with the uninjured contralateral muscle (Figure 7C). These results were similar to the effect of overexpression of the rhoGEF domain of obscurin (Figures 4 and 5). Together, these data indicate that large-strain lengthening contractions can activate and displace rhoA, in a manner similar to that observed when the rhoGEF domain of obscurin was overexpressed.
Figure 7.
Contraction-induced injury alters rhoA localization and activates rhoA in vivo. Tissue from injured or contralateral uninjured rat hindlimbs was collected immediately after large-strain lengthening contractions. (A) Longitudinal cryosections (20 μm) of rat TA were analyzed by confocal fluorescence microscopy. Top, control. Absence of FDX indicates lack of injury to the muscle fiber. RhoA localizes to the M-band (red), and obscurin's rhoGEF domain localizes to the M-band and the Z-disk (blue). Bottom, injured: FDX-positive fibers indicate that they were injured (green). RhoA localizes to the M-band, Z-disk and Z/I junction (red), whereas obscurin localizes to the M-band and the Z-disk (blue). (B) Quantification of rhoA intensity, measured with ImageJ software across one sarcomere length, from the insets shown in A. Top, rhoA in uninjured muscle, from Z-disk to M-band to Z-disk. Bottom, rhoA in injured muscle. (C) Homogenates from control (uninjured) or injured (D0) rat TA were analyzed for rhoA expression and activity. Top, Western blotting was used to determine the relative levels of rhoA in the homogenates. Bottom, RhoA activity in the homogenates shown in the top panel was assayed with a GST fusion protein containing the RBD (GST-RBD), which binds specifically to the GTP-bound form of rhoA, followed by Western blotting. Total active rhoA is greater in injured tissue than in control tissue, but the total amount of rhoA remains unchanged immediately after injury.
DISCUSSION
This is the first study to identify rhoA as a major ligand for the rhoGEF domain of obscurin that is directly involved in signaling in mammalian striated muscle. We show that obscurin's rhoGEF domain interacts with rhoA in vitro, but not with the closely related protein, cdc42, and that obscurin codistributes with rhoA at the M-band in vivo, in both developing myotubes and in adult skeletal muscle. We show further that overexpression of obscurin's rhoGEF domain in adult rat hindlimb muscle increases the expression of rhoA threefold but has little effect on the expression of cdc42 or rac1. This increase is accompanied by the appearance of rhoA at other sarcomeric structures, as well as by changes in expression and localization of two of rhoA's downstream effectors, ROCK1 and CRIK. We reported previously that the rhoGEF domain of obscurin binds ranBP9 (Bowman et al., 2008), a protein that was first identified as a ligand of the small GTPase, ran-GTP, and that, our results showed, can influence the assembly of Z-disks in developing sarcomeres. The results we report here support the idea that obscurin's rhoGEF domain also participates directly in signaling in muscle fibers, specifically by regulating the levels, activities, and subcellular localizations of rhoA and the amounts and subcellular localizations of two of its downstream effectors, ROCK1 and CRIK. Furthermore, muscle injury induced in vivo by large-strain lengthening contractions also activates rhoA and displaces it from M-bands, similar to the effects of obscurin's rhoGEF domain. These results suggest that obscurin-mediated activation of rhoA may play a physiological role in the response of muscle to contractile activity or contraction-induced injury.
Specificity
Obscurin's rhoGEF domain interacts with rhoA in vitro as well as in vivo in a distinctive and specific manner. Although rac1 localizes to the A-band in striated muscle (Figure 3C) and may associate at the M-band with obscurin, neither its expression nor its localization was affected by overexpression of obscurin's rhoGEF domain (Figure 5C; data not shown). Similarly, obscurin's rhoGEF domain failed to associate with either rac1 or cdc42 (Figure 1, A and B). The absence of an effect on rac1 or cdc42 following in vivo expression of the rhoGEF domain suggests that obscurin has a specific effect on rhoA, but we cannot exclude the possibility that obscurin's rhoGEF domain can activate other small GTPases under particular physiological conditions. During the preparation of this manuscript, a report by Qadota et al. (2008) was published demonstrating that the DH-PH region of unc-89, the C. elegans homologue of obscurin's rhoGEF-PH domains, has exchange activity for rho-1, the C. elegans homologue of rhoA, but not for the small GTPases homologous to cdc42 or rac. These findings are consistent with our results in mammals, indicating that the specificity of obscurin's rhoGEF domain for rhoA is likely to have been evolutionarily conserved from nematodes to man. Preliminary evidence from our laboratory suggests that, in addition to rhoA, the rhoGEF domain may also interact with rhoC and rac3 (data not shown). Further research is needed to determine whether these interactions, like those mediated by rhoA, can activate downstream effectors, and if so, how obscurin differentiates between activation of rhoA and other small GTPases. Given the significant levels of rhoA present in skeletal muscle, and its concentration near obscurin at the M-band, obscurin's ability to activate rhoA and its downstream effectors is likely to be both facilitated by their close association and physiologically relevant.
The form of obscurin that is most likely to interact with rhoA is obscurin A, which our earlier work indicates is present at the M-band and associates with the sarcoplasmic reticulum (Young et al., 2001; Agarkova et al., 2003; Bagnato et al., 2003; Kontrogianni-Konstantopoulos and Bloch, 2005; Lange et al., 2005a; Armani et al., 2006; Bowman et al., 2007; Carlsson et al., 2008). Although it seems most likely that this isoform is responsible for interacting with and activating rhoA, other isoforms of obscurin that contain the rhoGEF domain may do so as well. However, as we do not observe significant accumulation of rhoA at the A/I junction, where obscurin B is concentrated (Bowman et al., 2007), obscurin B seems unlikely to play such a role.
The specificity of obscurin's rhoGEF domain for rhoA is independent of its tandem PH domain, which is present in the A isoform of obscurin, as it is in many GEFs (Hoffman and Cerione, 2002; Rossman et al., 2005). In some GEFs, the tandem PH domain enhances guanine exchange activity (Rossman et al., 2002, 2003; Pruitt et al., 2003), but in others it is inhibitory (Han et al., 1998; Nimnual et al., 1998; Aghazadeh et al., 2000; Zheng, 2001). Although it is not required for interaction, the PH domain of obscurin is certainly not inhibitory, and in fact may help regulate guanine exchange on rhoA, because it seems to increase the efficiency of association of the rhoGEF domain with rhoA, thereby promoting its redistribution from M-bands (Figures 1B and 4C; data not shown).
Activation and Distribution
The presence of rhoA together with obscurin at M-bands, and its displacement when the rhoGEF domain is overexpressed, are consistent with the hypothesis that the ability of rhoA to bind to obscurin's rhoGEF domain is sufficient to anchor it at the M-band. Because the dominant-negative form of rhoA associates with the rhoGEF domain preferentially, it seems likely that the rhoA concentrated at the M-band is inactive and that its dissociation and redistribution to the myoplasm and other sarcomeric structures is associated with its activation. Indeed, overexpression of the rhoGEF domain both induces the activation and the redistribution of rhoA. Our experiments do not rule out the possibility that rhoA at M-bands also binds to proteins other than obscurin, or to a domain of obscurin other than its rhoGEF domain. Overexpression of the rhoGEF domain, which we have demonstrated can associate with and activate rhoA, would likely be sufficient to compete with any endogenous binding site at the M-band, be it a domain of obscurin or of another protein. Experiments with forms of obscurin or its rhoGEF domain that cannot bind or activate small GTPases will be needed to address this question definitively and are currently underway in our laboratory.
Although obscurin's rhoGEF domain can activate rhoA, a physiological role for obscurin in activating rhoA in vivo must still be established. Both the expression and the activation of rhoA are linked to muscle activity (Kawamura et al., 2003; McClung et al., 2003; McClung et al., 2004; Zhang et al., 2007), raising the possibility that muscle activity stimulates obscurin's rhoGEF activity. The presence of obscurin A at the periphery of M-bands, where contraction can lead to significant increases in the diameter of the sarcomere (Gonzalez-Serratos, 1975; Hegarty and Allen, 1977; Brown et al., 1984; Farman et al., 2007; Telley and Denoth, 2007), may allow sarcomeric shortening to be sensed by obscurin's rhoGEF domain. The M-band is also the site at which the C-terminal kinase domain of titin is located, putting obscurin in an unique position to integrate contraction with downstream signaling cascades, which in turn can regulate the cytoskeleton, myofibril assembly, and muscle growth or hypertrophy (Granzier and Labeit, 2004; Agarkova and Perriard, 2005; Lange et al., 2005b; Weinert et al., 2006; Carmignac et al., 2007; Fukuzawa et al., 2008). Our studies of muscle after contraction-induced injury indicate that lengthening contractions can activate and displace rhoA, similar to the effects caused by overexpression of obscurin's rhoGEF domain, suggesting that obscurin may be at least partially responsible for this effect. Further experiments with muscle expressing a form of obscurin lacking a functional rhoGEF domain will be needed to test this idea definitively. However, the similarity of the results obtained after lengthening contractions and after overexpression of the rhoGEF domain suggests the exciting possibility that obscurin responds to contractile activity by activating rhoA and, subsequently, ROCK1.
Downstream Signaling
The links between obscurin's rhoGEF domain, rhoA and ROCK1 are likely to be associated with changes in gene expression in cardiac and skeletal muscle linked to atrophy and hypertrophy. The expression of rhoA has been linked to the maintenance of homeostasis in skeletal muscle, because its levels are increased or decreased by disuse or functional overload, respectively (Aikawa et al., 1999; Finkel, 1999; Clerk and Sugden, 2000; Molkentin and Dorn, 2001; Chockalingam et al., 2002; McClung et al., 2003; McClung et al., 2004; Saka et al., 2006). Stretch also activates rhoA in cultured striated muscle cells (Kawamura et al., 2003; Clark et al., 2004; Zhang et al., 2007). Activation might occur by altering the conformation or accessibility of obscurin's rhoGEF domain, thereby promoting the dissociation of rhoA and facilitating the exchange of guanine nucleotides required for its activation. Contraction-related changes in other sarcomeric proteins that bind to obscurin, such as titin (Bang et al., 2001; Fukuzawa et al., 2008) or myomesin (Fukuzawa et al., 2008), may also alter its binding to rhoA. Although our results would suggest otherwise, it remains possible that obscurin constitutively activates rhoA at the M-band. Indeed, some activated rhoA is present in control muscles that overexpress GFP alone, but because the rats were mobile and active before the muscle tissue was collected, its activation may have been linked to physiological levels of muscle contraction and stretching, or to other mechanisms.
Obscurin's rhoGEF domain increases not only the activity but also the overall expression of rhoA in adult skeletal muscle (Figure 5A). This suggests that rhoA is controlled by a feed-forward mechanism, whereby an overall increase in its activity promotes further increases in its expression. This is likely to be a physiological response, as increases in both the expression and activity of rhoA have been reported in cardiac hypertrophy (Aikawa et al., 1999; Molkentin and Dorn, 2001; Grounds et al., 2005; Ahuja et al., 2007).
Our results indicate that obscurin is one of a growing number of large structural proteins, associated with particular sites within cells, that can interact with small GTPases to regulate their activity both locally and broadly in the cytoplasm. When up-regulated and activated by its interactions with obscurin's rhoGEF domain, rhoA has the potential to activate several downstream effectors. Here, we have shown that the increased expression and activation of rhoA by obscurin's rhoGEF domain increases ROCK1 expression and decreases CRIK expression. ROCK1 activity in striated muscle is associated with hypertrophy and myoblast fusion (Maekawa et al., 1999; Liu et al., 2003; Nishiyama et al., 2004; Castellani et al., 2006; Peters and Michel, 2007), whereas CRIK activity is associated with cytokinesis and Golgi organization (Camera et al., 2003, 2008; Shandala et al., 2004; Berto et al., 2007). RhoA signaling through ROCK1 is a well-characterized pathway that is up-regulated in cardiac hypertrophy, and is responsible for many of the associated morphological changes (Molkentin and Dorn, 2001; Ahuja et al., 2007; Peters and Michel, 2007). Many of these changes are the result of activation of a subset of genes by SRF, which contain a SRE in their promoters (Carnac et al., 1998; Wei et al., 1998; Schratt et al., 2001; Lin et al., 2002; Carson et al., 2003; Liu et al., 2003; Kuwahara et al., 2005; Charvet et al., 2006; Miano et al., 2007). Our results therefore suggest that obscurin's rhoGEF domain can activate rhoA, leading to its increased expression, and that this in turn may promote hypertrophic signaling in striated muscle by mobilizing rhoA's downstream effectors. Our future studies will test this idea by examining the effects of obscurin-mediated activation of rhoA on ROCK1 activity and the expression of sarcomeric proteins.
ACKNOWLEDGMENTS
We thank Dr. Susan Kandarian for expert guidance in using in vivo gene transfer. We thank A. O'Neill, B. Busby, and Drs. K. Kontrogianni-Konstantopoulos, M. Borzok, and P. Reed for useful discussions. Our research has been supported by National Institutes of Health grants HL-64304 and AR-056330 and the Muscular Dystrophy Association (to R.J.B.) and by fellowships to D.L.F.-S. provided by training grants T32 AR-O7592 (principle investigator, M. Schneider) and HL-072751 (principle investigator, T. Pallone). D.L.F.-S. is the recipient of a Development Award (114938) from the Muscular Dystrophy Association.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-10-1029) on July 15, 2009.
REFERENCES
- Agarkova I., Ehler E., Lange S., Schoenauer R., Perriard J. C. M-band: a safeguard for sarcomere stability? J. Muscle Res. Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- Agarkova I., Perriard J. C. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Aghazadeh B., Lowry W. E., Huang X. Y., Rosen M. K. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. doi: 10.1016/s0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Ahuja P., Perriard E., Pedrazzini T., Satoh S., Perriard J. C., Ehler E. Re-expression of proteins involved in cytokinesis during cardiac hypertrophy. Exp. Cell Res. 2007;313:1270–1283. doi: 10.1016/j.yexcr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Aikawa R., Komuro I., Yamazaki T., Zou Y., Kudoh S., Zhu W., Kadowaki T., Yazaki Y. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ. Res. 1999;84:458–466. doi: 10.1161/01.res.84.4.458. [DOI] [PubMed] [Google Scholar]
- Armani A., Galli S., Giacomello E., Bagnato P., Barone V., Rossi D., Sorrentino V. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp. Cell Res. 2006;312:3546–3558. doi: 10.1016/j.yexcr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Bagnato P., Barone V., Giacomello E., Rossi D., Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 2003;160:245–253. doi: 10.1083/jcb.200208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M. L., Centner T., Fornoff F., Geach A. J., Gotthardt M., McNabb M., Witt C. C., Labeit D., Gregorio C. C., Granzier H., Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- Benian G. M., Tinley T. L., Tang X., Borodovsky M. The Caenorhabditis elegans gene unc-89, required for muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J. Cell Biol. 1996;132:835–848. doi: 10.1083/jcb.132.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berto G., Camera P., Fusco C., Imarisio S., Ambrogio C., Chiarle R., Silengo L., Di Cunto F. The Down syndrome critical region protein TTC3 inhibits neuronal differentiation via RhoA and Citron kinase. J. Cell Sci. 2007;120:1859–1867. doi: 10.1242/jcs.000703. [DOI] [PubMed] [Google Scholar]
- Bloch R. J. Dispersal and reformation of acetylcholine receptor clusters of cultured rat myotubes treated with inhibitors of energy metabolism. J. Cell Biol. 1979;82:626–643. doi: 10.1083/jcb.82.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov A. B., Kontrogianni-Konstantopoulos A., Bloch R. J., Westfall M. V., Russell M. W. Dynamics of obscurin localization during differentiation and remodeling of cardiac myocytes: obscurin as an integrator of myofibrillar structure. J. Histochem. Cytochem. 2004;52:1117–1127. doi: 10.1369/jhc.3A6183.2004. [DOI] [PubMed] [Google Scholar]
- Borisov A. B., Raeker M. O., Russell M. W. Developmental expression and differential cellular localization of obscurin and obscurin-associated kinase in cardiac muscle cells. J. Cell. Biochem. 2008;103:1621–1635. doi: 10.1002/jcb.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov A. B., Sutter S. B., Kontrogianni-Konstantopoulos A., Bloch R. J., Westfall M. V., Russell M. W. Essential role of obscurin in cardiac myofibrillogenesis and hypertrophic response: evidence from small interfering RNA-mediated gene silencing. Histochem. Cell Biol. 2006;125:227–238. doi: 10.1007/s00418-005-0069-x. [DOI] [PubMed] [Google Scholar]
- Borzok M. A., Catino D. H., Nicholson J. D., Kontrogianni-Konstantopoulos A., Bloch R. J. Mapping the binding site on small ankyrin 1 for obscurin. J. Biol. Chem. 2007;282:32384–32396. doi: 10.1074/jbc.M704089200. [DOI] [PubMed] [Google Scholar]
- Bowman A. L., Kontrogianni-Konstantopoulos A., Hirsch S. S., Geisler S. B., Gonzalez-Serratos H., Russell M. W., Bloch R. J. Different obscurin isoforms localize to distinct sites at sarcomeres. FEBS Lett. 2007;581:1549–1554. doi: 10.1016/j.febslet.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A. L., Catino D. H., Strong J. C., Randall W. R., Kontrogianni-Konstantopoulos A., Bloch R. J. The rho-guanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9. Mol. Biol. Cell. 2008;19:3782–3792. doi: 10.1091/mbc.E08-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Del Re D. P., Sussman M. A. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ. Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Brown L. M., Gonzalez-Serratos H., Huxley A. F. Structural studies of the waves in striated muscle fibres shortened passively below their slack length. J. Muscle Res. Cell Motil. 1984;5:273–292. doi: 10.1007/BF00713108. [DOI] [PubMed] [Google Scholar]
- Bryan B. A., Li D., Wu X., Liu M. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell Mol. Life Sci. 2005a;62:1547–1555. doi: 10.1007/s00018-005-5029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan B. A., Mitchell D. C., Zhao L., Ma W., Stafford L. J., Teng B. B., Liu M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol. Cell. Biol. 2005b;25:11089–11101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera P., da Silva J. S., Griffiths G., Giuffrida M. G., Ferrara L., Schubert V., Imarisio S., Silengo L., Dotti C. G., Di Cunto F. Citron-N is a neuronal Rho-associated protein involved in Golgi organization through actin cytoskeleton regulation. Nat. Cell Biol. 2003;5:1071–1078. doi: 10.1038/ncb1064. [DOI] [PubMed] [Google Scholar]
- Camera P., Schubert V., Pellegrino M., Berto G., Vercelli A., Muzzi P., Hirsch E., Altruda F., Dotti C. G., Di Cunto F. The RhoA-associated protein Citron-N controls dendritic spine maintenance by interacting with spine-associated Golgi compartments. EMBO Rep. 2008;9:384–392. doi: 10.1038/embor.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L., Yu J. G., Thornell L. E. New aspects of obscurin in human striated muscles. Histochem. Cell Biol. 2008;130:91–103. doi: 10.1007/s00418-008-0413-z. [DOI] [PubMed] [Google Scholar]
- Carmignac V., et al. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann. Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- Carnac G., Primig M., Kitzmann M., Chafey P., Tuil D., Lamb N., Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson J. A., Culberson D. E., Thompson R. W., Fillmore R. A., Zimmer W. Smooth muscle gamma-actin promoter regulation by RhoA and serum response factor signaling. Biochim. Biophys. Acta. 2003;1628:133–139. doi: 10.1016/s0167-4781(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Castellani L., Salvati E., Alema S., Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 2006;281:15249–15257. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- Centner T., et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J. Mol. Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- Charrasse S., Causeret M., Comunale F., Bonet-Kerrache A., Gauthier-Rouviere C. Rho GTPases and cadherin-based cell adhesion in skeletal muscle development. J. Muscle Res. Cell Motil. 2003;24:309–313. [PubMed] [Google Scholar]
- Charrasse S., Comunale F., Grumbach Y., Poulat F., Blangy A., Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C., et al. New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways. Mol. Cell. Biol. 2006;26:6664–6674. doi: 10.1128/MCB.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam P. S., Cholera R., Oak S. A., Zheng Y., Jarrett H. W., Thomason D. B. Dystrophin-glycoprotein complex and Ras and Rho GTPase signaling are altered in muscle atrophy. Am. J. Physiol. Cell Physiol. 2002;283:C500–C511. doi: 10.1152/ajpcell.00529.2001. [DOI] [PubMed] [Google Scholar]
- Clark C. B., McKnight N. L., Frangos J. A. Stretch activation of GTP-binding proteins in C2C12 myoblasts. Exp. Cell Res. 2004;292:265–273. doi: 10.1016/j.yexcr.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Clerk A., Sugden P. H. Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ. Res. 2000;86:1019–1023. doi: 10.1161/01.res.86.10.1019. [DOI] [PubMed] [Google Scholar]
- Del Re D. P., Miyamoto S., Brown J. H. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J. Biol. Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- Farman G. P., Allen E. J., Gore D., Irving T. C., de Tombe P. P. Interfilament spacing is preserved during sarcomere length isometric contractions in rat cardiac trabeculae. Biophys. J. 2007;92:L73–L75. doi: 10.1529/biophysj.107.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Myocyte hypertrophy: the long and winding RhoA'd. J. Clin. Invest. 1999;103:1619–1620. doi: 10.1172/JCI7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa A., Lange S., Holt M., Vihola A., Carmignac V., Ferreiro A., Udd B., Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band—implications for hereditary myopathies. J. Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H. Graded activation of myofibrils and the effect of diameter on tension development during contractures in isolated skeletal muscle fibres. J. Physiol. 1975;253:321–339. doi: 10.1113/jphysiol.1975.sp011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ. Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- Grounds H. R., Ng D. C., Bogoyevitch M. A. Small G-protein Rho is involved in the maintenance of cardiac myocyte morphology. J. Cell. Biochem. 2005;95:529–542. doi: 10.1002/jcb.20441. [DOI] [PubMed] [Google Scholar]
- Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R. D., Krishna U. M., Falck J. R., White M. A., Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hegarty P. V., Allen C. E. A light microscope study of fibre diameter and sarcomere length relationships in rigor skeletal muscles. Experientia. 1977;33:505–507. doi: 10.1007/BF01922239. [DOI] [PubMed] [Google Scholar]
- Hilgers R. H., Todd J., Jr, Webb R. C. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J. Hypertens. 2007;25:1687–1697. doi: 10.1097/HJH.0b013e32816f778d. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hoffman G. R., Cerione R. A. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 2002;513:85–91. doi: 10.1016/s0014-5793(01)03310-5. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Miyamoto S., Brown J. H. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J. Biol. Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- Kjoller L., Hall A. Signaling to Rho GTPases. Exp. Cell Res. 1999;253:166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Bloch R. J. Obscurin: a multitasking muscle giant. J. Muscle Res. Cell Motil. 2005;26:419–426. doi: 10.1007/s10974-005-9024-7. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Catino D. H., Strong J. C., Bloch R. J. De novo myofibrillogenesis in C2C12 cells: evidence for the independent assembly of M bands and Z disks. Am. J. Physiol. Cell Physiol. 2006a;290:C626–C637. doi: 10.1152/ajpcell.00442.2005. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Catino D. H., Strong J. C., Randall W. R., Bloch R. J. Obscurin regulates the organization of myosin into A bands. Am. J. Physiol. Cell Physiol. 2004;287:C209–C217. doi: 10.1152/ajpcell.00497.2003. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Catino D. H., Strong J. C., Sutter S., Borisov A. B., Pumplin D. W., Russell M. W., Bloch R. J. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006b;20:2102–2111. doi: 10.1096/fj.06-5761com. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Jones E. M., Van Rossum D. B., Bloch R. J. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol. Biol. Cell. 2003;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K., Barrientos T., Pipes G. C., Li S., Olson E. N. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol. Cell. Biol. 2005;25:3173–3181. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Agarkova I., Perriard J. C., Ehler E. The sarcomeric M-band during development and in disease. J. Muscle Res. Cell Motil. 2005a;26:375–379. doi: 10.1007/s10974-005-9019-4. [DOI] [PubMed] [Google Scholar]
- Lange S., et al. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005b;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Li S., Czubryt M. P., McAnally J., Bassel-Duby R., Richardson J. A., Wiebel F. F., Nordheim A., Olson E. N. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. USA. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Wang D., Sadee W. Serum response factor activation by muscarinic receptors via RhoA. Novel pathway specific to M1 subtype involving calmodulin, calcineurin, and Pyk2. J. Biol. Chem. 2002;277:40789–40798. doi: 10.1074/jbc.M202745200. [DOI] [PubMed] [Google Scholar]
- Liu H. W., et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am. J. Respir. Cell. Mol. Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- Lovering R. M., Hakim M., Moorman C. T., 3rd, De Deyne P. G. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J. Biomech. 2005;38:1501–1507. doi: 10.1016/j.jbiomech.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R. M., Roche J. A., Bloch R. J., De Deyne P. G. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch. Phys. Med. Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Maekawa M., Ishizaki T., Boku S., Watanabe N., Fujita A., Iwamatsu A., Obinata T., Ohashi K., Mizuno K., Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- McClung J. M., Lee W. J., Thompson R. W., Lowe L. L., Carson J. A. RhoA induction by functional overload and nandrolone decanoate administration in rat skeletal muscle. Pflugers Arch. 2003;447:345–355. doi: 10.1007/s00424-003-1151-7. [DOI] [PubMed] [Google Scholar]
- McClung J. M., Thompson R. W., Lowe L. L., Carson J. A. RhoA expression during recovery from skeletal muscle disuse. J. Appl. Physiol. 2004;96:1341–1348. doi: 10.1152/japplphysiol.01015.2003. [DOI] [PubMed] [Google Scholar]
- Meriane M., Roux P., Primig M., Fort P., Gauthier-Rouviere C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell. 2000;11:2513–2528. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano J. M., Long X., Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- Mir L. M., Bureau M. F., Gehl J., Rangara R., Rouy D., Caillaud J. M., Delaere P., Branellec D., Schwartz B., Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir L. M., Bureau M. F., Rangara R., Schwartz B., Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad. Sci. III. 1998;321:893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Dorn I. G., 2nd. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- Musa H., Meek S., Gautel M., Peddie D., Smith A. J., Peckham M. Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation. J. Cell Sci. 2006;119:4322–4331. doi: 10.1242/jcs.03198. [DOI] [PubMed] [Google Scholar]
- Nimnual A. S., Yatsula B. A., Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Kii I., Kudo A. Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J. Biol. Chem. 2004;279:47311–47319. doi: 10.1074/jbc.M403546200. [DOI] [PubMed] [Google Scholar]
- O'Brien S. P., Seipel K., Medley Q. G., Bronson R., Segal R., Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. USA. 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. L., Michel M. C. The RhoA/Rho kinase pathway in the myocardium. Cardiovasc. Res. 2007;75:3–4. doi: 10.1016/j.cardiores.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Pruitt W. M., Karnoub A. E., Rakauskas A. C., Guipponi M., Antonarakis S. E., Kurakin A., Kay B. K., Sondek J., Siderovski D. P., Der C. J. Role of the pleckstrin homology domain in intersectin-L Dbl homology domain activation of Cdc42 and signaling. Biochim. Biophys. Acta. 2003;1640:61–68. doi: 10.1016/s0167-4889(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Qadota H., Blangy A., Xiong G., Benian G. M. The DH-PH region of the giant protein UNC-89 activates RHO-1 GTPase in Caenorhabditis elegans body wall muscle. J. Mol. Biol. 2008;383:747–752. doi: 10.1016/j.jmb.2008.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeker M. O., Su F., Geisler S. B., Borisov A. B., Kontrogianni-Konstantopoulos A., Lyons S. E., Russell M. W. Obscurin is required for the lateral alignment of striated myofibrils in zebrafish. Dev. Dyn. 2006;235:2018–2029. doi: 10.1002/dvdy.20812. [DOI] [PubMed] [Google Scholar]
- Reuveny M., Heller H., Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS Lett. 2004;569:129–134. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Roche J. A., Lovering R. M., Bloch R. J. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport. 2008;19:1579–1584. doi: 10.1097/WNR.0b013e328311ca35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman K. L., Cheng L., Mahon G. M., Rojas R. J., Snyder J. T., Whitehead I. P., Sondek J. Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J. Biol. Chem. 2003;278:18393–18400. doi: 10.1074/jbc.M300127200. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Worthylake D. K., Snyder J. T., Siderovski D. P., Campbell S. L., Sondek J. A crystallographic view of interactions between Dbs and Cdc42, PH domain-assisted guanine nucleotide exchange. EMBO J. 2002;21:1315–1326. doi: 10.1093/emboj/21.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Raeker M. O., Korytkowski K. A., Sonneman K. J. Identification, tissue expression and chromosomal localization of human Obscurin-MLCK, a member of the titin and Dbl families of myosin light chain kinases. Gene. 2002;282:237–246. doi: 10.1016/s0378-1119(01)00795-8. [DOI] [PubMed] [Google Scholar]
- Sahai E., Alberts A. S., Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka M., Obata K., Ichihara S., Cheng X. W., Kimata H., Noda A., Izawa H., Nagata K., Yokota M. Attenuation of ventricular hypertrophy and fibrosis in rats by pitavastatin: potential role of the RhoA-extracellular signal-regulated kinase-serum response factor signalling pathway. Clin. Exp. Pharmacol. Physiol. 2006;33:1164–1171. doi: 10.1111/j.1440-1681.2006.04508.x. [DOI] [PubMed] [Google Scholar]
- Schratt G., Weinhold B., Lundberg A. S., Schuck S., Berger J., Schwarz H., Weinberg R. A., Ruther U., Nordheim A. Serum response factor is required for immediate-early gene activation yet is dispensable for proliferation of embryonic stem cells. Mol. Cell. Biol. 2001;21:2933–2943. doi: 10.1128/MCB.21.8.2933-2943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandala T., Gregory S. L., Dalton H. E., Smallhorn M., Saint R. Citron kinase is an essential effector of the Pbl-activated Rho signalling pathway in Drosophila melanogaster. Development. 2004;131:5053–5063. doi: 10.1242/dev.01382. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A., Gineitis D., Copeland J., Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Sutter S. B., Raeker M. O., Borisov A. B., Russell M. W. Orthologous relationship of obscurin and Unc-89, phylogeny of a novel family of tandem myosin light chain kinases. Dev. Genes Evol. 2004;214:352–359. doi: 10.1007/s00427-004-0413-5. [DOI] [PubMed] [Google Scholar]
- Takano H., Komuro I., Oka T., Shiojima I., Hiroi Y., Mizuno T., Yazaki Y. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 1998;18:1580–1589. doi: 10.1128/mcb.18.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley I. A., Denoth J. Sarcomere dynamics during muscular contraction and their implications to muscle function. J. Muscle Res. Cell Motil. 2007;28:89–104. doi: 10.1007/s10974-007-9107-8. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhou W., Croissant J. D., Johansen F. E., Prywes R., Balasubramanyam A., Schwartz R. J. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 1998;273:30287–30294. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- Weinert S., Bergmann N., Luo X., Erdmann B., Gotthardt M. M line-deficient titin causes cardiac lethality through impaired maturation of the sarcomere. J. Cell Biol. 2006;173:559–570. doi: 10.1083/jcb.200601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Ehler E., Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J. Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. J., Truskey G. A., Kraus W. E. Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. Am. J. Physiol. Cell Physiol. 2007;292:C2057–C2069. doi: 10.1152/ajpcell.00493.2006. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Schaefer A. W., Burnette D. T., Schoonderwoert V. T., Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- Zuurveld J. G., Veerkamp J. H., Wirtz P. Skeletal muscle fibers in suspension: a new approach to the study of oxidative and glycolytic metabolism in differentiated muscle. Int. J. Biochem. 1984;16:1107–1114. doi: 10.1016/0020-711x(84)90002-8. [DOI] [PubMed] [Google Scholar]