Abstract
We investigated the role of regulatory light-chain (Rlc1p) and heavy-chain phosphorylation in controlling fission yeast myosin-II (Myo2p) motor activity and function during cytokinesis. Phosphorylation of Rlc1p leads to a fourfold increase in Myo2p's in vitro motility rate, which ensures effective contractile ring constriction and function. Surprisingly, unlike with smooth muscle and nonmuscle myosin-II, RLC phosphorylation does not influence the actin-activated ATPase activity of Myo2p. A truncated form of Rlc1p lacking its extended N-terminal regulatory region (including phosphorylation sites) supported maximal Myo2p in vitro motility rates and normal contractile ring function. Thus, the unphosphorylated N-terminal extension of Rlc1p can uncouple the ATPase and motility activities of Myo2p. We confirmed the identity of one out of two putative heavy-chain phosphorylation sites previously reported to control Myo2p function and cytokinesis. Although in vitro studies indicated that phosphorylation at Ser-1444 is not needed for Myo2p motor activity, phosphorylation at this site promotes the initiation of contractile ring constriction.
INTRODUCTION
Cytokinesis is the final step of the cell cycle when a dividing cell is pinched in two by the actomyosin contractile ring. The myosin-II motor is essential for contractile ring function and cytokinesis in animal cells (Mabuchi and Okuno, 1977; De Lozanne and Spudich, 1987). Fission yeast possesses two type-II myosins, Myo2p and Myp2p, both of which function in the contractile ring. Myo2p is essential for cytokinesis and cell growth (Kitayama et al., 1997; May et al., 1997; Balasubramanian et al., 1998), whereas Myp2p only becomes essential for growth at elevated temperatures or high salt concentrations (Bezanilla et al., 1997; Motegi et al., 1997). The amino acid sequences of these myosins both predict the characteristic structural organization of type-II myosins: an N-terminal globular motor domain, followed by a light-chain binding domain, and a coiled-coil tail. Cdc4p (McCollum et al., 1995; Naqvi et al., 1999; Motegi et al., 2000) and Rlc1p (Le Goff et al., 2000; Naqvi et al., 2000) represent the respective “essential” (ELCs) and “regulatory” (RLCs) light chains.
The assembly of the fission yeast actomyosin ring occurs in two stages (Naqvi et al., 1999; Motegi et al., 2000; Wu et al., 2003): at the G2/M transition, Mid1p/anillin directs Myo2p to the division site as a broad band of dots (“nodes”). By the start of anaphase B, Myo2p nodes and actin filaments compact into a tight actomyosin ring. Myp2p joins the contractile ring a short time after assembly (Bezanilla et al., 2000). Ring constriction is delayed until completion of chromosome segregation and mitotic exit (Wolfe and Gould, 2005). Fission yeast rings are highly dynamic, exchanging Myo2p and actin filaments with half-lives of ∼30 s (Pelham and Chang, 2002; Clifford et al., 2008), similar to the 30–60 s half-lives reported for myosin-II in mammalian cell contractile rings (Guha et al., 2005; Murthy and Wadsworth, 2005).
Although the cellular behavior of a number of fission yeast cytokinesis proteins has been well studied, it is not yet clear how all these proteins function or how their activity is regulated. The mechanisms that control the activity of the myosin-II motor during the cell cycle or at the different stages of a contractile ring's lifetime (assembly, preconstricting, and constricting phases) are not understood. Myo2p is attractive for mechanistic studies because it can be purified in full-length form (Lord and Pollard, 2004). In this study we used in vitro and in vivo experiments in parallel to understand how phosphorylation controls Myo2p motor activity and contractile ring function.
It is well established that the motor activity of some myosin-IIs can be regulated by phosphorylation. Smooth muscle myosin and a number of nonmuscle myosin-II motors are activated by phosphorylation of their RLCs (Trybus, 1991; Tan et al., 1992). In mammalian cells phosphorylated RLC accumulates at the cleavage furrow (DeBiasio et al., 1996; Matsumura et al., 1998), whereas nonphosphorylatable forms of RLC do not support cytokinesis (Komatsu et al., 2000). Although heavy-chain phosphorylation is generally thought to be restricted to regulating the self-assembly of myosin-II into filaments (Egelhoff et al., 1993; Bresnick, 1999), phosphorylation of the Acanthamoeba myosin-II tail inhibits motor activity (Collins et al., 1982; Kuznicki et al., 1983).
Myo2p was initially predicted to be phosphorylated at the end of its tail based on mutagenesis at Ser-1518. Although an S1518A mutant abolished Myo2p localization at the ring, replacement with a phospho-mimicking glutamate was found to support localization (Mulvihill et al., 2001). However, this was refuted by another study identifying Ser-1444 as the critical phosphorylation site. Dephosphorylation at Ser-1444 occurs during early mitosis and is essential for Myo2p function and contractile ring assembly based on the lethality of a phospho-mimicking mutant (Motegi et al., 2004).
More recently, Rlc1p was shown to be an in vivo and in vitro substrate for p21-activated protein kinase Pak1p (Loo and Balasubramanian, 2008). Phosphorylation at Ser-35 and -36 prevents premature contractile ring constriction when cells are delayed in anaphase. Ser-36 represents the activating phosphorylation site found in other RLCs based on sequence homology (Loo and Balasubramanian, 2008). However, Rlc1p is unusual in that a relatively long stretch of 35 amino acids precedes its activation site, as opposed to the shorter stretch (12–18 amino acids) typical of other RLCs.
In this study we utilized nonphosphorylatable and phospho-mimicking mutant forms of Rlc1p and Myo2p to investigate the role of phosphorylation in regulating Myo2p motor activity and contractile ring function during cytokinesis. Wild-type and mutant forms of Myo2p were purified from fission yeast. The influence of myosin-II phosphorylation on contractile ring dynamics was assessed after integration of relevant mutations into the genome by gene replacement.
MATERIALS AND METHODS
Yeast Strains and Plasmids
The yeast strains and plasmids used in this study are listed in Tables 1 and 2.
Table 1.
Fission yeast strains
| Strain | Genotype | Source |
|---|---|---|
| TP 150 | h−leu1-32 | M. Yanagida |
| MLP 237 | h−leu1-32 ura4-D18 ade6-M216 his3-D1 | |
| MLP 238 | h+leu1-32 ura4-D18 ade6-M210 his3-D1 | |
| MLY 83 | h−leu1-32 ura4-D18 natR:41nmt1prom-FLAG-myo2 rlc1Δ:kanR | This study |
| MLP 651 | h−leu1-32 ura4-D18 ade6-M216 rlc1-mCFP:kan | Jian-Qiu Wu |
| MLY 78 | h+leu1-32 ura4-D18 ade6-M216 rlc1∇ura4+-mCFP:kan | This study |
| MLY 80 | h−leu1-32 ura4-D18 ade6-M216 rlc1-S35A-S36A-mCFP:kan | This study |
| MLY 81 | h−leu1-32 ura4-D18 ade6-M216 rlc1-S35D-S36D-mCFP:kan | This study |
| MLY 29 | h+/h−leu1-32/leu-32 ura4-D18/ura4-D18 ade6-M210/ade6-216 his3-D1/his3-D1 myo2/myo2Δ::mYFP-ura4 | This study |
| MLY 66 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 | This study |
| MLY 88 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 rlc1∇ura4+ | This study |
| MLY 91 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 rlc1-S35A-S36A | This study |
| MLY 93 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 rlc1-S35D-S36D | This study |
| TP 5 | h−leu1-32 ura4-D18 ade6-M210 his7-366 myp2Δ::his7+ | Bezanilla et al. (1997) |
| MLY 142 | h−leu1-32 ura4-D18 ade6-M210 his7-366 myp2Δ::his7+rlc1∇ura4+ | This study |
| MLY 145 | h−leu1-32 ura4-D18 ade6-M210 his7-366 myp2Δ::his7+rlc1-S35A-S36A | This study |
| MLY 147 | h−leu1-32 ura4-D18 ade6-M210 his7-366 myp2Δ::his7+rlc1-S35D-S36D | This study |
| MLY 402 | h+leu1-32 ura4-D18 ade6 his3-D1 myp2Δ::his7+mYFP-myo2 | This study |
| MLY 402b | h+leu1-32 ura4-D18 ade6 his3-D1 myp2Δ::his7+mYFP-myo2 rlc1∇ura4+ | This study |
| MLY 445 | h+leu1-32 ura4-D18 ade6 his3-D1 myp2Δ::his7+mYFP-myo2 rlc1-S35A-S36A | This study |
| TP 73 | h−leu1-32 ura4-D18 ade6-M216 his7-366 myo2-E1 | M. Balasubramanian |
| MLY 121 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A | This study |
| MLY 123 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D | This study |
| MLY 226 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1518A | This study |
| MLY 230 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1518E | This study |
| MLY 223 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A-S1518A | This study |
| MLY 227 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A-S1518E | This study |
| MLY 231 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D-S1518A | This study |
| MLY 232 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D-S1518E | This study |
| MLY 515 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 rlc1-NΔ (Δ aa 3-36) | This study |
| MLP 715 | h+leu1-32 ura4-D18 ade6–210 cdc25-22 | Jian-Qiu Wu |
| MLY 184 | h+leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2 cdc25-22 | This study |
| MLY 185 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A cdc25-22 | This study |
| MLY 186 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D cdc25-22 | This study |
| MLY 352 | h−leu1-32 ura4-D18 ade6 his3-D1 natR:41nmt1prom-FLAG-myo2-S1444 rlc1Δ:kanR | This study |
| MLY 349 | h−leu1-32 ura4-D18 ade6 his3-D1 natR:41nmt1prom-FLAG-myo2-S1444D rlc1Δ:kanR | This study |
| MLY 392 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A rlc1∇ura4+ | This study |
| MLY 394 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D rlc1∇ura4+ | This study |
| MLY 399 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A rlc1-S35A-S36A | This study |
| MLY 395 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D rlc1-S35A-S36A | This study |
| MLY 398 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444A rlc1-S35D-S36D | This study |
| MLY 396 | h−leu1-32 ura4-D18 ade6 his3-D1 mYFP-myo2-S1444D rlc1-S35D-S36D | This study |
Table 2.
Plasmids
| Plasmid | Comment | Source |
|---|---|---|
| pGST-cdc4 | pDS473a-based (nmt1 promoter, LEU2 marker) | Lord and Pollard (2004) |
| pGST-rlc1 | pDS473a-based (nmt1 promoter, ura4+ marker) | Lord and Pollard (2004) |
| pGST-rlc1-S35A-S36A | pDS473a-based (nmt1 promoter, ura4+ marker) | This study |
| pGST-rlc1-S35D-S36D | pDS473a-based (nmt1 promoter, ura4+ marker) | This study |
| pGST-rlc1-N1Δ | pDS473a-based (nmt1 promoter, ura4+ marker) | Lord and Pollard (2004) |
| pGST-cam1 | pDS473a-based (nmt1 promoter, LEU2 marker) | Lord and Pollard (2004) |
| pFA6a-natR-nmt41prom | Template for integration of a medium-strength (nmt1-41) inducible promoter | Lord and Pollard (2004) |
| pFA6a-natR-nmt41prom-FLAG | pFA-natR-nmt41prom with N-terminal FLAG tag | This study |
| KS-ura4 | Template for gene replacement with ura4+ | Bahler et al. (1998) |
| pFA6a-mYFP-kanR | Template for integration of monomeric YFP | Jian-Qiu Wu |
| pYFP-myo2 | YFP-myo2 fusion in pDS473a-based vector (LEU2 marker); includes myo2 promoter and terminator | This study |
| pYFP-myo2-S1444A | This study | |
| pYFP-myo2-S1444D | This study | |
| pYFP-myo2-S1518A | This study | |
| pYFP-myo2-S1518E | This study | |
| pYFP-myo2-S1444A-S1518A | This study | |
| pYFP-myo2-S1444A-S1518E | This study | |
| pYFP-myo2-S1444D-S1518A | This study | |
| pYFP-myo2-S1444D-S1518E | This study |
Microscopy
A Nikon TE2000-E2 inverted microscope (Melville, NY) with motorized fluorescence filter turret and a Plan Apo 60× (1.45 NA) objective was used to capture differential interference contrast (DIC) and epifluorescence cell images. Fluorescence utilized an EXFO X-CITE 120 illuminator (EXFO, Mississauga, ON, Canada). NIS Elements (Nikon) software was used to control the microscope, two Uniblitz shutters (Uniblitz, Rochester, NY), a Photometrics CoolSNAP HQ2 14-bit camera (Tucson, AZ), and autofocusing. Time-lapse movies monitored contractile ring assembly and dynamics by capturing YFP-Myo2p fluorescence every 2 min for 2 h at room temperature. Autofocusing was performed on the DIC channel before captures. Cell suspensions (3 μl) were mounted on flat 30-μl media pads (solidified by 1% agarose) prepared on the slide surface. VALAP (1:1:1 petroleum jelly, lanolin, and paraffin) was used to seal slides and coverslips. Image analysis of contractile ring dynamics was performed using ImageJ (http://rsb.info.nih.gov/ij/), Microsoft Excel (Redmond, WA), and KaleidaGraph software (Synergy Software, Reading, PA).
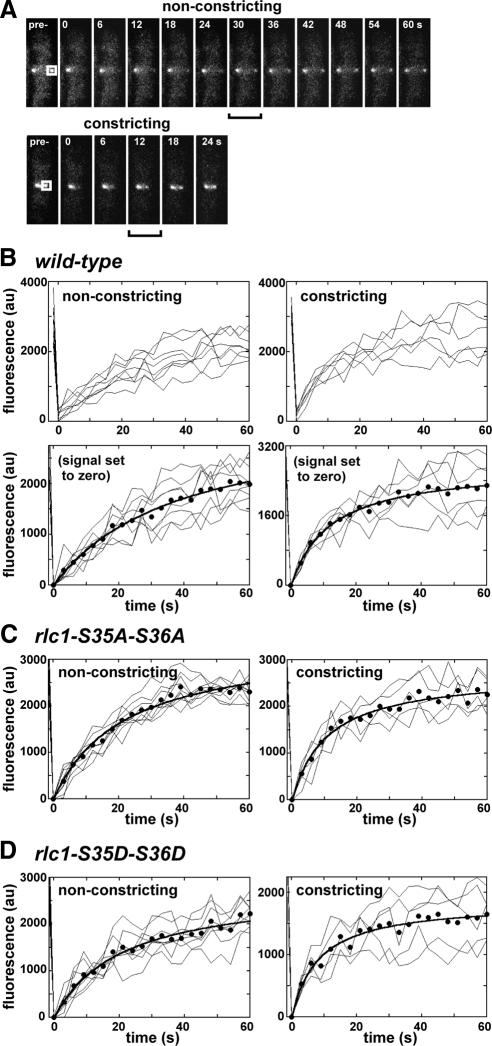
Fluorescence recovery after photobleaching (FRAP) experiments used confocal laser scanning microscopy with a Zeiss LSM 510 META system (Thornwood, NY) equipped with an argon laser, META detector, and a Plan Apo 100× (1.4 NA) objective. Cells were mounted on 1% agarose pads (as described above) before microscopy at room temperature. A region of interest (ROI) was selected on YFP-Myo2p contractile rings for directed bleaching using the LSM 510 (version 4.2) software. Photobleaching iterations were performed briefly at high laser power, ideally achieving 90–100% signal loss. Signal recovery was monitored by time-lapse analysis at low laser power with images collected every 3 s after bleach for 2 min. The LSM 510 software was used to perform data analysis (as detailed in the legend of Figure 5). Recovery curves of YFP-Myo2p signal versus time were plotted and fit using KaleidaGraph software.
Figure 5.
The rate of Myo2p exchange in the contractile ring increases during constriction. FRAP was used to measure YFP-Myo2p exchange rates in nonconstricting and constricting contractile rings using confocal laser scanning fluorescence microscopy. Cells were grown in YE5S media at 25°C before imaging at ambient temperature. (A) Micrographs charting recovery of YFP-Myo2p fluorescence in nonconstricting (top) and constricting (bottom) rings from representative rlc1+ cells. Panels on the far left show prebleached rings that were subsequently bleached at a region of interest (ROI, white box). Subsequent panels chart recovery of signal at the ring (0–60 s). Brackets indicate the point when recovery is half-maximal (t1/2). Panel width: 5 μm. (B–D) Plots charting fluorescence intensity versus time for ROIs on nonconstricting and constricting rings from rlc1+, rlc1-AA, and rlc1-DD cells. Fluorescence intensities measured before (−1.5 s) and after photobleaching (every 3 s, 0–60 s) are plotted. Individual ROI traces (thin lines) are shown (n = 5–10) along with an average fit (●, thick line). Datasets for each trace were corrected for additional bleaching encountered during time-lapse imaging by a control ROI (derived from an unbleached ring in the same field of cells). To facilitate curve fitting, zero signal intensity was set for each trace by subtracting residual YFP-Myo2p signal (detected at the ROI at the first time point after bleach, 0 s) from all trace values. Examples of datasets lacking this correction are provided for experiments with the rlc1+ strain (B, top panels).
ATPase and In Vitro Motility Assays
Actin-activated Myo2p ATPase assays were carried out at room temperature in 2 mM Tris-HCl, pH 7.2, 10 mM imidazole, 75 mM KCl, 0.1 mM CaCl2, 3 mM MgCl2, 2 mM ATP, and 1 mM DTT with 200–500 nM Myo2p and 0–100 μM actin. Malachite green was used to quantitate Pi release (Henkel et al., 1988). Controls omitting Myo2p were used and basal activity (detected in controls lacking actin) was subtracted to derive actin-activated ATPase rates. Curves were fit to Michaelis-Menten kinetics using KaleidaGraph software.
We used in vitro motility assays based on an established protocol (Kron and Spudich, 1986) with ∼0.5–2 μM Myo2p delivered into motility chambers. After Myo2p was adhered to the surface of a nitrocellulose-coated coverslip for 10 min, the chamber was washed three times with motility buffer (25 mM imidazole, pH 7.4, 50 mM KCl, 1 mM EGTA, 4 mM MgCl2, 2 mM DTT, 50 μg/ml catalase, 130 μg/ml glucose oxidase, and 3 mg/ml glucose) plus 0.5 mg/ml BSA, three times with motility buffer alone, twice with motility buffer containing 1 μM of unlabeled actin filaments, three times with motility buffer plus 1 mM ATP, twice with motility buffer plus 25 nM rhodamine phalloidin-labeled actin filaments, twice with motility buffer plus 20 mM DTT and 0.5% methyl-cellulose, and twice with motility buffer plus 20 mM DTT, 0.5% methyl-cellulose, and 1.5 mM ATP. Filaments were observed at room temperature by epifluorescence microscopy and recorded at 2-s intervals. ImageJ software was used to calculate filament velocities from time-lapse series.
Mass Spectrometry
A Coomassie-stained Myo2p heavy-chain band was excised from an SDS-PAGE gel and cut into ∼0.75-mm cubes; 500 μl of 50% acetonitrile was added, and samples were soaked for 2 h at room temperature. The solution was removed and discarded, and the process was repeated. The cubes were dried in a speed vacuum, and 100 μl of 50 mM ammonium bicarbonate containing 2 μg trypsin was added before incubating the sample on ice for 1 h. Thirty microliters of 50 mM ammonium bicarbonate was added before incubation at 30°C for 18 h. Formic acid, 7.5 μl, was added to inactivate the trypsin. Peptides were extracted with the addition and removal of four 100-μl aliquots of 25 mM ammonium bicarbonate in 50% acetonitrile. Samples were dried in a speed vacuum and reconstituted in 30 μl of 0.05% heptaflorobutyricacid.
All measurements were made by liquid chromatography electrospray ionization-mass spectrometry (LC-ESI-MS/MS). The liquid chromatography was performed using an Atlantis 1 × 150-mm i.d. column packed with 5 μm C18 (Waters, Milford, MA), with a Shimadzu MS pump and auto-sampler (Shimadzu Biotech, Columbia, MD). Five microliters of each sample was injected into 0.5% formic acid in 5.0% acetonitrile at a 25 μl/min flow rate. At 4 min, the flow was increased to 50 μl/min, and the gradient was ramped linearly to 0.5% formic acid in 45% acetonitrile over 30 min and held isocratic for 10 min. The ramp was increased to 0.5% formic acid in 60% acetonitrile for 6 min and then returned to 0.5% formic acid in 5% acetonitrile. The column was allowed to reequilibrate for 30 min before the next injection. The total run time was 86 min per analysis.
The LC eluant entered a LTQ ion trap mass spectrometer (Thermo Electron, Franklin, MA) connected to the LC by an ESI interface. The instrument was operated in positive electrospray ionization mode with a capillary temperature of 275°C and a spray voltage of 5.0 kV. Spectra were collected by scanning from 300 to 1800 Da using automatic gain control. Data-dependent MS/MS analyses were also performed using an isolation width of 1, collision energy of 35%, activation Q of 0.25, activation time of 30 ms, minimum MS signal 5 × 103, and a repeat count of 2 before a 60-s exclusion.
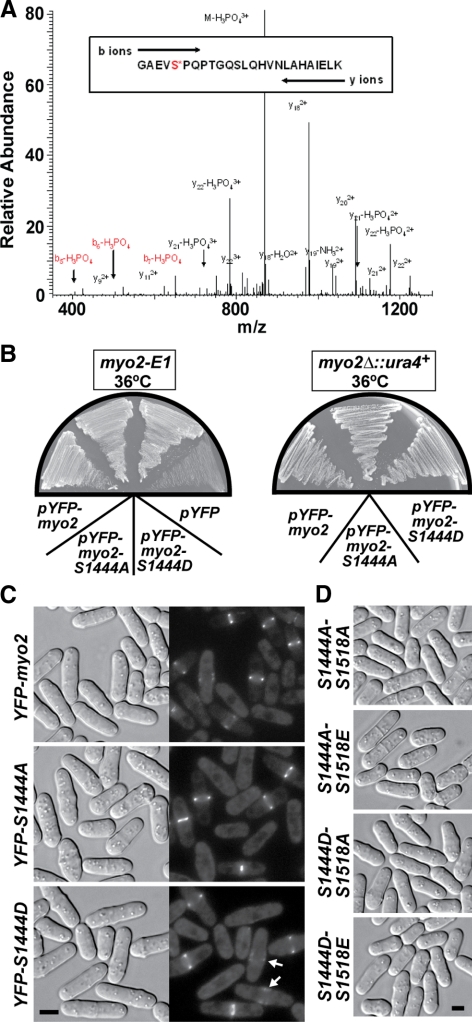
A SEQUEST search was performed via the Thermo-Finnigan BioWorks software (Austin, TX) using the Myo2p sequence. Phosphorylation was accounted for by the variable addition of 79.96 Da to each serine, threonine, and tyrosine residue. Once found, the data-dependent MS/MS spectra for m/z 902.35 (z = 3) was manually compared with theoretical fragment ions created using Protein Prospectors MS-Product (http://prospector.ucsf.edu/ucsfhtml4.0/msprod.htm) to provide additional identification.
Supplementary Material
Standard methods and details regarding yeast strain and plasmid construction are provided in the Supplementary Material. Protein purification and protein pulldown assay protocols can also be found in this section. Movies illustrating the effect Rlc1p-AA and -DD mutant forms have on Myo2p in vitro motility activity and contractile ring dynamics are provided (Supplementary Movies 1 and 2). Additional figures assessing the effects myo2 and rlc1 phosphorylation site mutations impart on Myo2p localization and cytokinesis are included (Supplementary Figures S1 and S2).
RESULTS
Rlc1p Phosphorylation Enhances the In Vitro Motility Rate of Myo2p
We purified Myo2p associated with nonphosphorylatable (S35A-S36A, AA) or phospho-mimicking (S35D-S36D, DD) forms of Rlc1p from fission yeast. The average rate of motility for Myo2p with Rlc1p-AA was ∼25% of that seen for Myo2p bound to Rlc1p-DD (Table 3, Figure 1A, Supplementary Movie 1) or wild-type Rlc1p (Table 3).
Table 3.
In vitro activity of wild type and mutant forms of Myo2p
| Myo2p samplea | Activity (± SD) |
|---|---|
| In vitro motility (μm · s−1)b | |
| Wild type | 0.42 ± 0.06 |
| Rlc1p-S35A-S36A | 0.12 ± 0.04 |
| Rlc1p-S35D-S36D | 0.41 ± 0.07 |
| Rlc1p-NΔ | 0.46 ± 0.04 |
| Myo2p-S1444A | 0.40 ± 0.06 |
| Myo2p-S1444D | 0.44 ± 0.08 |
| Myo2p-S1444A/Rlc1p-S35A-S36A | 0.14 ± 0.02 |
| Myo2p-S1444A/Rlc1p-S35D-S36D | 0.46 ± 0.11 |
| Myo2p-S1444D/Rlc1p-S35A-S36A | 0.16 ± 0.03 |
| Myo2p-S1444D/Rlc1p-S35D-S36D | 0.59 ± 0.05 |
| Actin-activated Mg2+-ATPasec |
||
|---|---|---|
| KM (μM) | Vmax (s−1)d | |
| Wild type | 19.9 ± 3.2 | 3.2 ± 0.2 |
| Rlc1p-S35A-S36A | 16.9 ± 4.2 | 2.7 ± 0.2 |
| Rlc1p-S35D-S36D | 18.6 ± 4.3 | 3.0 ± 0.2 |
a Each Myo2p sample is made up of the Myo2p heavy chain, ELC Cdc4p, and Rlc1p. Relevant mutations are indicated.
b n = 60 filaments. Motility was performed using one-step purified Myo2p samples.
c ATPase values for Rlc1p-AA and -DD samples are derived from the curves in Figure 6A.
d ATPase activity: moles of ATP hydrolyzed per motor per second.
Figure 1.
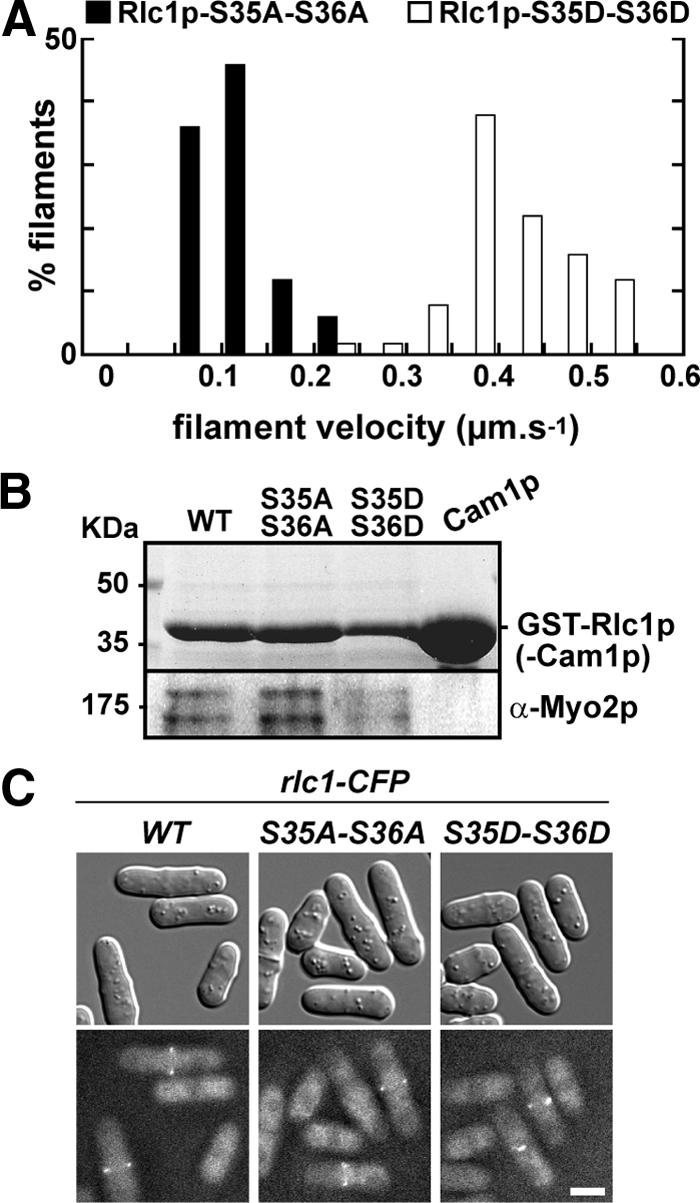
Phosphorylation of Rlc1p speeds up the in vitro motility rate of Myo2p. (A) Epifluorescence microscopy was used to generate time-lapse movies tracking movement of rhodamine-labeled actin filaments across the surface of Myo2p-coated coverslips. Myo2p samples were one-step purified. The histogram charts the distribution of Myo2p-driven actin filament gliding rates for preparations containing an unphosphorylatable form of Rlc1p (AA, ■) or a phospho-mimicking form (DD, □). Rates were derived from independent experiments using three separate preparations of Myo2p (n = 60 filaments). See Supplementary Movie 1. (B) Myo2p copurifies with both Rlc1p-AA and -DD isolated from cell extracts. Top panel, a Coomassie-stained gel indicating isolation and enrichment of wild-type GST-Rlc1p (positive control), GST-tagged mutants, and GST-Cam1p/calmodulin (negative control). Bottom panel, a Western blot indicating copurification of endogenous Myo2p with all three Rlc1p forms. (C) Wild-type, AA, and DD forms of Rlc1p-CFP localize to the contractile ring. Fusion proteins were visualized using fluorescence microscopy. Top panels, DIC images; bottom panels, the corresponding CFP images. Bar, 4 μm.
The motility rate of Myo2p with Rlc1p-AA (0.12 μm/s) is similar to that of Myo2p lacking Rlc1p (0.09 μm/s; Lord and Pollard, 2004). To determine whether unphosphorylated Rlc1p directly inhibits Myo2p motility or simply fails to remain bound to Myo2p, we compared the abilities of wild-type and mutant RLCs to associate with Myo2p. Rlc1p-AA was as effective as wild-type and DD forms in pulling down Myo2p from cells extracts (Figure 1B) and localizing at the contractile ring (Figure 1C). Thus, unphosphorylated Rlc1p imparts its inhibitory effect while bound to Myo2p.
Rlc1p Phosphorylation Regulates the Dynamics and Function of the Contractile Ring
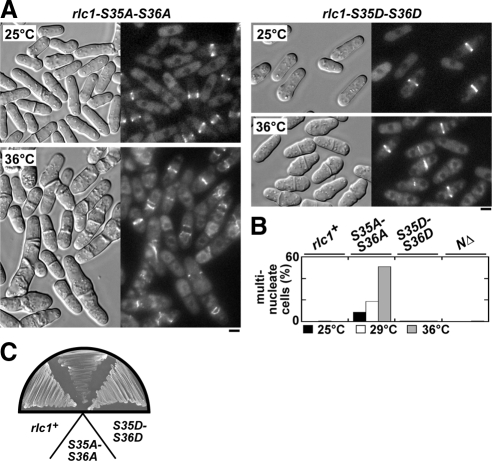
rlc1 phosphorylation site mutations were integrated into the genome by gene replacement to test the importance of Rlc1p phosphorylation in contractile ring dynamics and cytokinesis. Although rlc1-AA cells generally grew normally at 25°C, ∼10% of the population failed to complete cytokinesis (Figure 2, A and B). These defects became more apparent following growth at 36°C, with ∼50% of cells showing defects in contractile ring integrity leading to cytokinesis failure (Figure 2, A and B). In contrast, rlc1-DD cells were like wild type, exhibiting normal contractile rings that fully support cytokinesis (Figure 2, A and B). Preventing Rlc1p phosphorylation does not completely block growth (at any temperature), but inclusion of 1 M KCl in plates prevented growth of rlc1-AA cells at all temperatures tested (Figure 2C).
Figure 2.
Phosphorylation of Rlc1p promotes contractile ring integrity and cytokinesis. (A) Cell morphology and contractile rings viewed by DIC and epifluorescence imaging of a chromosomal YFP-Myo2p fusion are shown for rlc1-AA (left panels) and rlc1-DD (right panels) strains. Cells were grown in rich YE5S media at either 25°C (top) or 36°C (bottom) before imaging. Bars, 4 μm. (B) rlc1+, rlc1-AA, rlc1-DD, and rlc1-NΔ strains grown in YE5S media at 25, 32, and 36°C were treated with Hoechst stain to mark nuclei. The plot summarizes the cytokinesis phenotypes of cells quantitated by scoring multinucleate cells (3+ nuclei/cell) using fluorescence microscopy (n = 300 cells). (C) The ability of wild-type, rlc1-AA, and rlc1-DD cells to grow at 32°C on a YE5S plate containing 1 M KCl are compared.
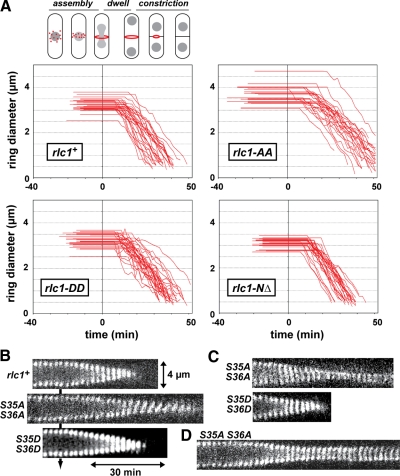
We compared contractile ring performance in rlc1+, rlc1-AA, and rlc1-DD cells grown at 25°C. Three different properties of the contractile ring were measured (Figure 3A): assembly (time taken for Myo2p to compact into a mature ring following its appearance as a broad band of nodes), dwell (time from completion of ring compaction until initiation of constriction), and constriction (change in ring circumference over time). Ring assembly took 50% longer in rlc1-AA cells versus wild-type or rlc1-DD cells (Figure 3A; Table 4). Although all three strains showed a similar dwell time (Figure 3A; Table 4), intact rlc1-AA rings constricted ∼1.5-fold slower than wild-type and rlc1-DD rings (Figure 3, A–C; Table 4). Notably, rlc1-AA rings often failed to complete constriction (16% of cells; Figure 3D), or began to slant or slip out of position in the cell cortex once constriction began (51% of cells; Figure 3, B and C; Supplementary Movie 2). These defects became more apparent when rlc1-AA cells were grown at higher temperatures, which presumably accounts for the more striking morphological phenotypes accompanying growth at these temperatures (Figure 2, A and B).
Figure 3.
Effect of Rlc1p phosphorylation on contractile ring dynamics. (A) Plots charting the assembly, dwell, and constriction phases of individual rings from rlc1+ (n = 30), rlc1-AA (n = 25), rlc1-DD (n = 30), and rlc1-NΔ (n = 30) cells recorded using time-lapse fluorescent microscopy of YFP-Myo2p. Only rlc1-AA cells that successfully completed constriction were included in the analysis. For each plot the length of flat-line traces shown at negative time represent assembly time, whereas the length of flat line traces shown at positive time correspond to the dwell time. Diagonal lines correspond to the constriction phase, derived from measuring ring diameter over time for each cell. Cells were grown in YE5S media at 25°C and imaged by time-lapse epifluorescence microscopy (at 23°C). The schematic of a fission yeast cell (shown above the rlc1+ plot) illustrates the timing of contractile ring (red) assembly, dwell, and constriction phases as they relate to the division and position of nuclei (gray) during mitosis. (B) Kymographs comparing YFP-Myo2p contractile ring dynamics of rlc1+, rlc1-AA, and rlc1-DD cells. Each kymograph is made up of a series of thin slices centered on the contractile ring using images captured every 2 min. Slice height: 4.4 μm. Kymographs start from the point at which rings have assembled, spanning dwell and constriction phases. Kymographs are aligned based on the time point at which constriction begins (indicated by the arrow). See Supplementary Movie 2. (C) Additional kymographs comparing contractile ring dynamics from rlc1-AA and -DD cells during the constriction phase. (D) Kymograph of a contractile ring from an rlc1-AA cell that fails to complete constriction.
Table 4.
Effect of rlc1 and myo2 mutations on YFP-Myo2p contractile ring dynamics
| Strain | Ring property (± SD)a |
||
|---|---|---|---|
| Assembly (min)b | Dwell (min)b | Constriction (μm/min)b | |
| Wild typec | 16.6 ± 2.4 | 14.3 ± 4.0 | 0.38 ± 0.06 |
| rlc1-S35A-S36A | 24.7 ± 4.2 | 13.1 ± 4.4 | 0.25 ± 0.05d |
| rlc1-S35D-S36D | 18.5 ± 3.5 | 12.8 ± 3.5 | 0.35 ± 0.06 |
| myp2Δ | 18.9 ± 3.2 | 20.6 ± 3.6 | 0.25 ± 0.06d |
| myp2Δ rlc1-S35A-S36A | 25.5 ± 3.5 | 16.3 ± 2.7 | 0.19 ± 0.04d |
| rlc1-NΔ | 17.7 ± 3.4 | 14.2 ± 3.4 | 0.39 ± 0.02 |
| Wild typec | 17.2 ± 1.6 | 13.3 ± 2.2 | 0.44 ± 0.02 |
| myo2-S1444A | 16.6 ± 2.4 | 18.4 ± 1.7 | 0.41 ± 0.06 |
| myo2-S1444D | 17.7 ± 1.6 | 8.1 ± 1.9 | 0.45 ± 0.01 |
a Ring properties were analyzed after growth of cells at 25°C. Assembly: time taken for Myo2p to compact into a mature ring after its appearance as a broad band of nodes; dwell: time from completion of ring compaction until initiation of constriction; constriction: change in ring circumference over time. n = 25–50 rings/strain. Dwell times and constriction initiation were discerned by charting ring diameter over time for each ring. Individual constriction rates were derived from the slopes of constricting ring circumferences.
b Paired Student's t tests were used to confirm the significance of changes in average assembly times, dwell times, and constriction rates between strains indicated (p < 0.05 indicates a significant difference between datasets). rlc1-AA vs. rlc1+ (assembly: <0.0001/constriction: <0.0001), vs. rlc1-DD (0.0001/0.012), vs. rlc1-NΔ (0.0001/ <0.0001); rlc1-NΔ vs. rlc1+ (0.44/0.10). myo2+ vs. myo2-S1444A (dwell: <0.0001), vs. myo2-S1444D (<0.0001).
c The duplicate entries for wild-type control strains reflect two separate datasets (rlc1 vs. myo2 mutants) gathered and analyzed independently.
d Rates were only derived for rings that successfully completed constriction in rlc1-AA, myp2Δ, and myp2Δ rlc1-AA mutants.
In addition to controlling Myo2p activity, Rlc1p phosphorylation most likely regulates Myp2p. To specifically test whether Rlc1p phosphorylation is important for Myo2p function in vivo, we assessed mutant phenotypes in a myp2Δ background. In the absence of Myp2p, rlc1-AA cells still exhibited cytokinesis defects that were absent in rlc1+ and rlc1-DD cells at 25°C (Figure 4, A and B). Phenotypic differences became less distinct at higher temperatures as the temperature-sensitive cytokinesis defects associated with loss of Myp2p (Motegi et al., 1997) began to dominate (Figure 4, A and B). Comparison of contractile ring dynamics in myp2Δ and myp2Δ rlc1-AA cells (that successfully completed constriction) revealed that ring assembly and constriction remained slower in the rlc1-AA background (Table 4). Thus, phosphorylation of Rlc1p promotes Myo2p function in cells, irrespective of whether Myp2p is present or not. Collectively our results suggest a correlation between the speed of Myo2p-driven in vitro motility and the rate of ring constriction in response to Rlc1p phosphorylation.
Figure 4.
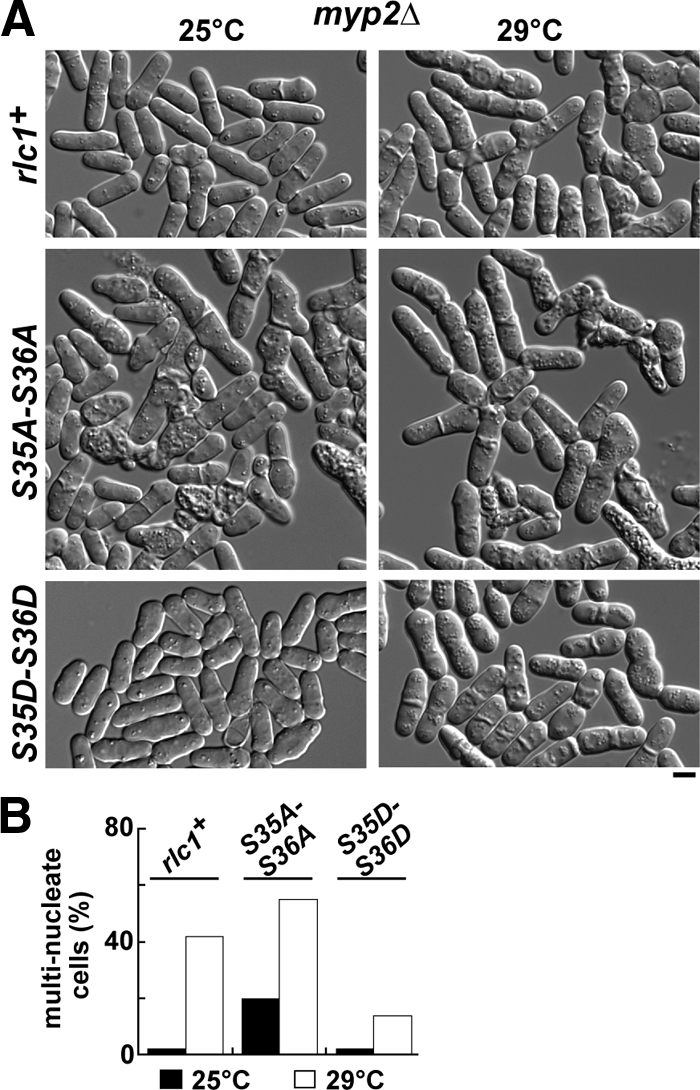
Phosphorylation of Rlc1p promotes Myo2p function in vivo. The phenotypes of rlc1+, rlc1-AA, and rlc1-DD strains lacking the nonessential myosin-II (Myp2p) were examined. (A) Representative fields of cells imaged by DIC microscopy following growth in YE5S media at 25 and 29°C. Bar, 4 μm. (B) Cells grown at 25 or 29°C were treated with Hoechst stain to mark nuclei. The plot summarizes the cytokinesis phenotypes of cells measured by scoring multinucleate cells using fluorescence microscopy (n = 300).
Rlc1p Phosphorylation Is Not Required for Recovery of Myo2p Fluorescence in Contractile Rings
Drugs that block myosin-II activity showed that continual, rapid exchange of actomyosin at the contractile ring relies upon myosin-II motor activity (Guha et al., 2005; Murthy and Wadsworth, 2005). We tested whether reducing myosin-II activity (by preventing Rlc1p phosphorylation) influences the exchange rate of Myo2p at contractile rings. FRAP analysis of nonconstricting and constricting rlc1+ rings indicated an average half-time (t1/2) for Myo2p recovery of ∼25 s (Table 5), similar to the 20 and 30 s half-times previously reported for Myo2p and its ELC (Pelham and Chang, 2002; Clifford et al., 2008). Interestingly, Myo2p exchange rates were different in nonconstricting versus constricting rings: Myo2p exchange was on average about threefold faster during constriction (Figure 5, A and B; Table 5). However, this difference in Myo2p's exchange rate cannot be attributed to Rlc1p phosphorylation. Comparison of rlc1-AA and -DD cells revealed similar slow and fast exchange rates in nonconstricting and constricting rings respectively (Figure 5, C and D; Table 5). Furthermore, Rlc1p phosphorylation did not influence the ratio of immobile versus mobile populations of Myo2p present in the ring (Table 5).
Table 5.
FRAP analysis of YFP-Myo2p exchange and recovery in nonconstricting and constricting contractile rings
| Strain | Nonconstricting ringsa |
Constricting ringsa |
||
|---|---|---|---|---|
| t1/2 (s)b | Recoveryc | t1/2 (s)b | Recoveryc | |
| rlc1+ | 36.9 ± 8.1 | 0.83 ± 0.13 | 12.9 ± 4.5 | 0.87 ± 0.15 |
| rlc1-S35A-S36A | 24.9 ± 9.1 | 0.86 ± 0.12 | 11.7 ± 6.6 | 0.89 ± 0.13 |
| rlc1-S35D-S36D | 23.1 ± 5.9 | 0.78 ± 0.11 | 8.7 ± 4.9 | 0.70 ± 0.12 |
a Paired Student's t tests were used to assess the significance of differences in average t1/2 and recovery values. Nonconstricting rings: rlc1+ vs. rlc1-AA (t1/2: 0.018/recovery: 0.55), vs. rlc1-DD (0.005/0.51), rlc1-AA vs. rlc1-DD (t1/2: 0.75). Constricting rings: rlc1+ vs. rlc1-AA (t: 0.87/recovery: 0.96), vs. rlc1-DD (0.22/0.32).
b Half-times of recovery were generated from the curves shown in Figure 5. The t1/2 values represent the mean generated from the fits of each individual FRAP experiment.
c Proportion of original (prebleached) signal recovered (mobile fraction of YFP-Myo2p in the ring). Values represent the mean generated from each individual FRAP experiment (by comparing maximal recovery and starting signals (from curves in Figure 5).
Phospho-regulation of Myo2p Motility Is Not Coupled to Actin-activated ATPase Activity
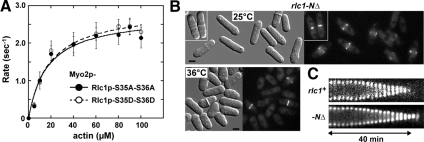
The decreased rate of Myo2p motility associated with unphosphorylated Rlc1p (Figure 1A) did not reflect a reduction in the motor's actin-activated ATPase activity. Myo2p bound to Rlc1p-AA or -DD exhibited similar, wild-type-like rates of ATP hydrolysis over a range of actin concentrations (Figure 6A; Table 3).
Figure 6.
The N-terminal extension of Rlc1p, but not Myo2p actin-activated ATPase activity, accounts for the reduced in vitro motility rate of dephosphorylated Rlc1p. (A) Actin-activated Mg2+-ATPase activity of two-step purified Myo2p as a function of actin concentration. The two curves represent Myo2p-Cdc4p associated with Rlc1p-AA (●) and Rlc1p-DD (○). Basal Myo2p ATPase activities (detected from control reactions lacking actin) and background Pi from actin (determined from controls lacking myosin) were subtracted from all measurements taken in the presence of 0–100 μM actin filaments. Each curve was generated from average values obtained from four different datasets and fit to Michaelis-Menten kinetics using KaleidaGraph software. (B) Cell morphology and contractile rings viewed by DIC and epifluorescence imaging of YFP-Myo2p are shown for the rlc1-NΔ strain in which Rlc1p lacks its regulatory N-terminus (amino acids 3–36). Cells were grown in rich media at either 25 or 36°C before imaging. Bar, 4 μm. (C) Representative kymographs comparing contractile ring dynamics of YFP-Myo2p in rlc1+ and rlc1-NΔ cells. Cells were prepared and kymographs generated from time-lapse fluorescence microscopy images as detailed in Figure 3B. Dwell and constriction phases are charted upon completion of ring assembly.
Intriguingly, the reduced rate of Myo2p motility seen in the presence of the nonphosphorylated Rlc1p-AA form was not observed when a truncated form of Rlc1p (Rlc1p-NΔ) lacking its N-terminal regulatory region (amino acids 3-36) was used (Table 3). The ability of plasmid-expressed Rlc1p- NΔ to localize to the ring and rescue an rlc1Δ mutant had previously led to the conclusion that Rlc1p phosphorylation was not important for cytokinesis (Lord and Pollard, 2004). However, based on current studies with Rlc1p-AA, phosphorylation is important in the context of the full-length RLC.
We examined the dynamics and function of contractile rings in cells expressing the truncated form of Rlc1p to test whether it was fully functional. rlc1-NΔ was integrated into the genome in place of the rlc1 gene. Like wild-type rlc1+ and the rlc1-DD mutant, rlc1-NΔ fully supported contractile ring function (Figures 2B and 6B) and normal ring dynamics (Figures 3A and 6C; Table 4). Thus, the ability of Rlc1p-NΔ to bypass the need for phosphorylation and support wild-type rates of Myo2p motility in vitro correlates with its ability to support normal contractile ring function in vivo without phosphorylation. Our results indicate that the extended N-terminus of Rlc1p acts to uncouple Myo2p's actin-activated ATPase activity and motility given that either phosphorylation or removal of this N-terminus alleviates the inhibition.
Phosphorylation of the Myo2p Heavy Chain at Ser-1444 Promotes Contractile Ring Constriction
We wanted to test whether heavy-chain phosphorylation was also capable of regulating Myo2p motor activity. We initially focused on Ser-1444 of Myo2p because mass spectrometry identified this as the sole phosphorylated residue in our purified Myo2p heavy chains (Figure 7A). Although there is no guarantee phospho-mimicking mutations perfectly reflect phosphorylated amino acids in vivo, substitution with negatively charged aspartate or glutamate residues is an appealing tactic when contrasting with phenotypes associated with nonphosphorylatable mutations. Although a myo2-S1444D mutant was reported to be lethal (Motegi et al., 2004), both of our YFP-Myo2p-S1444A and -S1444D constructs complemented the temperature-sensitive myo2-E1 mutant and rescued growth of a myo2 null (Figure 7B). In the course of engineering S1444A and S1444D mutations into a diploid genome (to facilitate overexpression and purification of the mutant forms) we confirmed our plasmid-based findings. Haploid segregants in which the myo2 gene was replaced in the genome by YFP-myo2, YFP-myo2-S1444A, or YFP-myo2-S1444D all maintained normal growth and cell morphology (Figure 7C). Dephosphorylation of Ser-1444 was not required for Myo2p assembly at the contractile ring given that broad bands of Myo2p-S1444D nodes were visible and assembled into mature rings at a similar rate to wild-type Myo2p (Figure 7C; Table 4).
Figure 7.
Cytokinesis is not dependent on the phosphorylation state of the Myo2p tail at Ser-1444 or -1518. (A) Electrospray ionization liquid chromatography ion trap mass spectrometry was performed on the Myo2p heavy chain. The Coomassie-stained heavy-chain band was cut out of an SDS-PAGE gel and analyzed. The phosphorylated peptide GAEVS*PQPTGQSLQHVNLAHAIELK produced a precursor ion at m/z 902.35 (z = 3) and was selected for MS/MS fragmentation. The largest MS/MS ion, m/z 869.93 (z = 3) corresponded to the precursor ion with the neutral loss of phosphate (−97.97 Da). The second and third largest MS/MS ions m/z 978.84 and 1091.83 (z = 2), corresponded to fragment ions y18 and y20, respectively. These ions are generated from cleavage of the precursor ion on the N-terminal side of Pro1445 or Pro1447, respectively, and their high abundance is anticipated (Breci et al., 2003). The MS/MS ions m/z 426.09, 523.22, and 651.21 (z = 1) correspond to fragment ions b5, b6, and b7 with the neutral loss of phosphate. These fragment ions indicate phosphorylation is located on Ser-1444 rather than Ser-1451. (B) pYFP-myo2-S1444A and -S1444D complement the temperature-sensitive lethality of the myo2-E1 mutant (left plate) and rescue growth of a myo2Δ strain (right plate). pYFP-myo2: positive control; pYFP: negative control lacking a myo2 insert. Cells were streaked out and grown at 36°C on EMM minimal media plates lacking leucine. (C) Gene replacement of myo2 with YFP-myo2-S1444A or -S1444D forms does not impact cytokinesis (left panels, DIC). Localization of integrated YFP-Myo2p, YFP-Myo2p-S1444A, and YFP-Myo2p-S1444D was captured by epifluorescence microscopy (right panels). Arrows in the bottom right panel highlight the presence of YFP-Myo2p-S1444D in the broad bands of assembling contractile rings. Bar, 4 μm. (D) Morphologies of YFP-myo2-S1444A-S1518A, YFP-myo2-S1444A-S1518E, YFP-myo2-S1444D-S1518A, and YFP-myo2-S1444D-S1518E double mutants. All cells were grown at 32°C in YE5S media before imaging. Bar, 4 μm.
We next tested whether Myo2p-S1444 mutants gave more obvious phenotypes when combined with mutations in another potential heavy-chain phosphorylation site (Ser-1518) predicted to regulate localization of Myo2p at the contractile ring (Mulvihill et al., 2001). In agreement with the findings of Motegi et al. (2004), YFP-myo2-S1518A and -S1518E mutants exhibited normal Myo2p localization and function in vivo (Supplementary Figure S1). Genomic myo2 was replaced by all possible combinations of S1444A/D and S1518A/E double mutants, but none showed any obvious morphological defects in cytokinesis (Figure 7D).
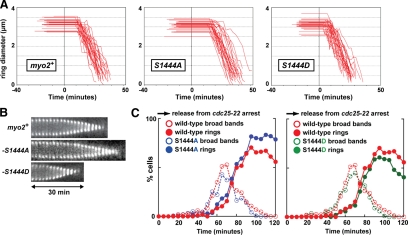
Time-lapse analysis of YFP-Myo2p-S1444A and -S1444D rings revealed differences in the timing of contractile ring dynamics. Compared with wild-type, S1444A rings dwelled longer before initiating constriction, whereas S1444D rings exhibited a shorter dwell (Figure 8, A and B; Table 4). This finding suggests one of two possibilities: 1) Myo2p tail phosphorylation regulates ring assembly. In this case, the absence of phosphorylation would promote premature initiation of ring assembly leading to a longer dwell time, whereas the phospho-mimic causes a delay in assembly leading to a shorter dwell time. 2) Myo2p tail phosphorylation regulates ring constriction. In this scenario, the absence of phosphorylation causes a delay in the initiation of ring constriction leading to a longer dwell time, whereas the phospho-mimic promotes the initiation of ring constriction leading to a reduced dwell time. To distinguish between these two possibilities we performed time courses in synchronized cells. Wild-type and mutant cells were arrested in the G2 phase of the cell cycle and released into mitosis (Figure 8C). We found that the timing of ring assembly was essentially identical in wild-type and mutant cells (Figure 8C), indicating that phosphorylation of the tail influences the initiation of ring constriction (as implied by the alignment of the kymographs in Figure 8B). However, these defects in contractile ring dynamics were not a consequence of altered Myo2p activity because purified Myo2p-S1444A and -S1444D exhibited wild-type rates of motility (Table 3).
Figure 8.
Phosphorylation of the Myo2p tail at Ser-1444 favors the initiation of ring constriction. (A) Plots charting the assembly, dwell, and constriction phases of individual rings from YFP-myo2, YFP-myo2-S1444A, and YFP-myo2-S1444D cells (n = 30; prepared as described in Figure 3A). (B) Representative kymographs generated from YFP-myo2, YFP-myo2-S1444A, and YFP-myo2-S1444D cells are displayed from the point at which rings have compacted from assembling broad bands. Cells were prepared and kymographs generated as detailed in Figure 3B. Nonconstricting and constricting phases are compared. (C) Time courses of broad band (assembling rings) and contractile ring appearance in cdc25-22 cells harboring a YFP-myo2, YFP-myo2-S1444A, or YFP-myo2-S1444D fusion. Cells were grown up in YE5S media at 25°C and then arrested in the G2 phase of the cell cycle by shifting cultures to 36°C (utilizing the temperature-sensitive cdc25-22 mutation). Synchronized cells were released into mitosis by growth at 25°C (time zero) and cell samples collected every 5 min from 0 to 120 min. The percentage of cells possessing broad bands or fully formed rings of YFP-Myo2p were scored using epifluorescence microscopy analysis. Plots for S1444A (left, blue circles) and S1444D (right, green circles) cells are overlaid on the wild-type time course. (For each strain n = 120–200 cells/time point).
Heavy-Chain Phosphorylation Does Not Influence Rlc1p-mediated Regulation of Myo2p
The fact Myo2p still supports a modest rate of actin filament gliding when associated with Rlc1p-AA (Table 3) led us to test whether the phosphorylation state of the Myo2p tail was involved in securing full regulation. myo2-S1444 and rlc1-S35-S36 double mutants were generated and morphological phenotypes assessed. When an rlc1-AA mutant was combined with a myo2-S1444A or -S1444D mutant its temperature-sensitive phenotype was no different from that observed in a myo2+ background (Supplementary Figure S2; Figure 2A). Likewise, no additive phenotypes were apparent when myo2-S1444A or -S1444D mutations were combined with the rlc1-DD mutant (Supplementary Figure S2). Consistent with the in vivo data, heavy-chain mutations at Ser-1444 had no effect on the in vitro motility rates of purified Myo2p associated with Rlc1p-AA or -DD (Table 3).
DISCUSSION
We have addressed the role of RLC and heavy-chain phosphorylation in the regulation of myosin-II motor activity and contractile ring function in fission yeast. Rlc1p phosphorylation promotes the in vitro motility activity of Myo2p and actomyosin ring function during cytokinesis. When Rlc1p is unphosphorylated, the speed of actin filament gliding is significantly reduced. This lower activity is largely reflected in vivo by defects in ring constriction, often leading to cytokinesis failure. Unlike other myosin-IIs regulated by RLC phosphorylation, regulation of Myo2p motility by Rlc1p phosphorylation is not the result of a reduction in the actin-activated ATPase activity of the motor. Although phosphorylation of the heavy chain at Ser-1444 has no effect on Myo2p motor activity or assembly at the contractile ring, this modification favors the initiation of ring constriction.
Role of Rlc1p Phosphorylation in Cytokinesis
The fact Rlc1p phosphorylation is not essential for viability allowed a detailed examination of this regulatory mechanism in the cell. As growth temperature was increased, the cytokinesis defects of cells harboring the nonphosphorylatable form of Rlc1p became more pronounced. Although Myo2p lacking Rlc1p or possessing Rlc1p-AA show similar rates of motility, the AA phenotype was distinct from that of an rlc1Δ strain in vivo. Although AA and rlc1Δ cells both fail to grow in the presence of 1 M salt, the cytokinesis phenotype of the AA mutant was not as severe as the phenotype of rlc1Δ cells (Le Goff et al., 2000; Naqvi et al., 2000). As with other type-II myosins, Myo2p lacking its RLC may lose function due to aggregation caused by the exposed hydrophobic RLC binding site (Vale et al., 1984; Pastra-Landis and Lowey, 1986). This scenario cannot account for defects associated with Rlc1p-AA because it still binds to Myo2p.
When Rlc1p phosphorylation is prevented, contractile ring assembly takes 1.5-fold longer, whereas the rate of ring constriction slows down 1.5-fold, implying that maximal Myo2p motility may promote these two phases. Strikingly, rlc1-AA rings often failed to complete constriction or slipped out of position in the division plane as they attempted to constrict. Thus, the major deficit caused by blocking Rlc1p phosphorylation is associated with ring constriction. A recent study revealed that the p21-activated kinase Pak1p phosphorylates Rlc1p at Ser-35 and -36 (Loo and Balasubramanian, 2008). However, this study compared contractile ring dynamics in rlc1+ and rlc1-S35A-S36A cells in an ase1Δ background where anaphase progression is slowed down. In contrast to our findings, the rlc1-AA mutant promoted premature ring constriction in this background (Loo and Balasubramanian, 2008). Presumably, maximal Myo2p motility helps maintain ring function during the nonconstricting phase when cells are forced to further delay constriction to ensure completion of chromosome segregation.
In contrast to the rlc1-AA mutant, some mutant forms of Myo2p exhibit low rates of motility that are not reflected by cytokinesis defects in vivo (Lord and Pollard, 2004). Notably, Myo2p lacking its entire light-chain binding domain “lever arm”) has no obvious cytokinesis phenotype (D'Souza et al., 2001). Thus, in addition to slowing down Myo2p motility, dephosphorylation of Rlc1p may also inhibit Myp2p, leading to a more pronounced cytokinesis phenotype when both of the type-II myosins are compromised. Myp2p motility is probably regulated by Rlc1p phosphorylation, but at this stage we are unable to confirm this given our inability to purify Myp2p.
Myosin-II motor activity controls the dynamic exchange of actomyosin at contractile rings (Guha et al., 2005; Murthy and Wadsworth, 2005). Intriguingly, we found that Myo2p exchanges much faster in constricting versus nonconstricting rings. However, this difference is not linked to Rlc1p phosphorylation and increased Myo2p motility. Given that Rlc1p phosphorylation has no effect on the actin-activated ATPase activity of Myo2p, changes in the rate of Myo2p exchange at the ring may reflect differences in the ATPase rate, rather than a change in the distance actin filaments are displaced per ATP hydrolysis cycle.
Regulation of Myo2p Motility by Rlc1p Phosphorylation
In cases where RLC phosphorylation regulates myosin-II, the extent to which phosphorylation regulates the motility of the motor can vary, but is always coupled to the actin-activated ATPase rate. For example, although dephosphorylation of smooth-muscle myosin switches off the ATPase and motility of the motor (Trybus, 1991; Sellers, 1999), dephosphorylation of Dictyostelium myosin-II reduces ATPase rate about fivefold, leading to a corresponding fivefold drop in the motility rate (Griffith et al., 1987; Ostrow et al., 1994; Chen et al., 1999). In contrast, although Rlc1p dephosphorylation reduces the rate of Myo2p motility about fourfold, it had no effect on the actin-activated ATPase rate. The extended N-terminus of Rlc1p is responsible for uncoupling Myo2p's ATPase and motility activities. Experiments with Rlc1p-NΔ and an rlc1-NΔ strain revealed that removal of the extension (including both phosphorylation sites) bypasses the requirement for phosphorylation in securing maximal rates of motility in vitro or fully functional contractile rings in vivo. In contrast, removal of the first 16 amino acids of smooth-muscle myosin RLC by either trypsin digestion or mutagenesis leads to motor inhibition irrespective of whether the RLC is phosphorylated (Ikebe and Morita, 1991; Ikebe et al., 1994). Thus, although the N-terminus of the smooth-muscle RLC functions in motor activation, the longer N-terminus of Rlc1p is inhibitory.
How does unphosphorylated Rlc1p uncouple Myo2p actin-activated ATPase and motility activities? One possible explanation is based on the unusually long N-terminus of Rlc1p and its relationship to other well-studied light chains. An N-terminal extension on the A1 isoform of the ELC from skeletal muscle has been shown to inhibit myosin-II motility without affecting actin-activated ATPase activity (Pastra-Landis et al., 1983; Lowey et al., 1993). The extension is thought to associate with actin filaments to impose an internal load on myosin, which retards motility (Andreev et al., 1999). A similar extension is found at the N-terminus of the Drosophila flight muscle myosin RLC, and this too has been proposed to contact actin to secure optimal muscle organization (Irving et al., 2001). It is possible that the N-terminal extension of Rlc1p associates with actin filaments, resulting in drag that slows down Myo2p's motility rate independent of its ATPase rate.
Alternatively, Rlc1p dephosphorylation may prevent maximal actin filament displacement by perturbing the motion or stiffness of the “lever-arm” or by limiting conformational changes within the motor required for lever arm motion. More in vitro studies will be needed to fully understand the mechanism by which Rlc1p phosphorylation promotes Myo2p motility.
Influence of Heavy-Chain Phosphorylation
We examined the importance of putative phosphorylation sites located in the Myo2p tail at Ser-1444 and -1518. Although the phospho-mimicking myo2-S1444D mutant was reported to be lethal (Motegi et al., 2004), both of our -S1444A and -S1444D myo2 constructs supported Myo2p function. This discrepancy may be explained by our use of plasmids employing the native myo2 promoter, as opposed to the maximum strength nmt1 promoter used in the plasmid of Motegi et al. Importantly, we confirmed our observations using a different approach (gene replacement in the genome). Furthermore, Myo2p-S1444A and -S1444D mutants still supported cytokinesis when combined with mutations at Ser-1518.
Mass spectrometry revealed that Myo2p is phosphorylated at Ser-1444. Although this phosphorylation did not influence Myo2p motility, time-lapse analysis of contractile rings indicated a role for Ser-1444 phosphorylation in promoting the initiation of ring constriction. It is unlikely that tail phosphorylation regulates self-assembly. Myo2p is insoluble at low ionic strength with and without tail phosphorylation, but the structures of these assemblies are not known (J. E. Friend and T. D. Pollard, personal communication). Dephosphorylation at S1444 was previously reported to be essential for targeting Myo2p to the medial division site via an interaction with Mid1p (Motegi et al., 2004). However, neither of our phosphorylation site mutants (S1444A and S1444D) showed defects in contractile ring placement or assembly. Therefore, phosphorylation may govern Myo2p's ability to associate with Mid1p or another ring component to help start ring constriction. Overall, our studies on the heavy chain and Rlc1p suggest that two pathways of phosphorylation regulate Myo2p function by distinct mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heather Truax, Ashley Truax, and Jessica Johnson for assistance with data analysis. We thank Mohan Balasubramanian (Temasek Life Sciences Laboratory, Singapore) for the myo2-E1 strain; Jian-Qiu Wu (The Ohio State University, Cleveland, OH) for the pFA6a-mYFP plasmid, and the rlc1-mCFP and cdc25-22 strains; and Mitsuhiro Yanagida (Kyoto University, Kyoto, Japan) for the protease deficient strain. We are indebted to Marilyn Wadsworth and Doug Taatjes from the University of Vermont Microscopy Imaging Facility for their expertise and assistance with FRAP experiments and accompanying data analysis and Jim Vigoreaux and Dwight Matthews for access to mass spectrometry facilities. We are grateful to the Lowey and Trybus labs for access to equipment. We thank Janice Friend and Tom Pollard (Yale University, New Haven, CT) for communicating unpublished results. We acknowledge Alex Hodges, Susan Lowey, Tom Pollard, and Kathy Trybus for their insightful comments on the manuscript. Work in the Lord laboratory is funded by a New Research Initiative Award from the University of Vermont and a Scientist Development grant (0835236N) from the American Heart Association.
Glossary
Abbreviations used:
- ELC
essential light chain
- FRAP
fluorescence recovery after photobleaching
- LC-ESI-MS
liquid chromatography electrospray ionization-mass spectrometry
- RLC
regulatory light chain
- ROI
region of interest.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-04-0346) on July 1, 2009.
REFERENCES
- Andreev O. A., Saraswat L. D., Lowey S., Slaughter C., Borejdo J. Interaction of the N-terminus of chicken skeletal essential light chain 1 with F-actin. Biochemistry. 1999;38:2480–2485. doi: 10.1021/bi981706x. [DOI] [PubMed] [Google Scholar]
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S., Gould K. L. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Forsburg S. L., Pollard T. D. Identification of a second myosin-II in Schizosaccharomyces pombe: Myp2p is conditionally required for cytokinesis. Mol. Biol. Cell. 1997;8:2693–2705. doi: 10.1091/mbc.8.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Wilson J. M., Pollard T. D. Fission yeast myosin-II isoforms assemble into contractile rings at distinct times during mitosis. Curr. Biol. 2000;10:397–400. doi: 10.1016/s0960-9822(00)00420-6. [DOI] [PubMed] [Google Scholar]
- Breci L. A., Tabb D. L., Yates J. R., 3rd, Wysocki V. H. Cleavage N-terminal to proline: analysis of a database of peptide tandem mass spectra. Anal. Chem. 2003;75:1963–1971. doi: 10.1021/ac026359i. [DOI] [PubMed] [Google Scholar]
- Bresnick A. R. Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- Chen P., Chaudoir B. M., Trybus K. M., Chisholm R. L. Expression of chicken gizzard RLC complements the cytokinesis and developmental defects of Dictyostelium RLC null cells. J. Muscle Res. Cell Motil. 1999;20:177–186. doi: 10.1023/a:1005405023020. [DOI] [PubMed] [Google Scholar]
- Clifford D. M., Wolfe B. A., Roberts-Galbraith R. H., McDonald W. H., Yates J. R., 3rd, Gould K. L. The Clp1/Cdc14 phosphatase contributes to the robustness of cytokinesis by association with anillin-related Mid1. J. Cell Biol. 2008;181:79–88. doi: 10.1083/jcb.200709060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Cote G. P., Korn E. D. Localization of the three phosphorylation sites on each heavy chain of Acanthamoeba myosin II to a segment at the end of the tail. J. Biol. Chem. 1982;257:4529–4534. [PubMed] [Google Scholar]
- D'Souza V., M., Naqvi N. I., Wang H., Balasubramanian M. K. Interactions of Cdc4p, a myosin light chain, with IQ-domain containing proteins in Schizosaccharomyces pombe. Cell Struct. Funct. 2001;26:555–565. doi: 10.1247/csf.26.555. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- DeBiasio R. L., LaRocca G. M., Post P. L., Taylor D. L. Myosin II transport, organization, and phosphorylation: evidence for cortical flow/solation-contraction coupling during cytokinesis and cell locomotion. Mol. Biol. Cell. 1996;7:1259–1282. doi: 10.1091/mbc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Lee R. J., Spudich J. A. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Downs S. M., Spudich J. A. Myosin light chain kinase and myosin light chain phosphatase from Dictyostelium: effects of reversible phosphorylation on myosin structure and function. J. Cell Biol. 1987;104:1309–1323. doi: 10.1083/jcb.104.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M., Zhou M., Wang Y. L. Cortical actin turnover during cytokinesis requires myosin II. Curr. Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Henkel R. D., VandeBerg J. L., Walsh R. A. A microassay for ATPase. Anal. Biochem. 1988;169:312–318. doi: 10.1016/0003-2697(88)90290-4. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Ikebe R., Kamisoyama H., Reardon S., Schwonek J. P., Sanders C. R., 2nd, Matsuura M. Function of the NH2-terminal domain of the regulatory light chain on the regulation of smooth muscle myosin. J. Biol. Chem. 1994;269:28173–28180. [PubMed] [Google Scholar]
- Ikebe M., Morita J. Identification of the sequence of the regulatory light chain required for the phosphorylation-dependent regulation of actomyosin. J. Biol. Chem. 1991;266:21339–21342. [PubMed] [Google Scholar]
- Irving T., Bhattacharya S., Tesic I., Moore J., Farman G., Simcox A., Vigoreaux J., Maughan D. Changes in myofibrillar structure and function produced by N-terminal deletion of the regulatory light chain in Drosophila. J. Muscle Res. Cell Motil. 2001;22:675–683. doi: 10.1023/a:1016336024366. [DOI] [PubMed] [Google Scholar]
- Kitayama C., Sugimoto A., Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S., Yano T., Shibata M., Tuft R. A., Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J. Biol. Chem. 2000;275:34512–34520. doi: 10.1074/jbc.M003019200. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc. Natl. Acad. Sci. USA. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki J., Albanesi J. P., Cote G. P., Korn E. D. Supramolecular regulation of the actin-activated ATPase activity of filaments of Acanthamoeba Myosin II. J. Biol. Chem. 1983;258:6011–6014. [PubMed] [Google Scholar]
- Le Goff X., Motegi F., Salimova E., Mabuchi I., Simanis V. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 2000;113(Pt 23):4157–4163. doi: 10.1242/jcs.113.23.4157. [DOI] [PubMed] [Google Scholar]
- Loo T.H., Balasubramanian M. Schizosaccharomyces pombe Pak-related protein, Pak1p/Orb2p, phosphorylates myosin regulatory light chain to inhibit cytokinesis. J. Cell Biol. 2008;183:785–793. doi: 10.1083/jcb.200806127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord M., Pollard T. D. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 2004;167:315–325. doi: 10.1083/jcb.200404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J. Biol. Chem. 1993;268:20414–20418. [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 1977;74:251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Ono S., Yamakita Y., Totsukawa G., Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May K. M., Watts F. Z., Jones N., Hyams J. S. Type II myosin involved in cytokinesis in the fission yeast, Schizosaccharomyces pombe. Cell Motil. Cytoskelet. 1997;38:385–396. doi: 10.1002/(SICI)1097-0169(1997)38:4<385::AID-CM8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K., Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Nakano K., Kitayama C., Yamamoto M., Mabuchi I. Identification of Myo3, a second type-II myosin heavy chain in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 1997;420:161–166. doi: 10.1016/s0014-5793(97)01510-x. [DOI] [PubMed] [Google Scholar]
- Motegi F., Nakano K., Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J. Cell Sci. 2000;113(Pt 10):1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Mulvihill D. P., Barretto C., Hyams J. S. Localization of fission yeast type II myosin, Myo2, to the cytokinetic actin ring is regulated by phosphorylation of a C-terminal coiled-coil domain and requires a functional septation initiation network. Mol. Biol. Cell. 2001;12:4044–4053. doi: 10.1091/mbc.12.12.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K., Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Naqvi N. I., Eng K., Gould K. L., Balasubramanian M. K. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N. I., Wong K. C., Tang X., Balasubramanian M. K. Type II myosin regulatory light chain relieves auto-inhibition of myosin-heavy-chain function. Nat. Cell Biol. 2000;2:855–858. doi: 10.1038/35041107. [DOI] [PubMed] [Google Scholar]
- Ostrow B. D., Chen P., Chisholm R. L. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J. Cell Biol. 1994;127:1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastra-Landis S. C., Huiatt T., Lowey S. Assembly and kinetic properties of myosin light chain isozymes from fast skeletal muscle. J. Mol. Biol. 1983;170:403–422. doi: 10.1016/s0022-2836(83)80155-7. [DOI] [PubMed] [Google Scholar]
- Pastra-Landis S. C., Lowey S. Myosin subunit interactions. Properties of the 19,000-dalton light chain-deficient myosin. J. Biol. Chem. 1986;261:14811–14816. [PubMed] [Google Scholar]
- Pelham R. J., Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature. 2002;419:82–86. doi: 10.1038/nature00999. [DOI] [PubMed] [Google Scholar]
- Sellers J. R. Myosins. 2nd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- Tan J. L., Ravid S., Spudich J. A. Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Trybus K. M. Regulation of smooth muscle myosin. Cell Motil. Cytoskelet. 1991;18:81–85. doi: 10.1002/cm.970180202. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Szent-Gyorgyi A. G., Sheetz M. P. Movement of scallop myosin on Nitella actin filaments: regulation by calcium. Proc. Natl. Acad. Sci. USA. 1984;81:6775–6778. doi: 10.1073/pnas.81.21.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 2005;15:10–18. doi: 10.1016/j.tcb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.