Abstract
The family of selenoproteins have a broad range of functions, including protection against oxidative damage. Previous studies have shown that elevated levels of oxidative damage can induce accelerated loss of telomeric DNA during proliferation of mammalian cells. The incorporation of selenocysteine (Sec) into proteins in mammalian cells requires the Sec insertion sequence (SECIS) binding protein 2 (SBP2). Thus in the present study we have assessed the effect of knocking down the expression of SBP2 on telomere length. Following knock-down of SBP2 expression in 2 different human cell lines, the MSTO mesothelioma cell line (~5 Kb average telomere length) and SY5Y neuroblastoma cell line (~4.2 Kb average telomere length), we observed a significant reduction (−0.6 to −1.1Kb; P≤0.01) in telomere length as compared to control cells. This reduction in telomere length was independent of affects on telomerase, since both telomerase activity levels and Tert mRNA expression levels were not altered by knockdown of SBP2 expression. Furthermore, telomeres were particularly sensitive to S1 nuclease digestion following SBP2 knock-down, indicating an increased frequency of oxidative damage induced lesions in the telomeric DNA in these cells. Together, these observations imply that selenoproteins may help protect telomeric reserve in mammalian cells.
Keywords: Telomere, selenoprotein, shRNA knockdown, oxidative damage, telomere shortening
INTRODUCTION
Mammalian cell senescence, defined as an irreversible state of growth arrest (Hayflick and Moorhead, 1961), can be induced by a number of events, including excessive oxidative stress, DNA damage, dysregulated mitogenic stimuli, and telomere shortening (Campisi and d'Adda di Fagagna, 2007). Even in an optimal growth environment devoid of external stressors, normal human somatic cells still ultimately succumb to cell senescence due to the incremental loss of telomeric DNA at each round of cell division and eventual shortening of the terminal TTAGGG tract below a critical threshold required for telomere function (Harley et al, 1990; Hemann et al, 2001). This proliferation-dependent loss of telomeric DNA is attributed to the absence of sufficient levels of the enzyme telomerase, a ribonucleoprotein complex which functions to complete the replication of telomeres during DNA replication [S phase]—over-expression of telomerase reverse transcriptase (Tert), the limiting component of the telomerase complex, in normal human cell strains is sufficient to restore telomerase activity and prevent both telomere shortening and cell senescence (Bodnar et al, 1998; Blackburn, 2005).
The rate of telomere shortening observed for mammalian cells in a stress-free environment either in vivo or in vitro is typically in the range of 50–100bp per cell division. A number of factors can lead to acceleration of the rate of telomere shortening, including inhibition or suppression of the levels of telomerase activity (Mitchell et al, 2001; Allsopp et al, 2003), defects in non-telomerase components of the telomeric replication machinery, such as the WRN helicase (Schulz et al, 1996), and increased oxidative damage at the telomere (von Zglinicki et al, 1995). In regards to this latter mechanism, telomeric DNA is particularly sensitive to oxidative damage in some types of human cells, wherein elevated levels of reactive oxygen species react with the terminal TTAGGG tract and create unrepaired single strand breaks (Petersen et al, 1998). It has been shown that accumulated oxidative damage at telomeres can lead to a >5-fold increase in the rate of telomere shortening, accompanied by a dramatic decrease in replicative lifespan (von Zglinicki et al, 1995). Elevated oxidative stress is a well established feature of inflammatory responses of the immune system as well as a number of age-related diseases, including Alzheimer’s, cancer and cardiovascular disease, and therefore accelerated telomere shortening induced by increased oxidative damage may have significant physiological relevance.
In higher eukaryotes, the family of selenoproteins, including thioredoxin reductases, glutathione peroxidases, methionine sulfoxide reductase and selenoprotein P, have a number of functions, including playing an important role in responding to oxidative stress (Papp et al, 2007). Recent studies have shown that Sec insertion sequence (SECIS) binding protein 2 (SBP2) differentially regulates the incorporation of Sec into selenoproteins, and that suppression of SBP2 expression causes dysregulation of the expression levels of selenoproteins (Low et al, 2000; Squires et al, 2007). These observations support a role for SBP2 in establishing the hierarchy in selenoprotein synthesis. Therefore, in the present study, we have examined the effects of knocking down SBP2 expression levels on telomere length homeostasis in human cells in vitro.
MATERIALS & METHODS
Cell Culture and transfection
The MSTO-211H human mesothelioma cell line and SY5Y neuroblastoma cell line were cultured in RPMI 1640 medium (Gibco-Invitrogen) supplemented with 10% fetal bovine serum and incubated with 5% CO2 at 37°C. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Stable shRNA transfectants derived from single cells were selected and maintained in puromycin-supplemented medium (2 ug/ml). Transient siRNA transfectants were harvested 96 h post-transfection. For hydrogen peroxide (H2O2) treatment, MSTO cells were cultured in serum free media containing 250uM H2O2 for 2 hours, followed by 48 hours continued growth in the absence of H2O2 in regular growth media.
siRNA and shRNA expression constructs
SBP2 small interfering RNAs (siRNAs) and nontargeting control siRNAs were purchased from Dharmacon. The retroviral expression vectors (pSM2) for expression of SBP2 or control, scrambled, short hairpin (sh) RNAs were purchased from Open Biosystems. The SBP2 shRNA is designed to target the sequence, AAGTATTTATCTTCTGAGATAA, near the 5’ end of the SBP2 mRNA transcript. To generate an SBP2 shRNA expression vector that co-expressed GFP, the mir30-shRNA cassette of the SBP2-pSM2 vector was PCR amplified and subcloned into the GIPZ lentiviral expression vector (Open Biosystems) upstream of an IRES GFP sequence.
RNA isolation, cDNA synthesis, and real-time qPCR analysis
RNA was isolated from the transfected cells using RNeasy spin columns and treated with RNase-free DNase I (QIAGEN). Concentration and purity of the extracted RNA were determined using the A260/A280 value measured on an ND1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). One microgram of the RNA was used for cDNA synthesis. All cDNA was synthesized using the Applied Biosystems high-capacity cDNA synthesis kit. Real-time PCR was performed using Platinum SYBR Green quantitative PCR (qPCR) SuperMix (Invitrogen) in a Light Cycler 2.0 (Roche). Cycling conditions were used as suggested in the SYBR Green kit instructions, and results were analyzed using relative quantification software (Roche). Hypoxanthine phosphoribosyltransferase (HPRT) was used as an internal standard.
TRAP assay
Telomerase extracts were prepared using CHAPS lysis buffer, for equal numbers of cells (200,000) per sample. The TRAP assay was performed using the TRAPeze telomerase detection kit (Chemicon) according to manufacturers’ protocol.
Analysis of telomere length
Telomere length was measured by Southern analysis of terminal restriction fragment (TRF) length, as previously described (Allsopp et al, 2007; Vaziri et al, 1993).
Analysis of sensitivity to S1 nuclease
Following transient knock-down of SBP2, DNA was digested with restriction enzymes HinfI and RsaI to generate TRFs (Allsopp et al, 2007; Vaziri et al, 1993), and then treated with S1 nuclease (Promega) at 1unit/ug of DNA for 45 min at 37C in the 1X S1 nuclease buffer provided. Reactions were stopped by adding 25mM EDTA, followed by phenol/chloroform extraction of the DNA. Southern analysis of TRF length was then performed as described (Allsopp et al, 2007; Vaziri et al, 1993).
RESULTS & DISCUSSION
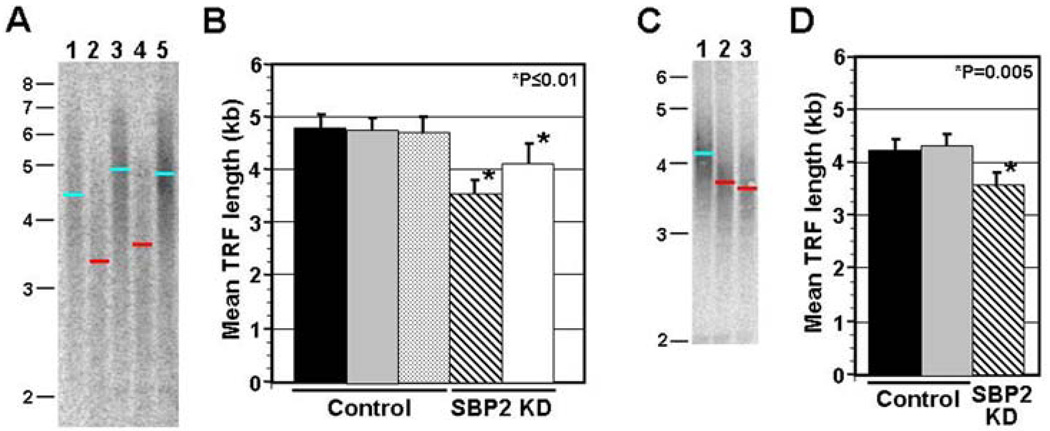
We have previously shown that SBP2 is expressed at relatively high levels in the mesothelioma cell line MSTO-211H (Squires et al, 2007). Furthermore, SBP2 expression levels were successfully knocked down in this cell line using siRNA technology. Therefore, MSTO-211H cells were also used in the present study to examine the effect of siRNA-mediated knock-down of SBP2 expression levels on telomere length. Sub-confluent MSTO-211H cells were either transiently or stably transfected with siRNA or an shRNA expression construct, respectively, that specifically targeted SBP2 mRNA. A typical southern blot showing analysis of terminal restriction fragment (TRF) length for 2 independent stable transfection experiments is shown in Figure 1A. Both of the MSTO cultures stably transfected with the SBP2 targeting shRNA construct showed substantial reduction in telomere length (~−1.1 Kb, ~48% of the total initial telomere length) as compared to untreated MSTO cells, or MSTO cells stably transfected with a non-specific shRNA expression construct. Quantitative analysis of mean TRF length for all transiently or stably transfected samples confirmed that telomere length is significantly reduced upon knock-down of SBP2 (Fig 1B; P≤0.01). Furthermore, slot blot analysis of telomeric signal intensity for these same samples revealed a ~45% reduction in total telomeric DNA in MSTO cells stably selected for SBP2 knock-down (P=0.002; data not shown).
Figure 1. Southern analysis of TRF length following knock-down of SBP2.
A. MSTO cells were stably transfected with shRNA expression vectors that either targeted SBP2 (lanes 2 and 4), or produced a control, scrambled, shRNA (lanes 1 and 3). A typical southern blot of TRF length analysis is shown for 2 independent transfection experiments (lanes 1 & 2 correspond to one transfection experiment, and 3 & 4 the other). Analysis were performed 3 weeks following initial transfection and Puro selection of stable transformants. A sample corresponding to untreated MSTO cells prior to transfection is also included (lane 5). The blue and red bars show mean TRF length for control samples and samples with stable SBP2 knock down respectively. Size of molecular weight markers is indicated at the side. B. Calculation of average mean TRF length following stable (Puro selection, striped bar) or transient (no Puro selection, white bar) knock-down of SBP2. Mean TRF length for control samples, untreated MSTO cells (solid bar) and MSTO cells stably or transiently transfected with the scrambled shRNA control (grey and stippled bars respectively) construct, are also shown (n≥3 for all analyses). Error bars represent ±1 standard deviation, and P value represents comparison of mean TRF length following knock-down of SBP2 expression relative to controls (Student’s t Test). C. Southern analysis of TRF length following knock-down of SBP2 expression in SY5Y neuroblastoma cells. A sample Southern blot is shown for control SY5Y cells transfected with a scrambled siRNA duplex (lane 1), and 2 independent SY5Y samples following transient knock-down of SBP2. D. Calculation of mean TRF length for untreated SY5Y cells (black bar), control SY5Y cells (grey bar) and SY5Y cells following transient knock-down of SBP2 (striped bar) (n=3 for all analyses).
To verify that this finding could be extended beyond a specific cell line, we repeated the analysis of the affect of knocking down SBP2 expression on telomere length in the SY5Y neuroblastoma cell line. Previous experiments had confirmed SBP2 expression in these cells (data not published). Southern analysis of mean TRF length following transient knock-down of SBP2 or transfection with a scrambled siRNA (control) in SY5Y revealed a reduction in telomere length following SBP2 knock-down (~−0.6Kb; Fig 1C,D; P=0.005). Importantly, this observation supports the potential broad applicability of the role of SBP2 in telomere length regulation.
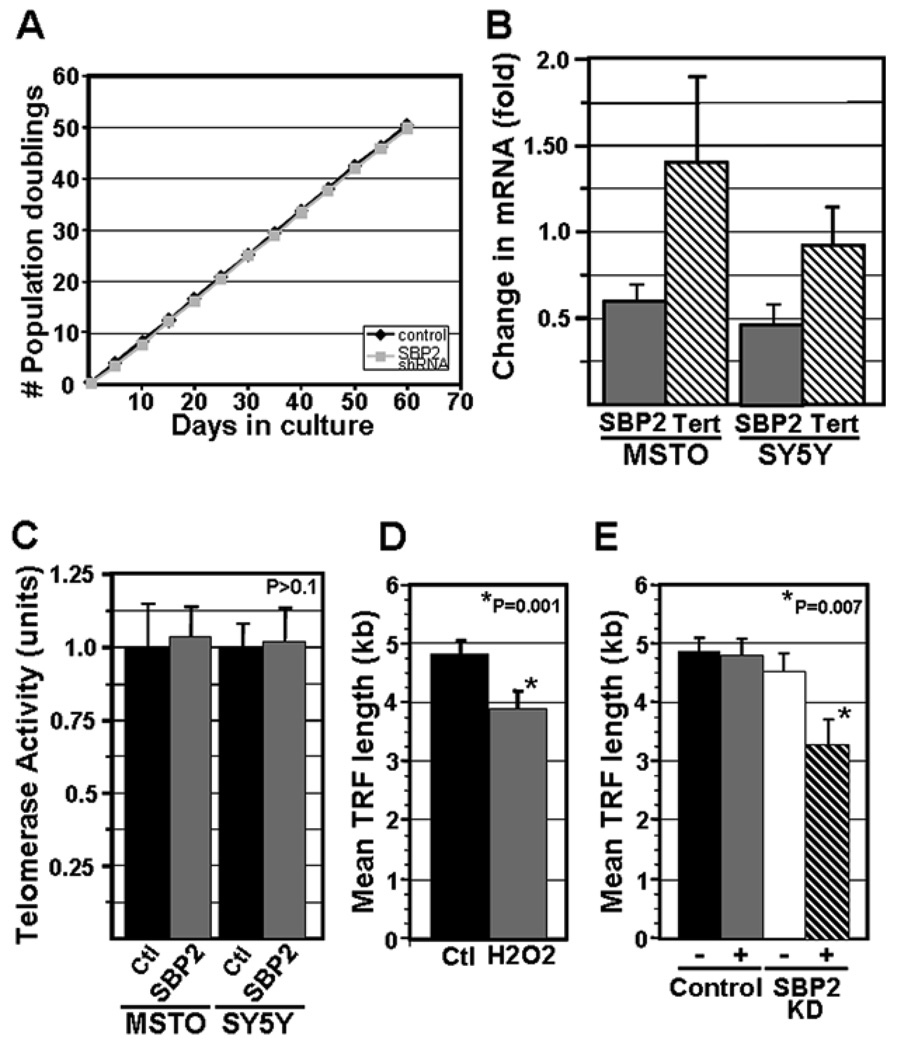
To examine whether accelerated cell turnover might contribute to the loss of telomeric DNA in MSTO cells transfected with the shRNA expression construct targeting SBP2, we compared the growth rate of MSTO cells transfected with either the SBP2 shRNA expression construct or the control shRNA expression construct. The growth rate of stable transformants over a 60 day period was similar for MSTO cells transfected with the SBP2 targeting construct or the control construct (Fig. 2A). We also assessed affect of SBP2 knock-down on the proportion of apoptotic cells, and did not observe any significant difference between control and SBP2 knock-down cells (data not shown). To assess whether inhibition of telomerase activity might contribute to the reduction in telomere length observed in the MSTO cells expressing the SBP2 targeting shRNA, we analyzed Tert mRNA expression levels and telomerase activity levels following transient or stable knock-down of SBP2. Real time RT-PCR analysis of SBP2 mRNA and TERT mRNA levels revealed a significant knock-down of SBP2 mRNA levels but not TERT mRNA levels in the SBP2 targeted MSTO cells and SY5Y cells compared to control (Fig 2B). Furthermore, telomerase activity was not affected following either transient or stable knock-down of SBP2 (Fig 2C). In previous experiments, we also showed that knocking down SBP2 expression in MSTO cells resulted in little or no detectable SBP2 protein, as assessed by Western analysis, compared to control cells (Squires et al, 2007 and data not shown). Together, these observations show that dysregulated cell growth, apoptosis, and inhibition of telomerase activity do not significantly contribute to the loss of telomeric DNA that accompanies knock-down of SBP2.
Figure 2. Analysis of the mechanism of telomere length reduction in MSTO cells following knock-down of SBP2 expression.
A. Growth curves for MSTO cells stably transfected with either the control, scrambled shRNA expression construct or shRNA expression construct targeting SBP2. B. Real time RT-PCR analysis of the expression level of SBP2 mRNA and TERT mRNA in MSTO cells and SY5Y cells following knock-down of SBP2. The fold change in expression levels, normalized to HPRT, is shown relative to control cells stably transfected with the control, scrambled shRNA construct (MSTO cells), or control cells transiently transfected with the control siRNA (SY5Y cells)(n≥3 for all samples). C. TRAP analysis of telomerase activity for MSTO cells and SY5Y cells stably transfected with either the control shRNA expression construct or shRNA expression construct targeting SBP2 (MSTO), or transiently transected with the control or SBP2 siRNA (SY5Y cells). Equal cell equivalents (1,000 cell equivalents) were used in all analyses (n≥3 for all samples). D. Analysis of the effect of exposure of cells to H2O2 on telomere length. MSTO cells were cultured in the presence of a non-toxic dose of H2O2 (250uM), or PBS (control), for 4 hours, followed by 4 hours of continued growth in the absence of H2O2. Telomere length was then assessed by Southern analysis of TRF length (n=3 for each sample). E. Analysis of the sensitivity of TRFs to S1 nuclease following SBP2 knock-down. MSTO cells were transiently transfected with either a scrambled siRNA (control) or a siRNA targeting SBP2. At 48 hours post-transfection, DNA was harvested, digested with restriction enzymes, and either treated with S1 nuclease (indicated by ‘+’) or not (indicated by ‘−’) prior to Southern analysis of TRF length. The calculated mean TRF lengths for each sample are shown ((n=3 for all samples). For panels D & E, P values for comparison of means were calculated using the Students’ t Test. For all bar graphs, error bars represent ±1 standard deviation.
Previous studies have shown that exposure of human cells to hydrogen peroxide during culture in serum free media induces oxidative damage and accelerated telomere shortening (von Zglinicki et al, 1995; von Zglinicki et al, 2000). Therefore, to assess whether oxidative stress could be contributing to the reduction in telomere length following SBP2 knock-down, we treated MSTO cells with a non-toxic level (250uM) of H2O2 and assessed the effect on telomeres by Southern analysis of TRF length. As shown in Figure 2D, we observed a significant reduction in mean TRF length in MSTO cells following exposure to H2O2 (−0.8Kb; P=0.001). This observation is consistent with elevated levels of oxidative damage to telomeres as the primary mechanism of telomere shortening following knock-down of SBP2 in MSTO cells.
To directly assess whether knock-down of SBP2 affects the amount of oxidative damage to telomeric DNA, we analyzed the sensitivity of telomeric DNA to S1 nuclease, a single-strand DNA endonuclease, following knock-down of SBP2. If oxidative damage-induced lesions are present at the telomere, S1 nuclease will cleave the telomeric DNA tract at these sites, resulting in a shift of the TRF to shorter sizes upon Southern analysis of TRF length (von Zglinicki et al, 1995). In cells targeted for transient knock-down of SBP2, a large amount of telomeric DNA is lost during the first 72 hours following transfection of the SBP2 siRNA (Fig 1B), and at 48 hours following transfection, telomeres are only slightly reduced in size as compared to control cells (Fig 2E). Therefore, we chose to assess sensitivity of telomeric DNA to S1 nuclease at 48 hours post-transfection during transient knock-down of SBP2 because lesions within the telomere tract are likely to have begun accumulating at this time point if oxidative damage is the primary mechanism accounting for the loss of telomeric DNA. In control MSTO cells transfected with the scrambled siRNA, we observed no significant sensitivity to S1 nuclease as assessed by comparing mean TRF length between S1 nuclease treated and untreated cells (Fig 2E; P>0.1), indicating minimal accumulation of oxidative damage-induced lesions in these cells. In MSTO cells transfected with the SBP2 siRNA, we observed a significant shift in mean TRF length to a shorter size (~−1.2Kb; P=0.007) following treatment of the DNA with S1 nuclease (Fig 2E). This result supports oxidative damage to telomeric DNA as a primary mechanism accounting for the shortening of telomeres induced by knocking down SBP2.
Interestingly, long term growth (>60 days culture) of MSTO cells stably transfected with the SBP2 targeting shRNA construct did not result in telomere-induced senescence (Fig 2A). Comparison of telomere length in these MSTO cells at 14 days and 60 days following selection of stable transformants, representing ~38 population doublings, showed no further reduction in telomere length (data not shown). This observation is consistent with findings from a number of independent studies that indicate telomerase, via a mechanism that is still poorly understood, preferentially maintains or lengthens very short telomeres (ie. critically short telomeres that have been reduced to the minimal length required to maintain telomere function) (Liu et al, 2002; Goldman et al, 2005). While we favor this hypothesis to explain the lack of telomere-induced senescence following long term knock-down of SBP2, other mechanisms may also come into play, including a possible increased resistance of the most proximal region of the telomere to oxidative stress.
In summary, the observations reported here have identified a new potential role for SBP2 and selenoproteins, in maintaining and protecting telomeric reserve in mammalian cells. Selenoproteins have been shown to be important for the normal physiological function of cells in highly proliferative organ systems, where telomere length maintenance is also imperative for long term function, including testis and the immune system (Papp et al, 2007; Hoffmann et al, 2007). Therefore it will be of interest in future studies to assess the effects of selenium depletion or aberrant expression of SBP2 on telomere length in male germ cells and hematopoietic cells, and also to specifically identify which selenoprotein(s) are important in the maintenance of telomeric reserve.
ACKNOWLEDGEMENTS
We thank Melissa Nagata and Alex Gurary for excellent technical assistance. This work was supported by a Castle Foundation award to RA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allsopp R, Shimoda J, Easa D, Ward K. Long telomeres in the human placenta. Placenta. 2007;28:324–327. doi: 10.1016/j.placenta.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cell senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, Mason PJ, Londoño-Vallejo A, Bessler M. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomere shortening during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead P. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, Hoge S, Li PA, Hoffmann F, Hashimoto A, Berry MJ. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007;35:3963–3973. doi: 10.1093/nar/gkm355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L. Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci U S A. 2002;99:3597–3602. doi: 10.1073/pnas.062549199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SC, Grundner-Culemann E, Harney JW, Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- Schulz VP, Zakian VA, Ogburn CE, McKay J, Jarzebowicz AA, Edland SD, Martin GM. Accelerated loss of telomeric repeats may not explain accelerated replicative decline of Werner syndrome cells. Hum Genet. 1996;97:750–754. doi: 10.1007/BF02346184. [DOI] [PubMed] [Google Scholar]
- Squires JE, Stoytchev I, Forry EP, Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Schächter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Döcke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med. 2000;28:64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]