Abstract
We investigated the impacts of tempo of early (days 14, 28, and 42) donor T-cell and natural killer (NK) cell engraftment, missing recipient killer cell immunoglobulin-like receptor (KIR) ligands, and numbers of donor inhibitory and activating KIR genes on hematopoietic cell transplantation (HCT) outcomes in 282 patients with hematological malignancies given nonmyeloablative conditioning. Modeling chimerism levels as continuous linear variable, we found that high early donor T-cell chimerism was significantly associated with acute GVHD (p=0.01), while high donor NK cell levels had no such association (p=0.38). Conversely, high donor NK cells levels were significantly associated with low relapse risk (p=0.0009), while no significant association was seen with high donor T-cell chimerism (p=0.10). The qualitative associations between donor T-cell and NK cell chimerism levels and GVHD and relapse did not change after adjustment for the presence of recipient KIR ligands or numbers of donor inhibitory or activating KIR genes. In conclusion, prompt engraftment of donor NK cells correlated with lessened risks of relapse but not with GVHD, while the converse was true for T-cells.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) after nommyeloablative conditioning relies nearly exclusively on graft-versus-tumor effects for tumor eradication [1-6]. While it is generally accepted that graft-versus-tumor effects after HLA-identical HCT are mainly due to T-cells [7-9], observations by the Perugia group showed that, after HLA-haploidentical HCT, graft-versus-host natural killer (NK) cell reactivity was associated not only with less relapse in patients with acute myeloid (but not lymphoblastic) leukemia but also lessened graft rejection and less acute graft-versus-host disease (GVHD) [10]. These findings renewed the interest for NK cells in the HCT setting.
The activities of NK cells are thought to be regulated by a quantitative balance between inhibitory signals mediated by inhibitory killer cell immunoglobulin-like receptor (KIRs) and CD94/NKG2A, and by activating signals mediated by natural cytotoxicity receptors (NCRs), NKG2D or DNAX accessory molecule-1 (DNAM-1, CD226) [11]. KIRs recognize allotypic determinants that are shared by different HLA-class 1 alleles: KIR2/DL2 and KIR2/DL3 recognize HLA-C group 1 alleles, KIR2/DL1 recognizes HLA-C group 2, and KIR3/DLI recognizes HLA-Bw4 alleles [11]. Conversely, CD94/NKG2A recognizes overall expression of HLA-class 1 molecules on target cells via HLA-E. Although HLA and KIR genes are inherited independently, clonal analyses have demonstrated that, in healthy individuals, each NK cell either expresses at least one inhibitory receptor for self HLA, either KIR or the non-specific CD94/NKG2A receptor, or they are developmentally immature [12,13].
After transplantation, donor NK cells arising from hematopoietic stem cells regenerate the same KIR repertoire as the donor, [14] leading to high frequencies of graft-versus-host reactive NK cells in those recipients who lack ligand for donor NK cell KIR [15]. Such alloreactive NK cells have been detectable for only a few months following HCT [15]. Thereafter, NK cells become tolerant to the host, probably in part through expression of KIR specific to recipient HLA [16]. NK alloreactivity has been associated with graft-versus-tumor effects after both HLA-mismatched and HLA-matched HCT [10,16-19].
We have previously analyzed the correlations between the tempo of engraftment of various peripheral blood cell subpopulations and HCT outcomes in 120 patients with various hematological malignancies given HCT after nonmyeloablative conditioning [20] and found a strong suggestion that rapid establishment of high levels of donor NK cell chimerism between days 14 and 100 after HCT predicted better progression-free survival [20]. Here, we examined the impact of both early donor T-cell and NK cell chimerism levels on transplant outcomes in the context of recipient HLA ligand and donor KIR receptor genetic data among 282 patients with cancer who underwent HCT from either related or unrelated donors after a minimal intensity conditioning regimen including 2 Gy total body irradiation (TBI).
Patients And Methods
Patients and Donors
Data from 282 patients given allogeneic HCT after non-myeloablative conditioning at the Fred Hutchinson Cancer Research Center, the University of Washington Medical Center, the Children's Hospital and Regional Medical Center and the Veterans Affairs Medical Center, all in Seattle, WA, between March 1998 and November 2006 were included in this study. Patient characteristics are listed in Table 1. Median patient age was 54 (range, 5–74) years. Diagnoses included hematological malignancies (n=274) and solid tumors (n=8). Patients were classified as having indolent or aggressive diseases as previously reported [21]. One hundred fifty-two patients received grafts from HLA-identical related donors, one from a one HLA-A antigen mismatched related donor, 95 from HLA-A, -B, -C, -DR, -DQ allele-matched unrelated donors, and 34 from one HLA class I allele and/or one antigen mismatched unrelated donors. Comorbidities at HCT were assessed using the hematopoietic cell transplantation comorbidity index (HCT-CI) score [22]. Prospective research protocols were approved by the Institutional Review Board of the FHCRC for the four participating institutions. Informed consent was obtained from all patients.
Table 1. Patients, diseases, and transplant characteristics (n=282).
| Characteristic | Value | ||
|---|---|---|---|
| Median patient age, years (range) | 54 (5-74) | ||
| Recipient gender (M/F), # pts (%) | 182 (65) / 100 (35) | ||
| Median donor age, years (range) | 45 (19-78) | ||
| Donor gender (M/F), # pts (%) | 147 (52) / 135 (48) | ||
| Diagnosis, # pts (%) | |||
| Acute myeloid leukemia | 63 (22.3) | ||
| Acute lymphoblastic leukemia | 8 (2.8) | ||
| Chronic myeloid leukemia | 15 (5.3) | ||
| Chronic lymphocytic leukemia | 33 (11.7) | ||
| Myelodysplastic syndrome | 31 (11.0) | ||
| Multiple myeloma | 44 (15.6) | ||
| Non-Hodgkin lymphoma | 52 (18.4) | ||
| Hodgkin disease | 25 (8.9) | ||
| Waldenström macroglobulinemia | 3 (1.0) | ||
| Renal cell carcinoma | 7 (2.5) | ||
| Cervical cancer | 1 (0.4) | ||
| Disease risk, # pts (%) | |||
| Indolent* | 123 (44) | ||
| Aggressive† | 159 (56) | ||
| Tandem autologous/allogeneic HCT, # pts (%) | 41 (14.5%) | ||
| Donor, # pts (%) | |||
| Related | |||
| HLA-identical | 152 (53.9) | ||
| 1 HLA-antigen mismatched | 1 (0.4) | ||
| Unrelated | |||
| 10/10 HLA allele-matched | 95 (33.7) | ||
| 1 HLA-allele mismatched | 16 (5.7) | ||
| 1 HLA-antigen mismatched | 13 (4.6) | ||
| 1 HLA-antigen + 1 HLA allele mismatched | 5 (1.8) | ||
| Numbers of inhibitory genes on donor NK cell KIR, # pts (%) | |||
| Unknown | 30 (11) | ||
| 2 | 6 (2) | ||
| 3 | 91 (32) | ||
| 4 | 104 (37) | ||
| 5 | 51 (18) | ||
| Number of activating genes on donor NK cell KIR, # pts (%) | |||
| Unknown | 53 (18.8) | ||
| 1 | 77 (27.3) | ||
| 2 | 46 (16.3) | ||
| 3 | 21 (7.4) | ||
| 4 | 37 (13.1) | ||
| 5 | 33 (11.7) | ||
| 6 | 15 (5.3) | ||
| Hematopoietic stem cell source, # pts (%) | |||
| G-PBMC | 271 (96) | ||
| Bone marrow | 11 (4) | ||
| Conditioning regimen, # pts (%) | |||
| 2 Gy TBI | 54 (19) | ||
| 2 Gy TBI + fludarabine | 228 (81) | ||
| Cell dose, median (range) (× 106/kg recipient) | |||
| CD34+ cells | 7.8 (0.8-42.6) | ||
| T cells | 312 (16-934) | ||
| Sustained engraftment / graft rejection, # pts (%) | 264 (94) / 18 (6) | ||
| Acute GVHD, # pts (%) | |||
| Grade | 0 /1 | 113 (40.1) | |
| 2 | 127 (45.0) | ||
| 3 | 31 (11.0) | ||
| 4 | 11 (3.9) | ||
| Chronic GVHD, # pts (%) | |||
| No | 135 (48) | ||
| Yes | 147 (52) | ||
| 3-year overall survival (%) | 50 | ||
| 3-year progression-free survival (%) | 37 | ||
Defined as acute myeloid leukemia in first complete remission, acute lymphoblastic leukemia in first complete remission, myelodysplastic syndrome-refractory anemia, chronic myeloid leukemia in first chronic phase, chronic lymphoblastic leukemia, low-grade non Hodgkin lymphoma, multiple myeloma in partial or complete remission, and Waldenstrom macroglobulinemia.
All other diagnoses.
M, male; F, female; R, recipient; D, donor; G-PBMC, G-CSF-mobilized peripheral blood mononuclear cells; TBI, total body irradiation; GVHD, graft-versus-host disease; KIR, killer cell immunoglobulin-like receptor.
Treatment and evaluation
Fifty-four recipients of related grafts were conditioned with 2 Gy TBI alone [3] while the remaining 99 related and all unrelated recipients were given, in addition, fludarabine, 30 mg/m2/day, on days -4, -3, and -2 [3,4,23-25]. Donor G-CSF-mobilized peripheral blood mononuclear cell (G-PBMC) (n=271) or marrow grafts (n=11) were infused without processing on day 0. Postgrafting immunosuppression included mycophenolate mofetil (MMF) and cyclosporine (CSP) or tacrolimus in all patients, as described previously [3,4,23,25,26].
Grading and treatment of acute and chronic GVHD were performed according to established criteria [27]. Disease-dependent restaging after HCT occurred monthly for the first 3 months and then at 6 months, 1 year, and yearly thereafter. Persistent or progressive malignancies in the absence of GVHD were treated by rapid taper and discontinuation of immunosuppression, and 24 patients received DLI [28]. Eight additional patients with low or failing T-cell chimerism received DLI 2 days after pentostatin (4 g/m2) administration in an attempt to prevent graft rejection, as previously reported [29].
Relapse and progression were defined as previously reported in C Kahl et al.[6] and M. Rotta et al.[30]. Relapse was defined as recurrence of malignancy based on one or more of the following parameters: marrow morphology, flowcytometry, cytogenetic studies, including fluorescence in situ hybridization, electrophoresis, immunofixation assays, polymerase chain reaction-based assays for disease markers, or imaging results. Disease progression was defined as an increase of at least 50% in disease burden or a 25% increase in any disease marker for patients with multiple myeloma.
Chimerism analyses
The different peripheral blood subpopulations were sorted by 3-color flow cytometry using a Vantage SE cytometer (BD, San Jose, CA USA). Cell types were defined as follows: T-cell, CD3+CD56- side scatterlow; and natural killer (NK) cell, CD56+CD3-CD14- side scatterlow. Percentages of donor chimerism in the different blood cell populations were assessed using polymerase chain reaction (PCR)-based analyses of polymorphic mini- or microsatellite regions (VNTR/STR), or by fluorescent in situ hybridization (FISH) for X and Y chromosomes if patients and donors were sex-mismatched, as previously reported [3,20].
HLA typing, KIR genotyping and missing KIR ligand algorithm
Sequence-specific oligonucleotide hybridization and/or sequencing-based typing methods were used to define exons 2 and 3 of HLA-A, -B, and -C alleles and exon 2 of -DRB1 and -DQB1 alleles in all unrelated donor–recipient pairs. Pairs with the same HLA-A, -B, -C, -DRB1 and -DQB1 alleles were defined as “10/10” allele matched; all other pairs were defined by the number of mismatched class I and/or class II alleles or antigens (Table 1). DNA for KIR genotyping was available for 264 of the 282 (94%) donors. The presence or absence of ten KIR genes (2DL1, 2DL2, 2DL3, 3DL1, 2DL5, 2DS2, 2DS3, 2DS4, 2DS4-22bp deletion and 2DS5) was determined using a commercial PCR-SSP kit (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Typing for the 2DS1 gene was performed using previously published PCR-SSP primers [31]. KIR pseudogenes (KIR2DP1 and 3DP1) were not typed. KIR framework genes (KIR2DL4, KIR3DL2 and KR3DL3) are present in all individuals [32] and hence were not genotyped. Recipient HLA-A, -B, -C alleles and donor KIR2DL1, KIR2DL2/3, KIR3DL1 and KIR3DL2 loci were evaluated as follows: the absence of recipient HLA-Bw4 epitopes in HLA-A and/or -B with the presence of donor KIR3DL1; absence of recipient Cw3 epitope (C1 group) in the presence of donor KIR2DL2/3; absence of recipient Cw4 epitope (C2 group) in the presence of donor KIR2DL1, and the absence of recipient HLA-A3/A11 alleles/antigens in the presence of donor KIR3DL2 [33]. The total number of donor activating KIR genes (2DS1, 2DS2, 2DS3, 2DS4 and 2DS5) and of donor inhibitory KIR genes (2DL1, 2DL2, 2DL3, 3DL1 and 2DL5) was determined for each pair.
Statistical analyses
Potential associations between chimerism levels and pre-transplant/transplant characteristics were determined by generalized estimating equations (GEE). The impacts of percent donor chimerism on rejection and acute GVHD were assessed using logistic regression, and the effects on progression-free survival, relapse, and non-relapse mortality were assessed using Cox regression. The association between KIR genetics and outcome was also examined with Cox regression. Chimerism was modeled in some cases as a time-dependent covariate (within the first 42 days following HCT for relapse, nonrelapse mortality, and progression-free survival), and in other cases the effects of chimerism values at a specified point in time on outcome (rejection and acute GVHD) were examined. Analyses looking at associations of chimerism levels and KIR with the outcomes relapse, non-relapse mortality and progression-free survival were adjusted for indolent/aggressive disease, HCT-CI score, donor relationship and prior autologous HCT. The impact of KIR genetics on the association between NK chimerism and outcome was assessed by including various models of KIR (as described below) in the appropriate regression models. Spearman's correlation coefficients were estimated to examine the correlations between donor cell subsets. P-values from regression models were derived from the Wald test.
Results
Kinetics of donor engraftment
Median donor T-cell and NK cell chimerism levels were 66 (range, 1–100)% and 78 (range, 3–100)% on day 14, 79 (range, 1–100)% and 90 (range, 2–100)% on day 28, and 81 (range, 1–100)% and 88 (range, 1–100)% on day 42, respectively. The Pearson correlation coefficients between donor T-cell and NK cell chimerism levels on days 14, 28 and 42 were 0.60, 0.76, and 0.75, respectively.
Factors affecting kinetics of donor engraftment
Transplant/pre-transplant factors (Table 2)
Table 2. Associations between transplant/pre-transplant factors and donor T-cell and NK cell chimerism levels*.
| Factor | NK cell chimerism | T-cell chimerism |
|---|---|---|
| # of CD34 transplanted | P=0.004 (higher # transplanted, higher chimerism levels) | P=0.002 (higher # transplanted, higher chimerism levels) |
| # of T-cells transplanted | P =0.06 (higher # transplanted, higher chimerism levels) | P =0.004 (higher # transplanted, higher chimerism levels) |
| BM (vs. G-PBMC) | P =0.007 (BM lower 30%) | P <0.0001 (BM lower 43%) |
| URD (vs. related donor) | P =0.49 (URD lower 2%) | P =0.45 (URD lower 2%) |
| Disease category | ||
| Lymphoid malignancies | --- | --- |
| Myeloid malignancies | P =0.23 (myeloid lower 4%) | P =0.0006 (myeloid lower 11%) |
| Solid Tumor | P =0.34 (solid lower 8%) | P =0.007 (solid lower 16%) |
P-values were obtained from generalized estimated equations (GEE), using chimerism values measured at days 14, 28 and 42 after hematopoietic cell transplantation. BM, bone marrow; G-PBMC, G-CSF-primed peripheral blood mononuclear cells; URD, unrelated donor.
High numbers of transplanted CD34+ cells correlated with high levels of donor T-cell (P=0.002) and NK cell (P=0.004) chimerism. Further, while high numbers of transplanted T-cells correlated closely with high levels of early donor T-cell (P=0.004) chimerism, an association with NK cell chimerism was weaker (P=0.06). Patients given marrow grafts had lower levels of donor T-cell (P<0.0001) and NK cell (P=0.007) chimerism than those given G-PBMC. Patients with lymphoid malignancies had higher levels of donor T-cell chimerism than those with myeloid malignancies (P=0.0006) or solid tumors (P=0.007), while the associations between disease categories and donor NK cell chimerism levels did not reach statistical significance.
Associations between KIR/KIR ligand and kinetics of donor NK cells engraftment
The absence of one or more recipient ligands for donor KIR did not have a statistically significant impact on average donor NK cell chimerism levels. Specifically, patients with all ligands present had, on average, donor NK cell chimerism levels of 5.6% less than that those missing one or more ligand (P=0.23) when comparing average chimerism levels from days 14, 28, and 42.
When the numbers of donor-inhibitory genes were modeled as continuous linear variables, increasing numbers of genes were associated with decreased donor NK cell chimerism, but the correlation was not statistically significant (P=0.11). Similarly, increasing numbers of donor-activating genes were associated with lower chimerism levels but the association was not statistically significant (P=0.10).
Associations between tempo of donor cell engraftment and HCT outcomes
Graft rejection
Eighteen of the 282 patients (6%) rejected their grafts between 13–1123 (median 74) days after HCT, with 4 rejections occurring despite pentostatin administration followed by DLI. After excluding patients given DLI for prevention of graft rejection, data on T-cell chimerism at day 14 existed for 7 rejectors and 170 non-rejectors, and similar data for NK cells were available for 6 rejectors and 142 non-rejectors. The mean day-14 T-cell chimerism levels were 14% and 65% among rejecting and non-rejecting patients, respectively (P<0.0001), and corresponding day-14 NK-cell chimerism levels were 34% and 73% (P<0.0001), respectively. Of the seven rejectors among patients with T-cell chimerisms available on day 14, five had values below 10%, one had a value between 10 and 50%, while the seventh had a value between 50 and 75%. Two of the 6 rejectors among patients with NK chimerisms available on day 14 had values below 10%, three had values between 10 and 50%, and the sixth rejector had a day-14 NK chimerism between 75% and 90%. The percentages of patients who were missing at least one ligand for donor NK-cell KIR were similar among patients who rejected (82%) and those who did not reject (90%).
GVHD
Grades II, III and IV acute GVHD occurring beyond day 14 was diagnosed in 45%, 10% and 4% of patients respectively. With chimerism modeled as a categorical variable, the probability of grades II-IV acute GVHD increased with increasing levels of donor T-cells (P=0.01, trend test) but not NK cells (P =0.38, trend test) (Table 3). When both day-14 donor T-cell and NK cell chimerism levels were included in a model for grades II-IV acute GVHD, the (non-statistically significant) association for donor NK cell chimerism levels became even less significant (P=0.83 in trend test), while the association for T-cell chimerism remained statistically significant (P=0.01 in trend test). These observations remained qualitatively the same after adjustments for the presence or absence of one or more ligands for donor NK cell KIR, as well as after adjusting for the number of activating or inhibitory genes (data not shown).
Table 3. Associations between day-14 donor chimerism levels and acute GVHD.
| # Patients with acute GVHD/# pts at risk§ (%) | ||
|---|---|---|
| % Donor chimerism on day 14 | T-cells | NK cells |
| 0-50 | 22/47 (47%) | 10/20 (50%) |
| 51-75 | 34/65 (52%) | 24/42 (57%) |
| 76-90 | 32/43 (74%) | 31/53 (58%) |
| 91-100 | 9/13 (69%) | 16/25 (64%) |
| Trend Test* | P=0.01† | P=0.38‡ |
P-values adjusted for donor type (related versus unrelated).
P=0.01 after adjusting for donor NK cell chimerism levels.
P=0.83 after adjusting for donor T cell chimerism levels.
Patients with GVHD or death before day 14 were excluded from the analyses.
Fifty-two percent of the patients had extensive chronic GVHD. When donor chimerism levels were modeled as average values for days 14, 28 and 42, there was no statistically significant association between NK cell levels and chronic GVHD (P=0.80, trend test with chimerism modeled as categorical variable), while there was a significant association between high T-cell levels and chronic GVHD (P=0.05 in trend test). Results were similar after adjustments for presence or absence of one or more ligands for donor NK cell KIR, as well as after adjusting for numbers of activating or inhibitory genes.
Taken together, these results suggest that the tempo of donor T-cell but not NK cell engraftment was associated with GVHD.
Relapse, achievement of complete remission, non-relapse mortality and progression-free survival
With a median follow-up of 4 years for surviving patients, 107 patients relapsed/progressed between 4 and 1978 (median 166) days. When donor chimerism levels were modeled as continuous variables, no significant association between donor T-cell chimerism levels and relapse was found (P=0.10), while high levels of donor NK cell chimerism were associated with decreased risk of relapse in time-dependent analyses (P=0.0009; Table 4). The magnitude of the association was similar if the analysis was restricted to patients whose day-14 chimerism was 25% or greater. Further, the associations between donor T-cell and NK cell chimerism levels and relapse risks were qualitatively the same when the analysis was restricted to patients with hematological malignancies. Conclusions were qualitatively the same after adjustments for presence or absence of one or more ligands for donor NK cell KIR, as well as for numbers of activating or inhibitory genes (data not shown). The risk of relapse was lower in patients who had all ligands for donor NK cell KIR compared to patients missing one or more ligands for donor NK cell KIR, but the difference was not statistically significant (adjusted hazard ratio [HR] = 0.74; 95% confidence interval [CI] 0.39–1.41; P=0.36) (Figure 1A). Summarized in Table 5 are the raw proportions of relapse according to the specific missing ligand [33]. A formal statistical analysis based on these specific ligands is not possible due to the large number of unique categories. However, the raw proportions in Table 5 do not suggest any striking associations.
Table 4. Associations between donor chimerism levels and relapse.
| HR (95% CI) | |||
|---|---|---|---|
| % Donor chimerism on day 14 | T-cells | NK cells | |
| 0-50 | 1 | 1 | |
| 51-75 | 0.35 (0.17-0.72, P=0.004) | 0.29 (0.10-0.82, P=0.02) | |
| 76-90 | 0.48 (0.25-0.91, P=0.03) | 0.29 (0.13-0.63, P=0.002) | |
| 91-100 | 0.50 (0.26-0.96, P=0.04) | 0.20 (0.09-0.45, P=0.0001) | |
| Trend Test* | P=0.10 | P=0.0009 | |
P-values obtained by Cox model. Chimerism was modeled as a time dependent covariate. All models were adjusted for donor relationship (unrelated vs. related), aggressive vs. indolent disease, HCT-CI, and prior autologous transplant.
Figure 1.
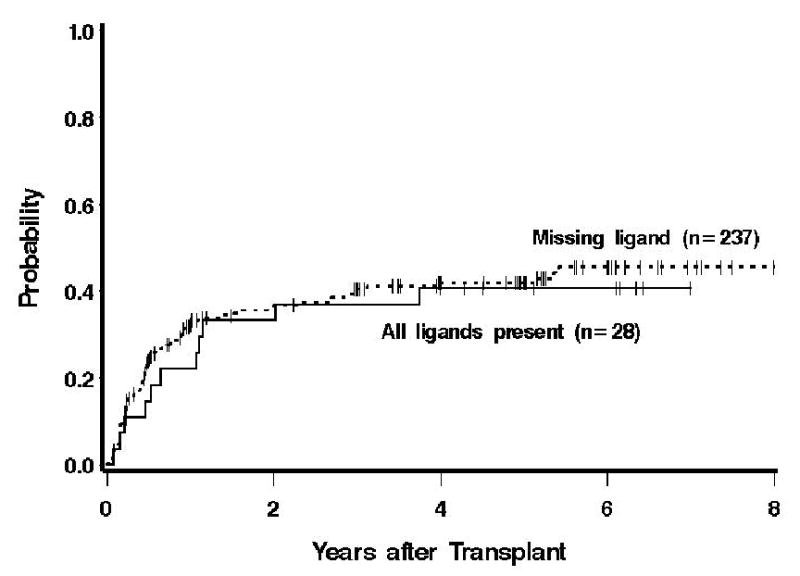
Probability of relapse among patients who had all ligands for donor NK cell KIR compared to those who lack one or more ligands.
Table 5. Associations between matching of recipient HLA class I ligand to donor NK cell KIR and proportion of relapse.
| # Patients (%) | # Relapse (%) | |
|---|---|---|
| Recipient has all ligands for Donor inhibitory KIR | 28 (9.9) | 11/28 (39) |
| Recipient misses one or more ligands for donor inhibitory KIR | 237 (84) | 91/237 (38) |
| Recipient A3 or 11− / Donor 3DL2+ | 67 (23.8) | 27/67 (40) |
| Recipient Bw4− / Donor 3DL1+ | 14 (5) | 5/14 (36) |
| Recipient C1− / Donor 2DL2/3+ | 7 (2.5) | 2/7 (29) |
| Recipient C2− / Donor 2DL1+ | 11 (3.9) | 2/11 (18) |
| Recipient A3/11− and Bw4− / Donor 3DL2+ and 3DL1+ | 11 (3.9) | 5/11 (45) |
| Recipient A3/11− and C1− / Donor 3DL2+ and 2DL2/3+ | 21 (7.4) | 7/21 (33) |
| Recipient A3/11− and C2− / Donor 3DL2+ and 2DL1+ | 43 (15.2) | 21/43 (49) |
| Recipient Bw4− and C1− / Donor 3DL1+ and 2DL2/3+ | 4 (1.4) | 1/4 (25) |
| Recipient Bw4− and C2− / Donor 3DL1+ and 2DL1+ | 26 (9.2) | 11/26 (42) |
| Recipient A3/11−- and Bw4− and C1− / Donor 3DL2+ and 3DL1+ and 2DL2/3+ | 3 (1.1) | 1/3 (33) |
| Recipient A3/11− and Bw4− and C2− / Donor 3DL2+ and 3DL1+ and 2DL2/3+ | 30 (10.6) | 9/30 (30) |
| Unknown KIR genotype | 17 (6) | 5/17 (29) |
A total of 172 patients had measurable disease at HCT, and 69 of them achieved complete remissions. The average CD3 donor chimerism among those who went into CR was 72%, and the average donor CD3 chimerism among those who did not enter CR was 67% (p=0.23).
Sixty-four patients died of non-relapse causes between 20 and 2466 (median 230) days after HCT. There were no statistically significant associations between donor T-cell (P=0.67), or NK cell (P=0.21) chimerism levels and non-relapse mortality when chimerism was modeled as a continuous linear variable. Results did not change after adjustments both for presence or absence of one or more ligands for donor NK cell KIR and numbers of activating or inhibitory genes (data not shown).
When chimerism levels were modeled as continuous linear variables, high levels of donor T-cell (P=0.01), and NK cell (P<.0001) chimerism were each associated with better progression-free survival. Results were similar after adjustments for presence of one or more ligands for donor NK cell KIR and numbers of activating or inhibitory genes (data not shown). The risk of failure for progression-free survival was higher among patients missing at least one ligand for donor NK cell KIR compared to patients not lacking such ligands, but the difference was not statistically significant (HR=1,33; 95% CI, 0.81–2.20; P=0.26).
Discussion
It has been commonly accepted that donor T-cells play important roles in engraftment, GVHD, and graft-versus-tumor effects after HLA-matched HCT in patients with malignancies [9]. Removing T-cells from hematopoietic grafts by various methods of T-depletion has generally been accompanied by higher graft rejection rates, less GVHD, and more frequent relapse/progression of underlying malignancies compared to patients given unmodified grafts [34,35]. Consistent with these previous observations, current patients with high numbers of grafted T-cells experienced rapid establishment of high donor T-cell chimerism which, in turn, was associated with a low rate of graft rejection, increases in both acute and chronic GVHD, and a slightly, but not significantly, lessened risk of relapse.
The role of donor NK cells in these transplant settings has been far less clear. One of the earliest studies in 175 patients given HLA-matched related marrow grafts after conventional conditioning showed relatively rapid recovery of NK cell function but failed to show correlations between the tempo of recovery and risks of GVHD, infections or recurrence of malignancies [7]. However, renewed interest in the role of NK cells in the HCT setting [10] was rekindled by observations from investigators in Perugia showing that, after HLA-haploidentical HCT, graft-versus-host NK cell reactivity was associated with less graft rejection, less acute GVHD and less relapse in those patients who had acute myeloid leukemia. However, the incidences of acute GVHD were similar in patients with or without graft-versus-host NK cell reactivity in a more recent paper [36].
The most robust predictor of donor versus recipient NK cell alloreactivity in the HCT setting has been extensively debated. Based on experimental observations, the Perugia investigators proposed that all mature NK cells expressed at least one inhibitory receptor for self HLA, and thus the presence or absence of functional KIRs could be deduced by HLA-genotype [10,37]. Based on this hypothesis, the authors presented a simple algorithm in which comparison between donor and recipient HLA class I genotype allowed prediction of NK alloreactivity (KIR ligand incompatibility model). Several groups have retrospectively tested the KIR ligand incompatibility model in patients given grafts from HLA-mismatched unrelated donors [17,18,38,39]. While some studies found lower risks of relapse in patients with KIR ligand incompatibility in the graft-versus-host direction [17,18], others failed to identify such an association [38-40].
Given that HLA and KIR genes are encoded on chromosomes 6 and 19, respectively, they are inherited independently [41]. Consequently, 75% of HCT performed between HLA-matched siblings, and almost all of unrelated HCT are KIR genotype mismatched [41]. These observations are the basis for the “missing KIR ligand model” in which donor-recipient NK cell alloreactivity was predicted by analyses of donor KIR genotype and recipient HLA genotype [42,43]. Both HLA-matched and HLA-mismatched donor-recipient pairs might have missing ligands. In support of that model, one study showed that, early after transplantation, engrafted donor stem cells gave rise to a NK cell wave which expressed the same repertoire as the donor cells [14] and contained high-frequencies of donor-versus recipient alloreactive NK cells in cases of HCT where recipients lacked ligand for donor NK cell KIRs [15]. Several studies have evaluated the missing KIR-ligand model in HLA-identical HCT and results were heterogeneous. While some studies showed lower risks of relapse in patients missing one or more KIR ligands [16,19], others failed to find such an association or even found a detrimental impact of missing KIR ligand [44,45].
Given our previously observed strong suggestion between rapid post-transplant establishment of high levels of donor NK cell chimerism and better progression-free survival [20], and data demonstrating the importance of KIR ligands in HLA-haploidentical transplantation [10], the current study sought to determine whether immunogenetic factors could provide additional information to better understand the mechanisms underlying donor NK chimerism and specifically, whether donor-recipient HLA and KIR genotype information could predict those patients who might have a higher probability of achieving robust donor NK chimerism. Interestingly, we did not observe an association between missing one or more ligands for donor NK cells and risk of relapse. Furthermore, the qualitative association between prompt NK cell engraftment and less relapse did not differ after adjustment for number of activating or inhibitory donor KIR genes (that served as a marker for KIR donor haplotype). Several important characteristics of our study population might help explain the lack of association of genotype with outcome. Most importantly, the conditioning and GVHD prophylaxis regimens and the use of T-replete donor stem cells in our patients provided a setting that favored T cell reconstitution [46,47] and was vastly different from the regimens employed in the HLA-haploidentical transplant setting [10]. The HLA and KIR genetic systems are each highly polymorphic [48] and the organization of KIR genes on haplotypes complex [33]; examination of the clinical impact of KIR ligands together with their cognate receptors resulted in very small numbers of donor-recipient pairs with each combination, and might have limited our ability to evaluate their clinical importance. Likewise, although we observed a lower risk of relapse in patients who had all ligands for donor KIR compared to patients missing one or more ligands, and a higher risk of failure for progression-free survival among patients missing at least one ligand for donor KIR compared to patients not lacking such ligands, these differences were not statistically significant. Examination of these questions in a larger transplant experience will be important in the future. Analyses of recipient and donor KIR haplotypes are ongoing to determine whether there is an association between KIR haplotype and HCT outcomes after nonmyeloablative conditioning, as it has been observed in the myeloablative setting [33,49].
One previous study, comparing KIR reconstitution in patients given T-cell depleted or unmanipulated grafts, showed that T-cells in the graft altered HLA-C binding KIR reconstitution, while, interestingly, reconstitution of KIR3/DL1, binding to Bw4, was less affected by the number of T-cells in the grafts [50]. This prompted us to investigate whether missing ligand for donor Bw4 would impact HCT outcomes. However, confirming observations when all KIR ligand were analyzed together, we failed to show an impact of missing ligand for KIR3/DLI on relapse risk and other HCT outcomes.
The main finding of our study was that prompt donor NK cell engraftment correlated with low risk of relapse. This association was not impacted by donor type (related versus unrelated) nor disease category. Results were in agreement with recent studies by Savani et al. showing that rapid versus slow NK cell recovery was associated with lower risk of relapse and better overall survival in chronic myeloid leukemia patients given T-cell depleted G-PBMC after myeloablative conditioning [51,52]. Interestingly, rapid NK cell recovery correlated not only with high numbers of CD34+ cells transplanted, but also with higher donor total (inhibitory and activating) KIR genes in their studies [51,52]. One might argue that prompt NK cell engraftment after nonmyeloablative conditioning could be a marker of “good graft function”, without implicating donor NK cells in graft-versus-tumor effects. However, this hypothesis was unlikely since previous studies failed to identify a correlation between kinetics of granulocyte engraftment and relapse risk [3,20,23]. Further, the associations between high donor NK chimerism levels and low risk of relapse remained quantitatively similar when the analyses were restricted to patients with day-14 NK chimerism levels > 25%, demonstrating that this association was not due to patients with very poor engraftment (who were likely to have graft rejection and thus relapse). Importantly, prompt donor NK cell engraftment was not associated with higher incidence of acute or chronic GVHD, suggesting that NK cell adoptive immunotherapy early after HCT could be a promising approach to separate graft-versus-tumor effects from GVHD.
In conclusion, robust engraftment of donor NK cells correlated with low risk of graft rejection, low risk of relapse, and high progression-free survival but not with acute GVHD. These associations did not depend on donor NK cell alloreactivity. The clinical importance of recipient ligand and donor KIR haplotypes on post-transplant donor NK chimerism and HCT outcomes merits further study.
Acknowledgments
We are grateful to Serina Gisburne, Sam Shin, Patrice Stroup, and Eustacia Zellmer for excellent technical assistance, and to Mohamed Sorror, M.D., for providing us with the HCT-CI scores. We thank Gresford Thomas and Heather Hildebrant for data processing; the research nurses Michelle Bouvier, Mary Hinds and John Sedgwick; physicians, physicians' assistants, and clerical staff for their dedicated care of the patients; and Bonnie Larson, Helen Crawford and Sue Carbonneau for help with manuscript preparation.
Grants: Supported in part by grants CA78902, CA92058, HL36444, CA18029, CA49605 and CA15704 from the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. D.G.M. was supported by a grant from the Gabrielle Rich Leukemia Foundation. R.S. also received support from the Laura Landro Salomon Endowment Fund. FB is research associate of the National Fund for Scientific Research (FNRS) Belgium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 2.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 3.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 4.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: a review of ten years experience with new transplant concepts and new therapeutic agents (Review) Leukemia. 2006;20:1661–1672. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 6.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livnat S, Seigneuret M, Storb R, Prentice RL. Analysis of cytotoxic effector cell function in patients with leukemia or aplastic anemia before and after marrow transplantation. J Immunol. 1980;124:481–490. [PubMed] [Google Scholar]
- 8.Goldman JM, Gale RP, Horowitz MM, et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase: increased risk of relapse associated with T-cell depletion. Ann Intern Med. 1988;108:806–814. doi: 10.7326/0003-4819-108-6-806. [DOI] [PubMed] [Google Scholar]
- 9.Kolb HJ, Schmidt C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–776. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 10.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 11.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Different checkpoints in human NK-cell activation (Review) Trends Immunol. 2004;25:670–676. doi: 10.1016/j.it.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Valiante NM, Uhrberg M, Shilling HG, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 13.Cooley S, Xiao F, Pitt M, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 16.Miller JS, Cooley S, Parham P, et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 18.Beelen DW, Ottinger HD, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105:2594–2600. doi: 10.1182/blood-2004-04-1441. [DOI] [PubMed] [Google Scholar]
- 19.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem cell transplantation for acute myelogenous leukemia (AML) predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 21.Panse JP, Heimfeld S, Guthrie KA, et al. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after nonmyeloablative conditioning. Br J Haematol. 2005;128:659–667. doi: 10.1111/j.1365-2141.2005.05363.x. [DOI] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 24.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 25.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 26.Baron F, Sandmaier BM, Storer BE, et al. Extended mycophenolate mofetil and shortened cyclosporine failed to reduce graft-versus-host disease after unrelated hematopoietic cell transplantation with nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2007;13:1041–1048. doi: 10.1016/j.bbmt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan KM. Graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. Oxford, UK: Blackwell Publishing Ltd; 2004. pp. 635–664. [Google Scholar]
- 28.Bethge WA, Hegenbart U, Stuart MJ, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103:790–795. doi: 10.1182/blood-2003-07-2344. [DOI] [PubMed] [Google Scholar]
- 29.Sandmaier BM, Maloney DG, Maris MB, et al. Conversion of low donor chimerism following nonmyeloablative conditioning for hematopoietic cell transplantation (HCT) using pentostatin and donor lymphocyte infusion (DLI) Blood. 2004;104(Part 1):57a. #186[abstr.] [Google Scholar]
- 30.Rotta M, Storer B, Sahebi F, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. doi: 10.1182/blood-2008-07-170746. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilches C, Castano J, Gomez-Lozano N, Estefania E. Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70:415–422. doi: 10.1111/j.1399-0039.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 32.Uhrberg M, Valiante NM, Shum BP, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 33.McQueen KL, Dorighi KM, Guethlein LA, Wong R, Sanjanwala B, Parham P. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Hum Immunol. 2007;68:309–323. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin PJ, Hansen JA, Torok-Storb B, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 35.Maraninchi D, Gluckman E, Blaise D, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet. 1987;2:175–178. doi: 10.1016/s0140-6736(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 36.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. Review. [DOI] [PubMed] [Google Scholar]
- 38.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 39.Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103:2860–2861. doi: 10.1182/blood-2003-11-3893. [DOI] [PubMed] [Google Scholar]
- 40.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry, and the Dutch Registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants (Review) Nature Reviews Immunology. 2003;3:108–122. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- 42.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 43.Bishara A, De Santis D, Witt CC, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63:204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 44.Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 45.Sun JY, Dagis A, Gaidulis L, et al. Detrimental effect of natural killer cell alloreactivity in T-replete hematopoietic cell transplantation (HCT) for leukemia patients. Biol Blood Marrow Transplant. 2007;13:197–205. doi: 10.1016/j.bbmt.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Baron F, Storer B, Maris MB, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Castermans E, Baron F, Willems E, et al. Evidence for neo-generation of T cells by the thymus after non-myeloablative conditioning. Haematologica. 2008;93:240–247. doi: 10.3324/haematol.11708. [DOI] [PubMed] [Google Scholar]
- 48.Marsh SG, Parham P, Dupont B, et al. Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Immunogenetics. 2003;55:220–226. doi: 10.1007/s00251-003-0571-z. [DOI] [PubMed] [Google Scholar]
- 49.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19:1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 50.Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 52.Savani BN, Rezvani K, Mielke S, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]


