Abstract
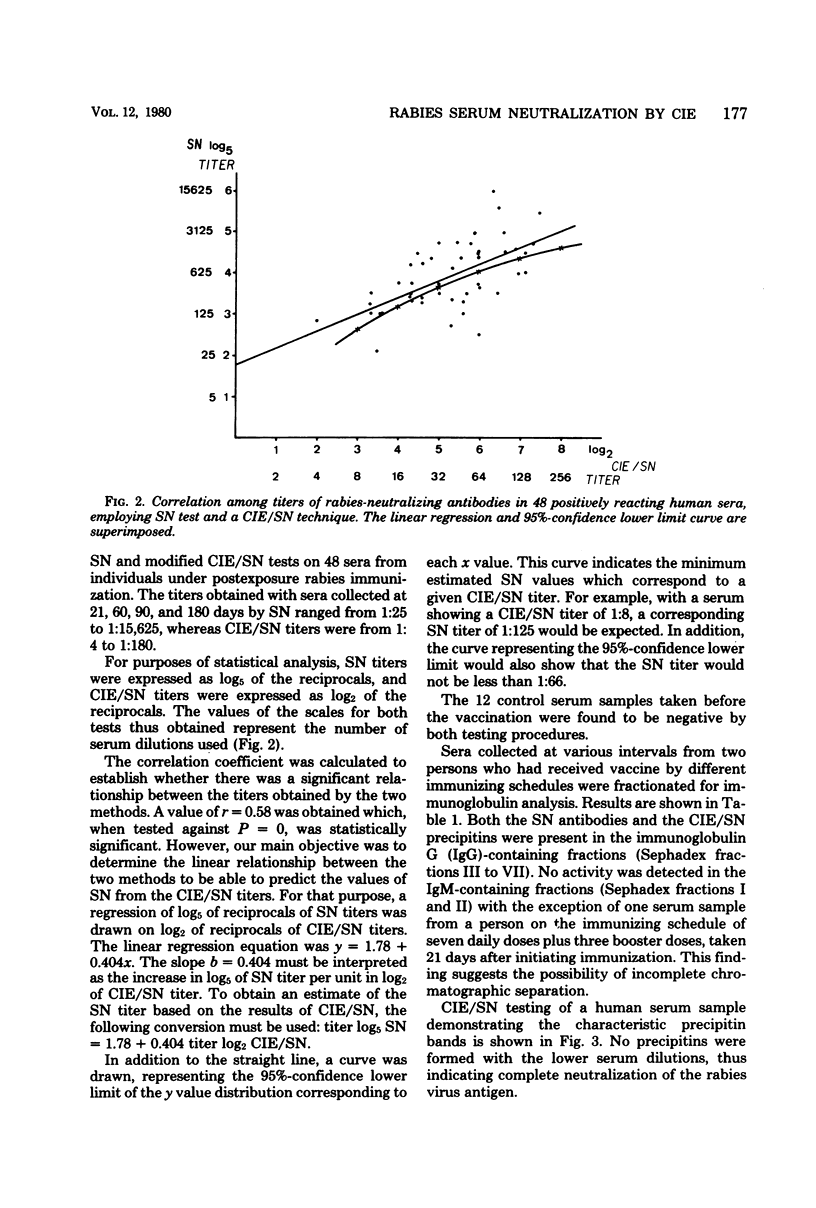
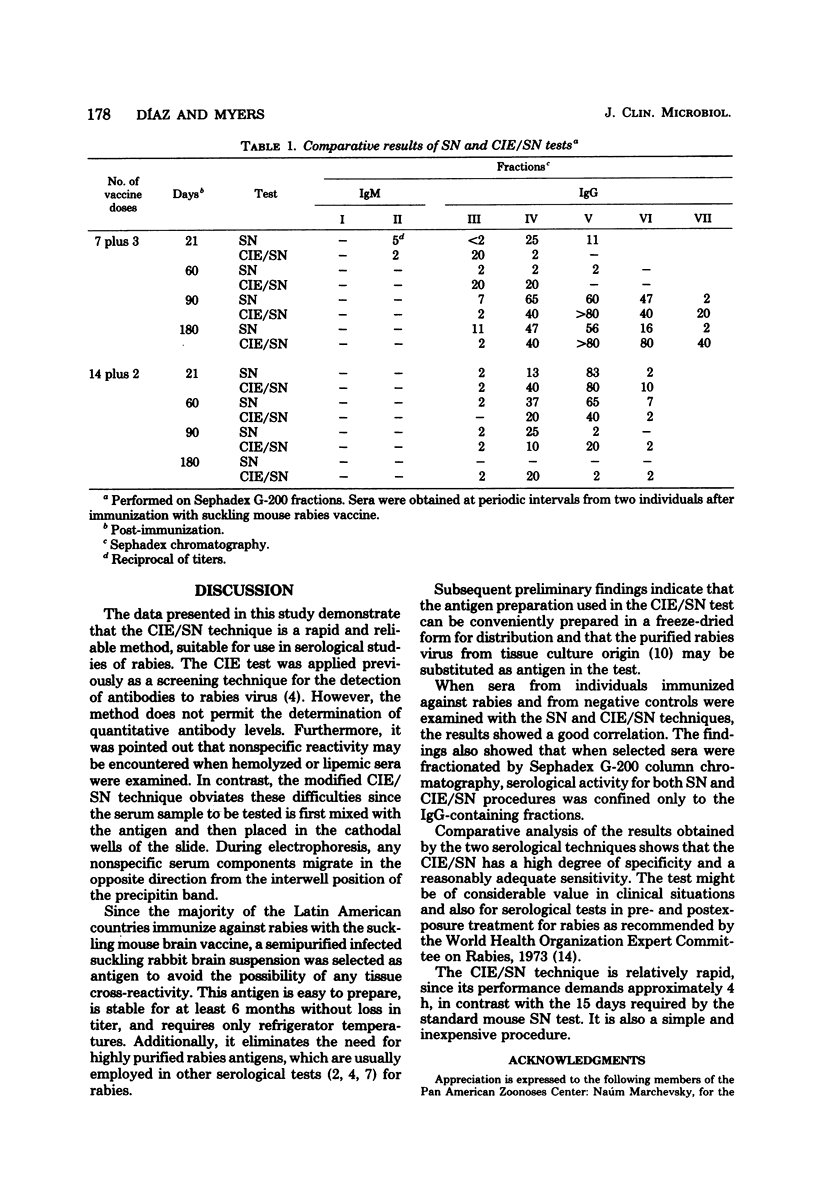
A modified counterimmunoelectrophoresis (CIE/SN) technique was developed as a diagnostic procedure for the detection of serum-neutralizing antibodies to rabies virus. CIE/SN test results with sera from individuals undergoing postexposure rabies immunization compared favorably with those obtained by the standard mouse neutralization test procedure. Fractionation of selected sera by Sephadex G-200 column chromatography demonstrated that serological activity involved in both procedures was present in the immunoglobulin G-containing fractions of the sera. The mean advantage of the CIE/SN over the standard mouse neutralization test consists in considerably reducing the time required for results, a factor which can be of clinical importance in evaluating antibody levels to rabies virus. In addition, the CIE/SN method is simple to perform and inexpensive.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATANASIU P., BAHMANYAR M., BALTAZARD M., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOMAROV A., KOPROWSKI H., LEPINE P. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. Bull World Health Organ. 1956;14(4):593–611. [PMC free article] [PubMed] [Google Scholar]
- Atanasiu P., Savy V., Perrin P. Epreuve immunoenzymatique pour la détection rapide des anticorps antirabiques. Ann Microbiol (Paris) 1977 May-Jun;128A(4):489–498. [PubMed] [Google Scholar]
- Diaz A. M., Varela-Diaz V. M. The counterimmunoelectrophoresis test for detection of antibodies to rabies virus. Ann Microbiol (Paris) 1977 Apr;128A(3):331–337. [PubMed] [Google Scholar]
- Díaz A. M., Gon'zalez Resigno G., Fern'andez Munilla A., Larghi O. P., Marchevsky N., Arrossi J. C. Vacuna antirrábica de cerebro de ratón lactante, Esquemas reducidos de inmunización post-exposicion. Rev Argent Microbiol. 1979 Jan-Apr;11(1):42–44. [PubMed] [Google Scholar]
- Hatch M. H. Modified counterelectrophoresis method for subtyping hepatitis B antigen. J Clin Microbiol. 1975 Sep;2(3):231–234. doi: 10.1128/jcm.2.3.231-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwert E. Laboratory techniques in rabies: hamgglutination and hemagglutination-inhibition tests. Monogr Ser World Health Organ. 1973;(23):135–146. [PubMed] [Google Scholar]
- Minor T. E., Helstrom P. B., Nelson D. B., D'Alessio D. J. Counterimmunoelectrophoresis test for immunoglobulin M antibodies to group B coxsackievirus. J Clin Microbiol. 1979 Apr;9(4):503–506. doi: 10.1128/jcm.9.4.503-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L. Aluminium phosphate method for rabies virus purification. Monogr Ser World Health Organ. 1973;(23):179–181. [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid tissue culture test for determining rabies neutralizing antibody. Monogr Ser World Health Organ. 1973;(23):354–357. [PubMed] [Google Scholar]
- THOMAS J. B., SIKES R. K., RICKER A. S. EVALUATION OF INDIRECT FLUORESCENT ANTIBODY TECHNIQUE FOR DETECTION OF RABIES ANTIBODY IN HUMAN SERA. J Immunol. 1963 Dec;91:721–723. [PubMed] [Google Scholar]




