Abstract
A comparison of the pathological profiles of two spongiform encephalopathies with a similar presumptive route of infection was performed. Archival kuru and recent variant Creutzfeldt–Jakob disease (vCJD) cases reveal distinct lesional differences, particularly with respect to prion protein, suggesting that the strain of agent is important in determining the phenotype. Genotype analysis of the polymorphism on codon 129 reveals (in conjunction with updated information from more kuru cases) that all three genotypes (VV, MV and MM (where M is methionine and V is valine)) are detected in kuru with some preference for MM homozygosity. The presence of valine does not therefore appear to determine peripheral selection of PrPCJD. vCJD remains restricted to date to MM homozygosity on codon 129. It remains to be determined whether this genotype is dictating a shorter incubation period.
Keywords: kuru, Creutzfeldt–Jakob disease, neuropathology
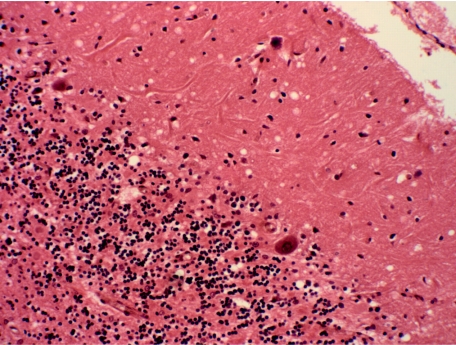
McLean et al. (1998) reviewed 11 archival cases of kuru held in the University of Melbourne. A comparative study of the pathology and the host genotype, particularly the naturally occurring (‘public’) polymorphism on codon 129, was conducted using these kuru cases and 11 new variant Creutzfeldt–Jakob disease (vCJD) cases from the UK. The triad of spongiform change, neuronal loss and gliosis was seen in all kuru cases in a distribution similar to that originally described, with prominent involvement of all cortical areas with the exception of the occipital cortex, hippocampus and insular gyri, and prominent changes also seen in the putamen, caudate and cerebellar cortex (figure 1; Fowler & Robertson 1958; Klatzo et al. 1959; Neumann et al. 1964; Kakulas et al. 1967; Beck & Daniel 1979).
Figure 1.
Kuru cerebellum showing spongiform change in the molecular layer, neuronal loss (Purkinje cells and granular cells) and gliosis (haematoxylin and eosin, ×200 actual magnification).
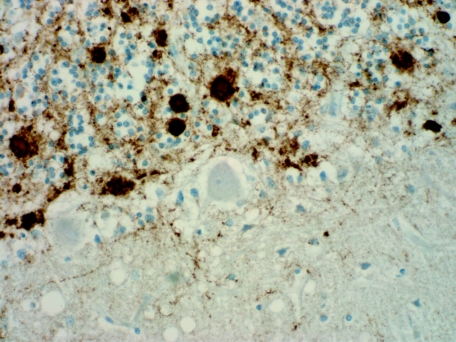
Neuropathological comparison of vCJD and kuru revealed that these diseases show distinct differences, particularly with PrPCJD immunohistochemistry, where there was a much greater PrP load in all brain areas in vCJD than in kuru, with the exception of the cerebellar granular layer (table 1). The patterns of immunohistochemical changes in the brain also varied between vCJD and kuru (table 2). A comparison of the phenotype of kuru with more recent subtyping of Creutzfeldt–Jakob disease (CJD) shows that the kuru cases resemble those cases of sporadic CJD with type 2 PrPCJD (Parchi et al. 1996). McLean et al. (1998) reported immunoperoxidase studies using the antibody 3F4 (SIGNET). A review of selected cases in 2008 using 12F10 (no. 189710 Cayman Chemical) highlights stronger and more pronounced reactivity (figure 2) than with the original 3F4 immunoperoxidase studies reported earlier.
Table 1.
PrPCJD load assessed on a scale of + to +++. (Comparison of the PrPCJD load in vCJD and kuru by immunohistochemistry.)
| PrPCJD load | vCJD | kuru |
|---|---|---|
| frontal cortex | +++ | + |
| basal ganglia | +++ | + |
| thalamus | +++ | + |
| cerebellar molecular layer | +++ | + |
| cerebellar granular layer | +++ | ++–+++ |
| basis pontis | ++ | + |
| spinal grey matter | ++ | + |
| substantia gelatinosa | + | + |
Table 2.
PrPCJD patterns either present (+) or not present (−). (Comparison of the PrPCJD patterns in vCJD and kuru by immunohistochemistry.)
| PrPCJD pattern | vCJD | kuru |
|---|---|---|
| florid plaques | + | − |
| linear/granular diffuse plaques | + | − |
| dendritic | + | + |
| perineuronal | + | + |
| cortical L3-5 laminar | − | + |
| accentuation | ||
| linear white matter in the brain stem and the spinal cord | + | + |
| granular intracytoplasmic | + | − |
Figure 2.
Kuru cerebellum showing strong immunoreaction with a granular pattern and plaque deposit in the granular layer and linear deposition within the molecular layer (12F10 prion protein, ×400 actual magnification).
The underlying differences in neuropathological profiles were thought to be in keeping with the different clinical features in these two disorders. Although both diseases exhibit prominent cerebellar ataxia, vCJD lacks the chronic emotional lability characteristic of kuru, and is characterized instead by psychiatric and sensory symptoms at onset with subsequent myoclonus and other movement disorders followed by dementia and akinetic mutism (Will et al. 1996).
The 11 cases of kuru showed a homogeneous pattern of prion protein deposition with an accentuation of cerebellar changes. Of the five cases in which the codon 129 could be assessed, there were two MM and three VV genotypes (M, methionine; V, valine; McLean et al. 1998). Analyses of codon 129 in kuru have shown that those patients with an MM genotype were preferentially affected and those with MV and VV genotypes appeared to be predisposed to a lower risk of disease development and longer incubation times (Cervenáková et al. 1998; Lee et al. 2001; see also Hainfellner et al. 1997; Lantos et al. 1997). Similarly, Collinge et al. (2006), in a study of 11 identified patients with kuru with assessabled incubation periods of 39–56 years, found they were heterozygous at the polymorphic codon 129 and believe this genotype is associated with an extended incubation period. The presence of MM homozygotes in kuru also suggests that it is not the presence of valine 129 that determines peripheral selection of the PrPCJD, as previously postulated to account for the high frequency of a valine 129 in other cases of iatrogenic CJD (Collinge et al. 1996). In vCJD, all cases to date have been MM homozygotes; however, vCJD has occurred within a very limited time frame compared with kuru (Cousens et al. 1997; Collinge et al. 2006). It therefore remains to be determined whether homozygosity at codon 129 dictates a shorter incubation period in vCJD. Moreover, the prolonged incubation period in kuru (over 50 years in some cases) may be significant when attempting to estimate future numbers of vCJD.
Footnotes
One contribution of 15 to a Theme Issue ‘The end of kuru: 50 years of research into an extraordinary disease’.
References
- Beck E., Daniel P. Kuru and Creutzfeldt–Jakob disease: neuropathological lesions and their significance. In: Prusiner S.B., Hadlow W.J., editors. Slow transmissible diseases of the nervous system. Academic Press; New York, NY: 1979. pp. 253–270. [Google Scholar]
- Cervenáková L., Goldfarb L.G., Garruto R., Lee H.S., Gajdusek D.C., Brown P. Phenotype–genotype studies in kuru: implications for new variant Creutzfeldt–Jakob disease. Proc. Natl Acad. Sci. USA. 1998;95:13 239–13 241. doi: 10.1073/pnas.95.22.13239. doi:10.1073/pnas.95.22.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J., Sidle K.C.L., Meads J., Ironside J., Hill A.F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. doi:10.1038/383685a0 [DOI] [PubMed] [Google Scholar]
- Collinge J., Whitfield J., McKintosh E., Beck J., Mead S., Thomas D.J., Alpers M.P. Kuru in the 21st century—an acquired human prion disease with very long incubation periods. Lancet. 2006;367:2068–2074. doi: 10.1016/S0140-6736(06)68930-7. doi:10.1016/S0140-6736(06)68930-7 [DOI] [PubMed] [Google Scholar]
- Cousens S.N., Vynnycky E., Zeidler M., Will R.G., Smith P.G. Predicting the CJD epidemic in humans. Nature. 1997;385:197–198. doi: 10.1038/385197a0. doi:10.1038/385197a0 [DOI] [PubMed] [Google Scholar]
- Fowler M., Robertson E.G. Observations on kuru. III. Pathological features in five cases. Australas. Ann. Med. 1958;8:16–26. doi: 10.1111/imj.1959.8.1.16. [DOI] [PubMed] [Google Scholar]
- Hainfellner J.A., Liberski P.P., Guiroy D.C., Cervenáková L., Brown P., Gajdusek D.C., Budka H. Pathology and immunocytochemistry of a kuru brain. Brain Pathol. 1997;7:547–553. doi: 10.1111/j.1750-3639.1997.tb01072.x. doi:10.1111/j.1750-3639.1997.tb01072.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas B.A., Lecours A.-R., Gajdusek D.C. Further observations on the pathology of kuru: a study of the two cerebra in serial section. J. Neuropathol. Exp. Neurol. 1967;26:85–97. doi: 10.1097/00005072-196701000-00007. doi:10.1097/00005072-196701000-00007 [DOI] [PubMed] [Google Scholar]
- Klatzo I., Gajdusek D.C., Zigas V. Pathology of kuru. Lab. Invest. 1959;8:799–847. [PubMed] [Google Scholar]
- Lantos P., Bhatia K., Doey L.J., Al-Sarraj S., Doshi R., Beck J., Collinge J. Is the neuropathology of new variant Creutzfeldt–Jakob disease and kuru similar? Lancet. 1997;350:187–188. doi: 10.1016/s0140-6736(05)62355-0. doi:10.1016/S0140-6736(05)62355-0 [DOI] [PubMed] [Google Scholar]
- Lee H.S., Brown P., Cervenáková L., Garruto R.M., Alpers M.P., Gajdusek D.C., Goldfarb L.G. Increased susceptibility to kuru of carriers of the PRNP129 methionine/methionine genotype. J. Infect. Dis. 2001;183:192–196. doi: 10.1086/317935. doi:10.1086/317935 [DOI] [PubMed] [Google Scholar]
- McLean C.A., Ironside J.W., Alpers M.P., Brown P.W., Cervenáková L., Anderson R.McD., Masters C.L. Comparative neuropathology of kuru with the new variant of Creutzfeldt–Jakob disease: evidence for strain of agent predominating over genotype of host. Brain Pathol. 1998;8:429–437. doi: 10.1111/j.1750-3639.1998.tb00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M.A., Gajdusek D.C., Zigas V. Neuropathological findings in exotic neurologic disorders among natives of the highlands of New Guinea. J. Neuropathol. Exp. Neurol. 1964;23:486–507. doi: 10.1097/00005072-196407000-00007. doi:10.1097/00005072-196407000-00007 [DOI] [PubMed] [Google Scholar]
- Parchi P., et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt–Jakob disease. Ann. Neurol. 1996;39:767–778. doi: 10.1002/ana.410390613. doi:10.1002/ana.410390613 [DOI] [PubMed] [Google Scholar]
- Will R.G., Ironside J.W., Zeidler M., Cousens S.N., Estibeiro K., Alperovitch A., Poser S., Pocchiari M., Hofman A. A new variant of Creutzfeldt–Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. doi:10.1016/S0140-6736(96)91412-9 [DOI] [PubMed] [Google Scholar]