Abstract
Recent studies have suggested that bone marrow-derived multipotent mesenchymal stem cells (MSCs) may have therapeutic applications in multiple clinical disorders including myocardial infarction, diabetes, sepsis, and hepatic and acute renal failure. Here, we tested the therapeutic capacity of human MSCs to restore alveolar epithelial fluid transport and lung fluid balance from acute lung injury (ALI) in an ex vivo perfused human lung preparation injured by E. coli endotoxin. Intra-bronchial instillation of endotoxin into the distal airspaces resulted in pulmonary edema with the loss of alveolar epithelial fluid transport measured as alveolar fluid clearance. Treatment with allogeneic human MSCs or its conditioned medium given 1 h following endotoxin-induced lung injury reduced extravascular lung water, improved lung endothelial barrier permeability and restored alveolar fluid clearance. Using siRNA knockdown of potential paracrine soluble factors, secretion of keratinocyte growth factor was essential for the beneficial effect of MSCs on alveolar epithelial fluid transport, in part by restoring amiloride-dependent sodium transport. In summary, treatment with allogeneic human MSCs or the conditioned medium restores normal fluid balance in an ex vivo perfused human lung injured by E. coli endotoxin.
Keywords: alveolar fluid clearance, keratinocyte growth factor, pulmonary edema, acute respiratory distress syndrome
Despite extensive research into the pathogenesis of acute lung injury and the acute respiratory distress syndrome (ALI/ARDS), mortality remains high at approximately 40% (1, 2). Current treatment is supportive with lung protective ventilation and a fluid conservative strategy (3, 4). Pharmacologic therapies that reduce the severity of lung injury in experimental studies have not yet been translated to effective clinical treatment options. Therefore, innovative therapies are needed.
Experimentally and clinically, the ability of the lung epithelium to remove alveolar edema is quantified as alveolar fluid clearance (AFC). Impaired AFC in patients with ALI/ARDS is associated with higher morbidity and mortality (5, 6). In the alveolar environment, basal AFC is determined predominately by amiloride-sensitive and insensitive epithelial sodium channels (ENaC) on the apical membrane as well as Na-K ATPase, located on the basolateral membrane, in both alveolar epithelial type I and II cells (7, 8). Several stimuli can up-regulate AFC including cAMP-dependent and -independent mechanisms. In the mouse, rat and human lung, cAMP dependent alveolar epithelial fluid transport is dependent on ENaC, CFTR, and Na-K ATPase (9). Catecholamine-independent pathways can also up-regulate AFC including growth factors, thyroid hormone, and glucocorticoids (7, 10, 11). Multiple pathways can reduce alveolar epithelial fluid transport and impair the resolution of alveolar edema, including hypoxia, high tidal volume ventilation, and pro-inflammatory cytokines in the pulmonary edema fluid (7, 12–16).
Recent studies have suggested that bone marrow-derived multipotent mesenchymal stem cells (MSCs) may have therapeutic applications in several clinical disorders including myocardial infarction (17), diabetes (18), sepsis (19), hepatic (20), and acute renal failure (21). Recently, allogeneic MSC have been studied in several in vivo models of lung disease (22–25). Despite initial interest in their multipotent properties (26, 27), engraftment in the lung does not appear to play a major role. The beneficial effects of MSCs in the other organ systems derive from their capacity to secrete paracrine soluble factors that modulate immune responses as well as alter the responses of endothelium or epithelium to injury through the release of growth factors (19, 28–31).
Currently, little is known regarding the effect of bone marrow-derived MSCs in experimental models of ALI and pulmonary edema. However, we recently reported that intrapulmonary (via the trachea) treatment with MSCs 4 h after endotoxin delivery to the lung improved survival and reduced pulmonary edema formation in endotoxin-induced ALI in mice, although the exact mechanisms of benefit were not identified in the study (23).
Here, we tested the therapeutic role of human allogeneic MSCs in resolving pulmonary edema in an ex vivo perfused human lung preparation (32) injured by E. coli endotoxin. Intra-bronchial instillation of endotoxin into the distal airspaces resulted in an increase in extravascular lung water and a complete loss of AFC. The addition of allogeneic human MSCs or its conditioned medium 1 h following endotoxin-induced lung injury fully restored AFC. Using siRNA knockdown of potential paracrine soluble factors, we found that the secretion of keratinocyte growth factor (KGF) was essential for the restorative effect of MSCs.
Results
Ex Vivo Human Lung Preparation.
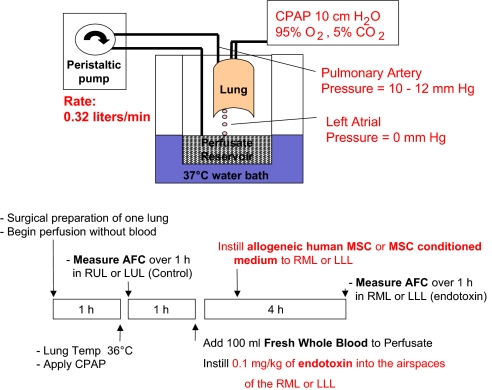
Human lungs donated by the Northern California Transplant Donor Network were used for perfusion and experimentation once the exclusion criteria were met. Baseline demographic data and ischemia time for the 38 donor lungs are listed in Table S1, including the initial blood gas values with perfusion. The details of the preparation and the protocol for the experiments are provided in Fig. 1. The cell counts of the whole blood added are listed in Table S2.
Fig. 1.
Schematic diagram of the ex vivo perfused human lung and experimental protocol.
Effect of Endotoxin on Alveolar Fluid Clearance With and Without Fresh Human Blood.
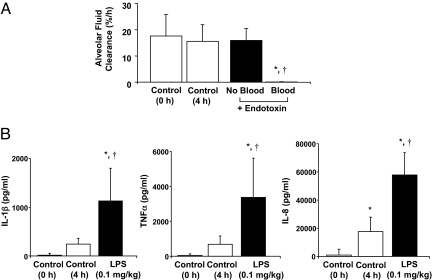
Following rewarming, the right middle lobe (RML) or left lower lobe (LLL) was injured with the intra-bronchial instillation of endotoxin at a dose of 0.1 mg/kg. For the initial experiments shown in Fig. 2A, human lungs were perfused only with a medium (DME H-21) containing 5% albumin; for the remainder of the experiments, perfusion was carried out with the same solution plus fresh whole human blood. AFC was measured at 4 h following endotoxin-induced lung injury. AFC was significantly reduced for the endotoxin-injured lung lobe only in the presence of fresh human blood. In this model, impaired AFC can reflect both an alteration in paracellular permeability as well as a decrease in transcellular ion and fluid transport. There was no significant difference in control AFC rates, measured at 0 and 4 h to reflect the lung lobes before and after the addition of whole blood (Fig. 2A). In addition, instillation of endotoxin into one lung lobe with whole human blood in the perfusate led to a sharp increase in IL-1β, TNFα, and IL-8 levels in the alveolar fluid from the endotoxin-injured lung lobe at 4 h. For the control lung lobe at 4 h, there was small increase in the proinflammatory cytokines (Fig. 2B).
Fig. 2.
Effect of endotoxin on alveolar fluid clearance and inflammation. Instillation of endotoxin into the lung lobe was associated with a significant decrease in (A) alveolar fluid clearance (AFC) and (B) an increase in inflammatory cytokine secretion. (A) The decrease in AFC in the endotoxin-injured lung lobe was dependent on the presence of fresh whole human blood in the perfusate. AFC in the endotoxin-injured lung lobe with fresh whole blood was significantly decreased compared to control AFC at 0 or 4 h measurements without endotoxin or to experiments with endotoxin without blood in the perfusate at 4 h. AFC was measured by the change in protein concentration of a 5% albumin instillate in the lung lobe over 1 h and expressed as mean AFC (%/h per 150 mL alveolar fluid) ± SD. For each condition, n = 3–6; *, P < 0.0001 vs. control AFC (0 h), †, P < 0.0013 vs. control AFC (4 h) by ANOVA (Bonferroni). (B) The addition of endotoxin into the lung lobe was associated with a significant increase in IL-1β, TNFα, and IL-8 levels in the alveolar fluid compared to the control lobe at 0 or 4 h. n = 3–6; *, P < 0.0001 vs. control (0 h), †, P < 0.0001 vs. control (4 h) for IL-1β, TNFα, and IL-8 by ANOVA (Bonferroni).
Lung Histology and Neutrophil Counts.
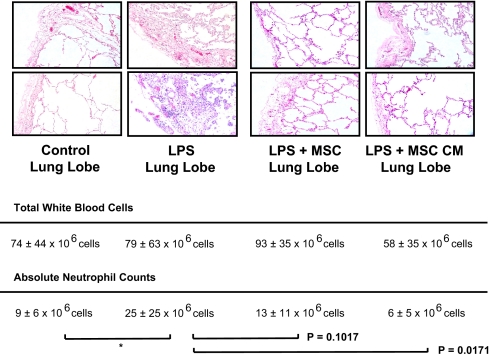
Representative histology from a lung lobe injured by endotoxin demonstrated an increase in edema and cellularity compared to histology from a control lobe. Histology from a representative sample of a lung lobe treated with the instillation of either 5 × 106 allogeneic human MSCs or MSC-conditioned medium (CM) given 1 h after instillation of endotoxin showed a reduction in the inflammatory cell infiltration and septal thickening at 4 h (Fig. 3). The number of white blood cells and neutrophils in the alveolar fluid of the endotoxin-injured lung lobe with and without treatment with MSCs or MSC-CM are shown (Fig. 3). There was a significant increase in the influx of neutrophils in the endotoxin-injured lung lobe compared to the control lobes. Although the mean number of neutrophils was reduced in the MSCs and MSC-CM-treated lobes, these differences did not quite achieve statistical significance when the Bonferroni adjustment was applied for multiple comparisons.
Fig. 3.
Histology sections and neutrophil counts. Human lungs exposed to endotoxin with and without MSC or its CM were fixed in 10% formalin at 4 h. Sections were stained with hematoxylin and eosin (magnification 10×). The instillation of MSC or its CM 1 h after endotoxin injury reduced the degree of edema and cellularity in the endotoxin-injured lung lobe. Total white blood cell and neutrophil counts in the alveolar fluid of each lung lobe are shown as mean ± SD, n = 3–6. *, P < 0.004 vs. control by ANOVA (Bonferroni). Measurement of WBC in MSC-treated lung lobes was confounded by the presence of the MSC themselves. Although not quite statistically significant by ANOVA (Bonferroni), P values are shown for the comparisons between LPS vs. LPS + MSCs and LPS vs. LPS + MSC-CM.
Lung Endothelial Permeability and Lung Water Content in Endotoxin-Injured Lung Lobe.
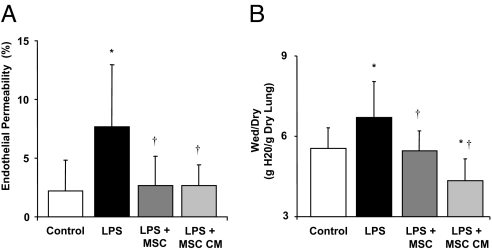
Instillation of allogeneic human MSCs or the MSC-CM 1 h following endotoxin-induced injury restored lung endothelial permeability to control levels (Fig. 4A). Instillation of endotoxin increased the water content (expressed as the wet/dry ratio of g H2O/g dry lung) by 21% (P < 0.002). The addition of MSCs or MSC-CM reduced the water content to control levels (Fig. 4B).
Fig. 4.
Effect of human MSCs or its CM on lung endothelial permeability to protein and Wet/Dry Ratio. Instillation of MSCs or its CM into the endotoxin injured RML or LLL 1 h later restored lung endothelial permeability to protein (A) and wet/dry (W/D) ratio (B) to control values. Data are expressed as mean % endothelial permeability or W/D ratio ± SD, n = 4–5 lungs; *, P < 0.0001 vs. control lobe, †, P < 0.0011 vs. LPS (0.1 mg/kg) injured lobe for lung endothelial permeability and *, P < 0.0014 vs. control lobe, †, P < 0.005 vs. LPS (0.1 mg/kg) injured lobe for the W/D ratio by ANOVA (Bonferroni).
Alveolar Fluid Clearance in the Endotoxin-Injured Lung Lobe.
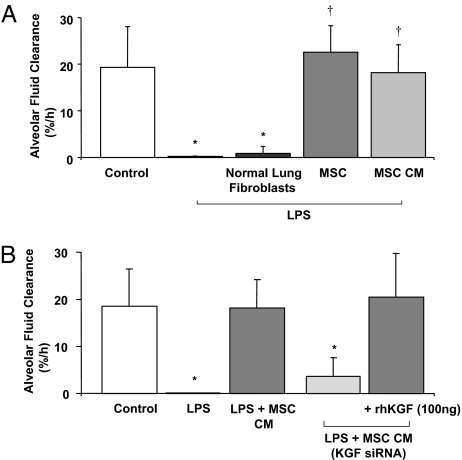
Either allogeneic human MSCs or the CM of MSCs instilled 1 h following endotoxin-induced lung injury normalized AFC. Instillation of 5 × 106 normal adult human lung (NHL) fibroblasts given 1 h following endotoxin-induced injury had no effect on AFC (Fig. 5A).
Fig. 5.
Effect of human MSCs or its CM on alveolar fluid clearance (A). MSCs or its CM restored the decrease in alveolar fluid clearance in the lung lobe injured by endotoxin to control values at 4 h. n = 3–4; *, P < 0.0006 vs. control AFC; †, P < 0.0001 vs. LPS (0.1 mg/kg) AFC by ANOVA (Bonferroni). Effect of the CM of human MSCs pretreated with a KGF siRNA on alveolar fluid clearance (B). Administration of the CM of MSCs grown for 24 h pretreated with the KGF siRNA (#10818, Ambion) into the endotoxin injured lung lobe after 1 h prevented the restoration of AFC with the CM alone. The addition of recombinant KGF (100 ng) to the CM pretreated with KGF siRNA restored the decrease in AFC to control values. Data are expressed as mean AFC ± SD, n = 4–5 lungs; *, P < 0.0012 vs. control lobe by ANOVA (Bonferroni).
CM of MSCs Pretreated with KGF siRNA and AFC.
When the MSCs were pretreated with the KGF siRNA (Fig. S1), 80% of the protective effect of the MSC-CM on AFC in endotoxin-injured lung lobes was abolished (Fig. 5B). The CM of MSCs pretreated with a non-targeting, non-specific siRNA (Neg Control #1, Ambion) maintained the protective effect of the CM alone on AFC (24 ± 12% for MSC-CM pretreated with negative control siRNA vs. 18 ± 6% AFC/h for the CM alone, mean rate ± SD% AFC/h, P<NS).
Alveolar Fluid KGF Levels.
KGF levels were measured in the alveolar fluid with and without MSCs or MSC-CM treatment following endotoxin-induced lung injury. The median control level was 0.0 pg/mL [ (0, 2), 25%–75% percentile] and increased to 3.5 pg/mL (0, 212) following endotoxin exposure. There was a trend toward increased levels of KGF in the alveolar fluid of endotoxin-injured lungs treated with MSCs or MSC-CM. KGF was 33 pg/mL (4, 156) with MSCs and 12 pg/mL (0, 35) with MSC-CM.
Effect of Recombinant KGF.
The addition of recombinant KGF (100 ng, R&D Systems) to the MSC-CM (4 ng/mL in 25 mL instillate), pretreated with KGF siRNA before instillation into the lung, restored the protective effect of the CM alone on AFC in the endotoxin-injured lung lobes compared to MSC-CM pretreated with the KGF siRNA (Fig. 5B). RhKGF by itself partially restored the decrease in the AFC rate as well as the increase in the levels of the inflammatory cytokines, suggesting the presence of other soluble factors with an effect on net fluid clearance (Fig. S2).
Effect of MSC on Net Fluid Transport in Primary Cultures of Injured Human Alveolar Epithelial Type II Cells.
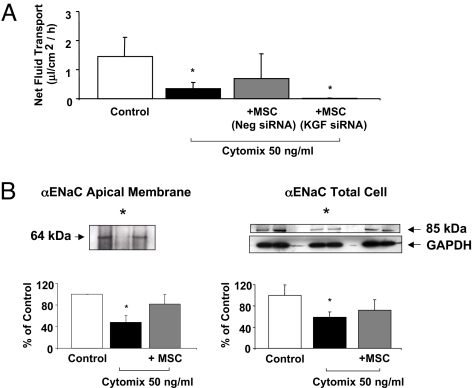
To test one mechanism that could account for the therapeutic effect of KGF in these experiments, we used an in vitro model of alveolar epithelial human type II cells, as in our prior studies (33). The addition of cytomix, a mixture of the most biologically active pro-inflammatory cytokines present in ALI pulmonary edema fluid (IL-β, TNFα, and IFNγ) (34), reduced net fluid transport by 70% in primary cultures of human alveolar epithelial type II cells. The simultaneous addition of allogeneic MSCs (250,000 cells per well) in the bottom chamber of the Transwell plate partially restored the loss of net fluid transport caused by the cytomix, whereas pretreatment of MSCs with a KGF siRNA abrogated the therapeutic benefit of MSCs on net fluid transport (Fig. 6A). These in vitro studies replicated the beneficial effects of human MSCs and KGF on alveolar fluid transport from the ex vivo perfused human lung experiments (Fig. 5B).
Fig. 6.
Effect of MSC on net fluid transport among primary cultures of human alveolar epithelial type II cells (A). Human alveolar epithelial type II cells were exposed to cytomix with and without MSC grown in the bottom chamber for 24 h. Cytomix reduced net fluid transport among the type II cells. The presence of allogeneic MSCs pretreated with a non-specific, non-targeting negative control siRNA partially restored the decrease in net fluid transport induced by cytomix. Pretreatment of MSCs with a KGF siRNA reduced the protective effect of MSCs. n = 3; *, P < 0.003 vs. control lobe by ANOVA (Bonferroni). Changes in the apical membrane and total cellular protein levels of the major sodium transport protein, αENaC, by human alveolar epithelial type II cells (B). Human alveolar epithelial type II cells exposed to cytomix with or without MSCs in the bottom chamber for 24 h. Total cellular protein or apical membrane protein levels, biotinylated using the Cell Membrane Isolation Kit (Pierce), were isolated. αENaC protein levels were measured by Western blot and expressed as the percent protein levels of control ± SD of each sample, measured in triplicates. *, P < 0.02 compared to controls for biotinylated proteins; *, P < 0.008 compared to controls for total protein by ANOVA (Bonferroni). A representative Western blot is depicted above each figure.
Effect of MSCs on the Apical Membrane Expression of ENaC.
Because previous studies demonstrated that KGF increased αENaC expression at the mRNA level (35, 36), we tested the effect of MSCs on both trafficking of ENaC subunits to the apical surface using biotinylation and on total cellular protein levels in the cultured alveolar type II cells injured with cytomix. Cytomix reduced both the total cellular level and the apical membrane expression of αENaC by 41% and 50%, respectively, at 24 h in primary cultures of human alveolar epithelial type II cells. The addition of human MSCs in the bottom chamber of the Transwell plate partially restored both the total cellular level and the apical membrane expression of αENaC (72% and 83%, respectively, of control values, Fig. 6B). In contrast, there was no statistically significant difference in the apical membrane expression of γENaC. We were not able to detect βENaC at either the total cellular protein level or on the apical membrane.
Effect of Amiloride on the CM of MSCs on AFC.
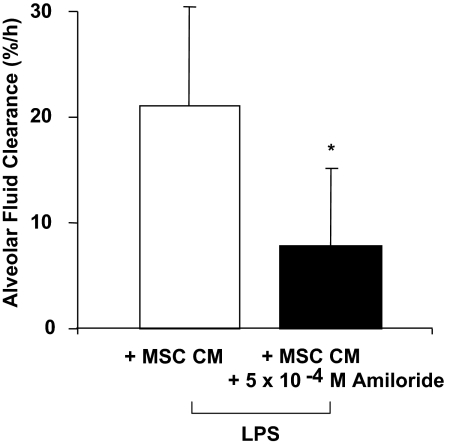
Because the in vitro experiments suggested a role for ENaC in the KGF-mediated restoration of AFC in the endotoxin-injured lung lobe, we tested the effect of amiloride, an inhibitor of ENaC, in the perfused human lung. The protective effect of MSC-CM on AFC in the lung lobe injured by endotoxin was significantly reduced when amiloride (5 × 10−4 M) was added to the medium. Overall, AFC was decreased by 56% compared to the MSC-CM alone (Fig. 7).
Fig. 7.
Effect of the addition of amiloride on the therapeutic benefit of CM on AFC. The addition of amiloride (5 × 10−4 M) to the CM (the medium of 1 × 106 cells grown for 24 h) given 1 h following endotoxin injury reduced the therapeutic effect of the MSC-CM on AFC in the lung lobe injured by endotoxin. n = 4; *, P < 0.03 vs. LPS with MSC-CM on AFC rate.
Discussion
The major findings of these experiments can be summarized as follows: (1) Following E. coli endotoxin-induced acute lung injury, treatment with either allogeneic human MSCs or human MSC-CM reduced pulmonary edema, improved lung endothelial barrier integrity and normalized alveolar epithelial fluid transport in an ex vivo perfused human lung; (2) the effect was mediated in part by the secretion of KGF; and (3) the beneficial effect of KGF was mediated in part by restoring sodium dependent alveolar fluid transport.
To study the potential therapeutic effect of human allogeneic MSCs, we developed a reproducible E. coli endotoxin-induced ALI model in an ex vivo human lung preparation perfused partially with whole blood. Instillation of endotoxin into the distal airspaces of one lung lobe resulted in acute pulmonary edema, an increase in lung vascular permeability and an almost complete loss of AFC. These effects were associated with an acute neutrophilic inflammatory response within the injured alveolus, as well as elevated levels of IL-1β, TNFα, and IL-8, the most biologically active cytokines found in ALI pulmonary edema fluid (1). The presence of fresh whole blood was essential for these effects (Fig. 2). PMNs in the fresh whole blood were likely responsible for the effect of endotoxin-induced lung injury although monocytes and platelets in the blood could also play a role. By histology, endotoxin increased the degree of edema and cellularity within the injured alveolus (Fig. 3). Lung endothelial permeability and the wet/dry ratio (a measure of lung water) increased as well following endotoxin-induced injury (Fig. 4). Instillation of either allogeneic human MSCs or its CM 1 h following endotoxin-induced lung injury fully restored normal fluid balance by three criteria: Alveolar lung endothelial permeability to protein was normalized (Fig. 4A), the wet/dry ratio returned to control levels (Fig. 4B) and alveolar epithelial fluid transport was restored to a normal level (Fig. 5A).
As suggested by recent mouse injury models [intra-tracheal LPS (23), intra-tracheal bleomycin (28), cecal ligation and puncture (19)], the secretion of pro- and anti-inflammatory cytokines, such as IL-1RA and IL-10, may account for some beneficial effects of MSCs. In the alveolar fluid of the LPS injured lung lobe, the instillation of allogeneic human MSCs or its CM had no statistically significant effect on the levels of TNFα and IL-8, although the level of IL-1β was decreased to control levels in MSC-CM experiments (Table S3). The levels of the anti-inflammatory cytokines, IL-1RA and IL-10, were not elevated in the LPS-injured lung lobe treated with MSCs or MSC-CM, similar to the findings of Ortiz et al. in bleomycin-injured mouse lungs treated with MSCs (28). These results suggest that the therapeutic benefit of MSCs or its CM does not derive primarily from an anti-inflammatory effect. However, in this ex vivo human lung model, the levels of circulating monocytes and neutrophils are significantly lower than in an intact animal (19, 23). In addition, the low levels of IL-1RA and IL-10 measured may reflect an early anti-inflammatory effect or a lung in recovery. Nevertheless, it is interesting that the beneficial effect of the MSCs or MSC-CM in this model was not primarily mediated by effects on the levels of pro- or anti-inflammatory cytokines.
Because MSCs are known to produce several epithelial specific growth factors, we tested the capacity of allogeneic human MSCs to secrete keratinocyte growth factor, the seventh member of the fibroblast growth factor family. We were particularly interested in KGF because of work from our group as well as other investigators who have reported that KGF can reduce lung injury in small animal models of pulmonary edema. Recombinant KGF pretreatment reduced mortality following intra-tracheal instillation of hydrochloric acid (37), bleomycin (38), hyperoxia (39) and Pseudomonas aeruginosa (40). In models of acute permeability edema such as α-naphthylthiourea (41), P. aeruginosa (40) or ventilator-induced lung injury (42), KGF reduced lung edema and bronchoalveolar lavage protein levels. In addition, KGF improved alveolar fluid transport in part by up-regulating αENaC gene expression (35) and Na-K ATPase activity (36) in rat lung. We found that cultured allogeneic human MSCs produced substantial quantities of KGF.
To determine the potential contribution of KGF secretion by MSC in these studies, we pretreated MSCs with KGF siRNA (Fig. S1) and instilled the CM into the endotoxin-injured ex vivo perfused human lung. The therapeutic benefit of MSC-CM on AFC was reduced by 80% when the MSCs were pretreated with a KGF siRNA, thus eliminating KGF secretion (Figs. 5B). The addition of rhKGF to the same CM (as rescue therapy) restored the therapeutic effect on AFC to the endotoxin-injured lung lobe. These results indicate that secreted KGF is an important paracrine soluble factor that mediates the therapeutic effect of MSCs on AFC. More significantly, MSCs or rhKGF can be given following the injury in the ex vivo perfused human lung and still maintain therapeutic efficacy. The addition of rhKGF itself partially restored AFC following endotoxin-induced lung injury, suggesting the presence of other important soluble factors with a therapeutic effect on AFC as well (Fig. S2).
To further test potential mechanisms of benefit of MSCs on alveolar fluid transport, we exposed primary cultures of human alveolar epithelial type II cells grown on a Transwell plate with an air-liquid interface to cytomix (33), a mixture of the most biologically active cytokines in ALI pulmonary edema fluid (34). Cytomix reduced vectorial fluid transport across epithelial type II cells by 70% over 24 h, an effect that was not associated with an increase in apoptosis or necrosis of the type II cells, as previously measured by flow cytometry (33). The addition of human MSCs to the lower chamber restored the cytomix-induced decrease in net fluid transport through a cell contact independent mechanism. As in the perfused human lung studies, pretreatment of MSCs with the KGF siRNA before its addition to the Transwell plate reduced the therapeutic benefit of MSCs on cytomix-injured net fluid transport in epithelial type II cells, suggesting an important contribution of this growth factor on AFC (Fig. 6A).
Several properties of KGF could explain the therapeutic effect of human MSCs on restoring AFC, including alveolar epithelial type II cell hyperplasia, surfactant production (43), anti-apoptotic effects (10) and increased transcription and/or translation of the major sodium and chloride transport proteins (35). Because the effect of MSC therapy in the E. coli endotoxin-induced lung injury in the ex vivo perfused human lung occurred over a 3 h time period, the therapeutic benefit of KGF is less likely explained by type II cell hyperplasia or transcriptional effects. Alternatively, we and other investigators have found that increases in vectorial fluid transport across the alveolar epithelium can be mediated by an increase in trafficking of sodium transport proteins to the cell surface in a short time frame (12, 44). To test this hypothesis, we carried out biotinylation studies to determine the effect of MSCs on the delivery of ENaC to the apical membrane in alveolar epithelial type II cells treated with cytomix. The results indicated that MSCs partially restored apical membrane protein levels of αENaC, the most significant ENaC isoform, suggesting the beneficial effect of MSCs or its CM was mediated in part through increased sodium transport on the alveolar epithelium (Fig. 6B). Currently, it remains unclear what portion of the increase in apical membrane levels of αENaC reflect an increase in membrane trafficking or a restoration of total αENaC proteins levels. In addition, it is difficult to extrapolate the in vitro mechanistic data to the perfused human lung due to the different models, form of injury (endotoxin vs. cytomix) and time points (4 h vs. 24 h) that were used. However, in support of the in vitro data, the addition of amiloride, a pharmacologic inhibitor of ENaC activity, to the CM reduced the therapeutic effect of the MSC-CM by 56% on AFC in endotoxin-injured lung lobe in the ex vivo perfused lung (Fig. 7). In addition to restoring normal sodium uptake across the apical membrane, it is also possible that the beneficial effect of MSCs or the CM could be also mediated by improved trafficking of Na-K ATPase subunits to the basolateral membrane (44).
MSCs may also normalize lung fluid balance and AFC through therapeutic effects on the lung endothelium. The integrity of the lung microvascular endothelium is essential to prevent the influx of protein-rich fluid from the plasma as well as inflammatory cells which may further aggravate the ability of the lung epithelium to remove alveolar edema. In these experiments, instillation of MSCs or its CM into an endotoxin-injured lung lobe normalized lung endothelial permeability to protein (Fig. 4A). The role of KGF is intriguing given the previous studies of ALI in animal models and a recent study by Murakami et al. (45) who reported that fibroblast growth factors (FGF) are key mediators responsible for the maintenance of endothelial barrier homeostasis. In addition, in this model, the impact of MSCs on paracellular permeability cannot be separated out from its impact on transcellular ion and fluid transport on AFC measurements. In the future, it will be important to understand the therapeutic role of MSCs on lung endothelial permeability in ALI.
There are some limitations to the current study. MSCs secrete multiple paracrine factors that may affect AFC (20, 29). In the experiments that used siRNA knockdown, AFC was abolished by 80% with the addition of CM pretreated with the KGF siRNA, indicating the other protective paracrine factors are present in the medium. This ex vivo perfused human lung preparation is short term (4 h) and does not include the influence of perfusion of other systemic organs such as the liver or spleen, which may mount a significant inflammatory response. For instance, the beneficial effect of human MSCs could also be explained in part by the secretion of interleukin-1 receptor antagonist (IL-1RA). Recently, Ortiz et al. (28) found that IL-1RA mediated the anti-inflammatory and anti-fibrotic effect of MSCs during bleomycin-induced lung injury in mice. It is also unclear why absolute neutrophil counts were decreased in the alveolar fluid in the endotoxin-injured lung lobe following MSC or CM treatment. Although FGF may be involved in lung endothelial permeability, the role of KGF or other paracrine soluble factors in neutrophil transmigration will need to be studied in the future.
In conclusion, intra-pulmonary instillation of human MSCs or its CM following E. coli endotoxin-induced lung injury restored normal alveolar epithelial fluid transport in an ex vivo perfused human lung preparation. The results demonstrate that (1) allogeneic MSCs or MSC-CM can be used to restore lung fluid balance in the setting of a clinically relevant lung injury (endotoxin) in an ex vivo perfused human lung; (2) the mechanism for the beneficial effect of human MSCs on AFC is mediated in large part through secretion of the epithelial specific growth factor, KGF; (3) the MSC therapy was effective as a treatment strategy. Cell-based therapy with MSCs or its CM needs to be tested further for its potential value in treatment of human ALI.
Methods
Ex Vivo Perfused Human Lung and Primary Cultures of Human Alveolar Epithelial Type II Cells.
A detailed description of the perfused human lung model (preparation, CM development) (32) as previously described and the measurement of AFC, lung endothelial permeability and W/D Ratio can be found in the SI Text. A detailed description of the Transwell model of human alveolar Type II cells and the measurement of net fluid transport (33) as previously described can be found in the SI Text. All other techniques (western blot, biotinylation, and histology) as well as reagents used are standard and can be found in the SI Text as well.
Data Analysis Plan.
Results were expressed as the mean ± SD if the data were normally distributed. Comparisons between two groups were made using the unpaired, two-tailed Student's t test. Comparisons with a sample over time were made by repeated measures of analysis of variance (ANOVA) using the Bonferroni correction for multiple-comparison testing using Statview (SAS Institute Inc.). For abnormally distributed data (Table S3), results were expressed as median with 25th and 75th confidence intervals, and, for statistical analysis, we used the Kruskal-Wallis tests for overall P value and the Mann-Whitney U tests with Bonferroni-corrections for pairwise comparisons.
Supplementary Material
Acknowledgments.
We thank the Northern California Transplant Donor Network and all of the transplant coordinators involved for their assistance in obtaining human research lungs; Drs. Raphael Briot, Arne Neyrinck, and James Frank for their aid with the human lung preparation; and Hanjing Zhuo, for her help with statistical analyses. This work was supported by the National Heart, Lung, and Blood Institute Grants HL-51856 and HL-51854 (to M.A.M.) and HL093026 (to J.W.L.) and the Foundation of Anesthesia Education & Research (to J.W.L.), and HL092059 (to N.G.) and Parker B. Francis Grant (to N.G.). The abstract was presented in part at the American Thoracic Society Meeting in Toronto, May 2008. Some of the materials (human allogeneic mesenchymal stem cells) used in this work were provided by the Tulane Center for Gene Therapy through National Center for Research Resources of the National Institutes of Health, Grant P40RR017447.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907996106/DCSupplemental.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.The Acute Respiratory Distress Syndrome Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 7.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MD, et al. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol. 2002;119:199–207. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 11.Guney S, et al. Dexamethasone prevents transport inhibition by hypoxia in rat lung and alveolar epithelial cells by stimulating activity and expression of Na+-K+-ATPase and epithelial Na+ channels. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1332–1338. doi: 10.1152/ajplung.00338.2006. [DOI] [PubMed] [Google Scholar]
- 12.Planes C, Blot-Chabaud M, Matthay MA, Couette S, Uchida T, Clerici C. Hypoxia and beta 2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem. 2002;277:47318–47324. doi: 10.1074/jbc.M209158200. [DOI] [PubMed] [Google Scholar]
- 13.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP- dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2003;284:L791–798. doi: 10.1152/ajplung.00331.2002. [DOI] [PubMed] [Google Scholar]
- 14.Roux J, et al. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–18589. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 15.Dagenais A, et al. Downregulation of ENaC activity and expression by TNF-alpha in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L301–311. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- 16.Frank J, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem. 2003;278:43939–43950. doi: 10.1074/jbc.M304882200. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara Y, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekkadan B, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 22.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 24.Rojas M, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: Potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz LA, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 32.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JW, et al. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem. 2007;282:24109–24119. doi: 10.1074/jbc.M700821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittet JF, et al. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol. 2001;166:6301–6310. doi: 10.4049/jimmunol.166.10.6301. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Folkesson HG, Jayr C, Ware LB, Matthay MA. Alveolar epithelial fluid transport can be simultaneously upregulated by both KGF and beta-agonist therapy. J Appl Physiol. 1999;87:1852–1860. doi: 10.1152/jappl.1999.87.5.1852. [DOI] [PubMed] [Google Scholar]
- 36.Guery BP, Mason CM, Dobard EP, Beaucaire G, Summer WR, Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997;155:1777–1784. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 37.Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15:433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 38.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol. 1998;186:90–98. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viget NB, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1199–1209. doi: 10.1152/ajplung.2000.279.6.L1199. [DOI] [PubMed] [Google Scholar]
- 41.Mason CM, Guery BP, Summer WR, Nelson S. Keratinocyte growth factor attenuates lung leak induced by alpha-naphthylthiourea in rats. Crit Care Med. 1996;24:925–931. doi: 10.1097/00003246-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Welsh DA, Summer WR, Dobard EP, Nelson S, Mason CM. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med. 2000;162:1081–1086. doi: 10.1164/ajrccm.162.3.9908099. [DOI] [PubMed] [Google Scholar]
- 43.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1146–1158. doi: 10.1152/ajplung.2000.279.6.L1146. [DOI] [PubMed] [Google Scholar]
- 44.Dada LA, Sznajder JI. Mechanisms of pulmonary edema clearance during acute hypoxemic respiratory failure: role of the Na,K-ATPase. Crit Care Med. 2003;31:S248–252. doi: 10.1097/01.CCM.0000057895.22008.EC. [DOI] [PubMed] [Google Scholar]
- 45.Murakami M, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.