Abstract
Transposable elements impact genome function by altering gene expression and causing chromosome rearrangements. As a result, organisms have evolved mechanisms, such as RNA-interference, to minimize the level of transposition. However, organisms without the conserved RNAi pathways, like Saccharomyces cerevisiae, must use other mechanisms to prevent transposon movement. Here, we provide evidence that antisense (AS) RNAs from the retrovirus-like element Ty1 inhibit retrotransposition posttranslationally in Saccharomyces. Multiple Ty1AS transcripts overlap Ty1 sequences necessary for copy number control (CNC) and inhibit transposition in trans. Altering Ty1 copy number or deleting sequences in the CNC region that are required for reverse transcription affect Ty1AS RNA level and Ty1 movement. Ty1AS RNAs are enriched in virus-like particles, and are associated with a dramatic decrease in the level of integrase, less reverse transcriptase, and an inability to synthesize Ty1 cDNA. Thus, Ty1AS RNAs are part of an intrinsic mechanism that limits retrotransposition by reducing the level of proteins required for replication and integration.
Keywords: retrotransposon, virus-like particles, RNA interference, Saccharomyces
Retrotransposons replicate through an RNA intermediate and can comprise over half the genome in many eukaryotes. To maintain genome stability, forms of RNAi have evolved to inhibit transposition (1). However, Saccharomyces cerevisiae lacks the genes required for RNAi, yet still maintains control over Ty1 retrotransposition. Ty1 elements and retroviruses have similar structures and strategies for gene expression, and encode functionally equivalent Gag and Pol proteins protease (PR), integrase (IN), and reverse transcriptase (RT) (2). Ty1 mRNA has unique subterminal 5′ (U5) and 3′ (U3) sequence motifs and a terminally repetitious (R) region. These sequences are required for reverse transcription and integration and form the U3-R-U5 segments of the LTR that bracket the element in the genome. Adjacent to U5 is the primer-binding site (pbs), where tRNA-Meti anneals with Ty1 mRNA to initiate reverse transcription within cytoplasmic virus-like particles (VLPs). Although not completely defined, sequences within GAG also enhance Ty1 mRNA dimer formation and packaging into VLPs (3, 4). The primary translation products are Gag-p49 and Gag-Pol-p199 that are cleaved by PR to form mature Gag-p45, PR-p20, IN-p71, and RT-p63. An association between IN and RT is required for reverse transcription in vivo and RT activity in vitro (5–7). Retrotransposition is completed by IN-mediated integration of Ty1 cDNA into the genome.
Despite high levels of Ty1 mRNA and many functional elements in the genome, transposition occurs at a low rate (8–10). Cells also contain a low level of mature Ty1 proteins (11), and few VLPs are present (12). When an active Ty1 element is fused to the GAL1 promoter and carried on a multicopy pGTy1 plasmid (13), galactose induction overcomes the barriers to transposition. However, the mechanism underlying this observation remains to be determined.
We have used a Ty1-less strain of Saccharomyces paradoxus (14) to study a Ty1 copy number control (CNC) system that acts posttranscriptionally and may inhibit reverse transcription (15). CNC requires Ty1 sequences, but occurs when a multicopy pGTy1 element is repressed or contains a minimal segment of mostly GAG sequence. Induction of a WT or defective pGTy1 element also overcomes CNC. These results suggest that a factor produced by Ty1 affects transposition in trans.
S. cerevisiae contains antisense (AS) and noncoding RNAs (16, 17), and specific noncoding transcripts affect transcription (18, 19). Analyses of expressed sequences indicate that Ty1AS RNAs are synthesized (16). Ty1AS RNAs have been reported to partially repress Ty1 transcription (20), which stands in contrast to results indicating that Ty1 CNC occurs posttranscriptionally (15). Here, we show that Ty1AS RNAs are necessary for CNC. Our results also suggest that AS transcripts associated with VLPs block reverse transcription by preventing the accumulation of mature IN and RT.
Results
Ty1 CNC and AS RNAs Are Related.
Northern blot analysis was performed using total RNA from isogenic S. paradoxus strains containing 0, 1, and 25 Ty1 elements, and a S. cerevisiae strain containing 30 Ty1 elements. The level of Ty1 mRNA increased 19-fold when S. paradoxus containing 25 chromosomal elements was compared with a strain containing a single element using a strand-specific probe from GAG (Fig. 1). S. cerevisiae also contained Ty1 mRNA, the transcription of which is reduced in an spt3 mutant (21). Two size classes of Ty1 AS RNAs were detected in S. paradoxus repopulated with Ty1 elements and in S. cerevisiae. The first contained 0.5–1.0 Kb Ty1AS RNAs that hybridized with the GAG, but not with a POL probe. The smaller Ty1AS RNAs were present in the 1 and 25 copy S. paradoxus strains, and their abundance was 20-fold greater in the 25-copy strain. These Ty1AS transcripts were also present in both wild type S. cerevisiae and the spt3 mutant, suggesting that the production of the smaller AS RNAs does not require transcription of full-length mRNA. A genome-length Ty1AS RNA of ≈6 Kb was present in the 25-copy S. paradoxus and 30-copy S. cerevisiae strains (Fig. S1A); however, this transcript was difficult to detect, and was not analyzed further. The levels of Ty1AS RNA and mRNA varied between the repopulated S. paradoxus strain and S. cerevisiae, suggesting that expression levels may be species or element-specific. Our results show that genomic Ty1AS RNAs are produced in a copy-dependent manner in two different genomic environments; a repopulated genome containing essentially identical copies of recent Ty1-H3 transposition events (15), and a S. cerevisiae strain whose natural history with respect to Ty1 is more complex (22–24).
Fig. 1.

Ty1 mRNA and AS RNAs increase with copy number. Northern blot analysis of total RNA from S. paradoxus strains containing 0 (DG1768), 1 (DG2454), and 25 Ty1 elements (DG2451), a S. cerevisiae strain (BY4742; 30 Ty1 elements), and an isogenic spt3 mutant. Samples were hybridized with 32P-labeled probes from GAG (Fig. S1 and Table S2). The levels of Ty1 mRNA and the 0.5–1.0 Kb Ty1AS RNAs were normalized to 18S and 25S rRNA. RNA size standards are alongside the blot. ND, not determined.
Ty1 transposition at preferred integration targets was determined using a qualitative PCR-based assay in strains with different Ty1 copy numbers (Fig. S1B). Because a single copy strain had a higher level of Ty1 transposition, increasing the amount of Ty1 mRNA and AS RNAs was associated with a decrease in the overall level of transposition. Mobility of a genetically tagged Ty1his3-AI element (9) also decreases when similar strains are repopulated with an increasing number of Ty1 elements (15).
Ty1AS RNAs Map Within the CNC Region.
Ty1AS RNAs were mapped by Northern blot and RACE analyses from S. paradoxus containing 25 Ty1 elements, and from S. cerevisiae. Probes spanning the Ty1 CNC region hybridized with three 0.5–1 Kb Ty1AS RNAs (denoted I, II, and III) from the repopulated S. paradoxus strain (Fig. 2A). Ty1AS RNAs I and II were also detected in S. cerevisiae, but III was not (Fig. S2). Therefore, Ty1AS RNA III may be specific to Ty1-H3, which is present in the pGTy1 plasmid used to repopulate S. paradoxus. Ty1AS RNA II also appeared to be the most abundant in both species, and may correspond to a previously characterized Ty1AS RNA (20).
Fig. 2.
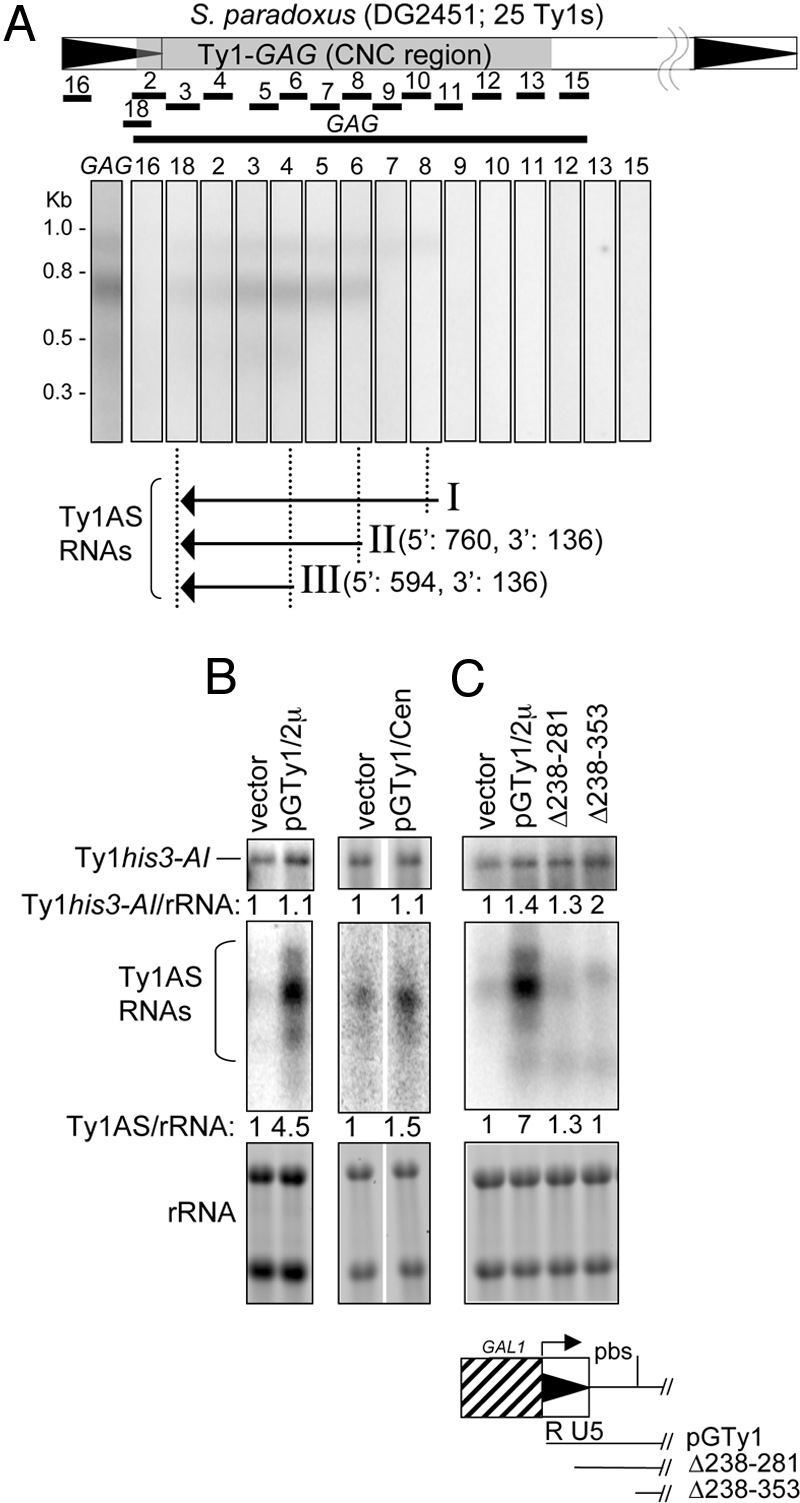
Ty1AS RNAs and CNC. (A) Ty1AS RNAs coincide with the CNC region (15). Total RNA from S. paradoxus repopulated with 25 Ty1 elements (DG2451) was subjected to Northern blot analysis. The probes used for mapping the Ty1AS RNAs (Table S2) are shown below the Ty1 element. At the bottom are the positions of Ty1AS RNAs I, II, and III. (B) Analysis of Ty1AS and mRNA levels in S. paradoxus strain DG2196 containing an empty vector (DG2254), pGTy1 on a multicopy (pGTy1/2μ; DG2255), or a low-copy vector (pGTy1/Cen; DG2283); and (C) strain DG2196 containing pGTy1Δ238-281/2μ (DG3284) or pGTy1Δ238-353/2μ (DG3285). At the bottom of the figure is the location of pGTy1/2μ Δ238-281 and Δ238-353.
RACE mapping of polyA+ RNA identified 5′ ends for Ty1AS RNA II (position 760) and III (position 594) from the repopulated S. paradoxus strain (DG2451) and Ty1AS RNA II (position 760) from S. cerevisiae (Fig. 2A; Fig. S2). The 3′ ends were obtained at position 136 within the 5′ LTR in S. paradoxus and at both 136 and 178 in S. cerevisiae, which may reflect sequence heterogeneity among the Ty1 elements. Therefore, the 3′ ends of the chromosomal Ty1AS RNAs extend past the start site of the mRNA, which begins at position 241. The location and sizes of Ty1AS RNAs generated by Northern blotting and RACE mapping from S. paradoxus suggests that Ty1AS RNAS II and III have distinct 5′ ends and sizes of ≈624 and 450 nt, respectively. However, Ty1AS RNA I was poorly represented in the 5′ RACE analysis, most likely because the 5′ ends were not well defined. These results show that the location of the Ty1AS RNAs coincides with the minimal region required for CNC (15).
Ty1AS RNAs Are Necessary for Posttranscriptional CNC.
S. paradoxus strains containing a chromosomal Ty1his3-AI element and a GAL1-promoted Ty1 element carried on multicopy (2μ) or low-copy centromere (Cen) vectors were grown in glucose-medium and assessed for Ty1his3-AI mobility (Table 1). Note that pGTy1/2μ confers CNC when Ty1 mRNA transcription from the plasmid is repressed by growth in glucose (15). Ty1his3-AI mobility decreased 22.5-fold when the strain containing pGTy1/2μ was compared with an empty vector. However, a pGTy1/Cen plasmid did not confer CNC due to its low copy number. We also analyzed pGTy1/2μ plasmids containing deletions in the previously defined CNC region. Deleting the terminally repetitious R-region of the 5′ LTR (Δ238-281) and the adjacent U5 and pbs regions (Δ238-353) eliminated CNC.
Table 1.
Ty1his3-AI mobility in S. paradoxus strains undergoing CNC
| Strain | Plasmid | Ty1his3-AI mobility (×10−6) | Relative mobility (fold change) |
|---|---|---|---|
| DG2254 | pGAL1 (vector) | 27 ± 8.7 | 1 |
| DG2255 | pGTy1/2μ | 1.2 ± 0.4 | 22.5 ↓ |
| DG2283 | pGTy1/Cen | 37 ± 1.4 | 1.4 ↑ |
| DG3284 | pGTy1/2μ Δ238-281 | 19 ± 5.6 | 1.4 ↓ |
| DG3285 | pGTy1/2μ Δ238-353 | 19 ± 3.2 | 1.4 ↓ |
Northern blot analysis using GAG probes was performed to determine whether the Ty1AS RNAs produced by pGTy1 (Fig. 2 B and C) were associated with the decrease in Ty1his3-AI mobility (Table 1). The relative amount of Ty1his3-AI mRNA remained unchanged in strains containing pGTy1/2μ or pGTy1/Cen (Fig. 2B). The 22.5-fold decrease in Ty1his3-AI mobility was accompanied by a 4.5-fold increase in the level of AS RNAs in cells containing pGTy1/2μ. When cells contained pGTy1/Cen, Ty1AS RNAs decreased to almost the same level as that observed with an empty vector. The Ty1AS RNAs from the deleted pGTy1 plasmids Δ238-281 and Δ238-353 also decreased to the level observed with an empty vector, whereas the Ty1his3-AI mRNA level remained constant (Fig. 2C). The aberrant Ty1AS RNAs from the deleted pGTy1 plasmids were not characterized further. Therefore, the increased level of the Ty1AS RNAs expressed from pGTy1/2μ is necessary for inhibiting Ty1his3-AI mobility posttranscriptionally, and sequences responsible for synthesis or stability of Ty1AS RNAs I, II, and III are located within the R-region of the 5′ LTR. Synthesis of active Ty1AS RNAs from pGTy1/2μ in the absence of GAL1-promoted expression of Ty1 mRNA also strengthens the observation that CNC occurs in trans (15).
To determine whether specific Ty1AS RNAs conferred CNC, Ty1AS RNA II identified here, or an AS RNA from a previous study (20), was expressed from the GAL1-promoter on low-copy plasmids p760-1 and p661-1, respectively (Fig. S3). Ty1his3-AI mobility decreased ≈2-fold in S. cerevisiae and remained about the same in a single-copy S. paradoxus strain expressing specific Ty1AS RNAs from the GAL1 promoter. Also, the level of Ty1 and Ty1his3-AI mRNA did not change or increased slightly in cells expressing individual Ty1AS RNAs. Our results suggest that ectopic expression of multiple Ty1AS RNAs or additional factors are required to confer CNC, and that synthesizing active Ty1AS RNAs may require specialized transcription or processing events.
Ty1AS RNAs Associate with VLPs.
RNA was isolated from a galactose-induced S. cerevisiae spt3 mutant expressing pGTy1his3-AI/2μ or an empty vector and from purified VLPs. The spt3 mutation decreases the level of full-length mRNA from chromosomal Ty1 elements, but not from an induced pGTy1 plasmid (25), and does not alter the level of Ty1AS RNAs (Fig. 1A). Northern blot analysis was performed to determine the level of Ty1 mRNA, AS RNAs, and the actin (ACT1) transcript (Fig. 3), which served as a control for specificity. The relative level of an RNA associated with the VLPs was estimated by dividing the hybridization signal from VLP-RNA by the signal from total RNA with each probe to obtain a “VLP-fraction.” The VLPs from cells expressing pGTy1his3-AI contained a large amount of Ty1his3-AI and Ty1HIS3 mRNAs (VLP-fraction = 0.53). Note that even though pGTy1 expression from a multicopy vector overcomes CNC (15), RNA from these VLPs was enriched for Ty1AS RNAs (VLP-fraction = 1.15). ACT1 mRNA was present in total RNA isolated from the galactose-induced culture, and a small amount was detected in the VLP preparation (VLP-fraction = 0.02), as shown previously (3). Thus, Ty1 mRNA and AS RNAs associated with VLPs were enriched >26-fold when compared with the ACT1 transcript. The ACT1 mRNA in the VLP fractions probably results from its high abundance and not from a specific association or packaging mechanism (26). The relative amounts of the Ty1AS RNAs, as well as the ACT1 transcript, were greatly diminished when equivalent subcellular fractions were analyzed from a spt3 mutant containing an empty vector. Therefore, Ty1AS RNAs, as well as Ty1 mRNA, are enriched in VLPs.
Fig. 3.
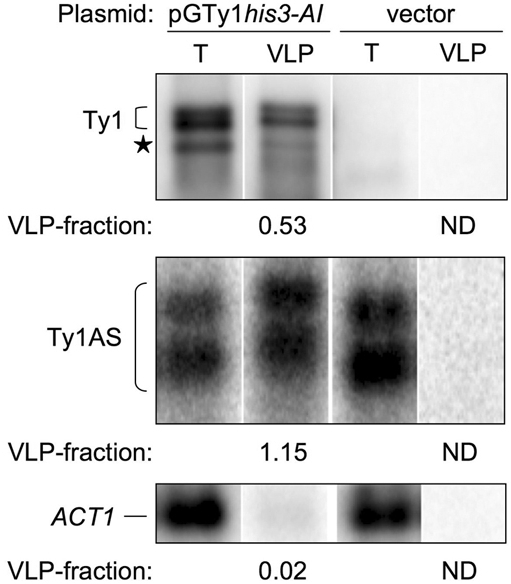
Association of Ty1 mRNA and AS RNAs with VLPs. Northern blot analysis was performed with equal amounts of total RNA (T; 5 μg) or VLP-RNA (VLP; 0.5 μg) from galactose-induced S. cerevisiae strains expressing pGTy1his3-AI (YSM505) or an empty vector (DG2082). 32P-labeled probes were used that are specific for the Ty1, mRNAs, Ty1AS RNAs (derived from GAG), and the actin (ACT1) transcript. A smaller Ty1 mRNA detected in an spt3 mutant is noted (★) (21). The VLP-fraction is shown below each panel.
Ty1AS RNAs Decrease the Level of Gag and Mature IN and RT, and Inhibit Reverse Transcription in VLPs.
It would be useful to have closely related strains that produce biochemically relevant amounts of Ty1-VLPs containing inhibitory levels of AS RNAs or not. Therefore, we constructed S. paradoxus strains containing a chromosomally-integrated pGTy1 plasmid to minimize transcription of Ty1AS RNAs and reduce the level of galactose-induced expression due to its low copy number, a chromosomal Ty1his3-AI element, a spt3 mutation, and either no additional Ty1 elements (CNC−) or 37 chromosomal Ty1-H3 elements (CNC+). Galactose-induction of pGTy1 increased Ty1his3-AI mobility and caused a mutator phenotype at the CAN1 locus in the CNC−, but not the CNC+ strain (Fig. S4), which is evidence of CNC. Encouraged by these phenotypes, we isolated VLPs from galactose-induced CNC− and CNC+ strains. The sucrose gradient fractions containing Ty1 VLPs were identified by RT activity with the exogenously added primer/template poly rC/oligo dG. The CNC− and CNC+ VLPs had comparable exogenous RT activities of 0.235 ± 0.003 and 0.39 ± 0.01, respectively, and displayed similar fractionation profiles.
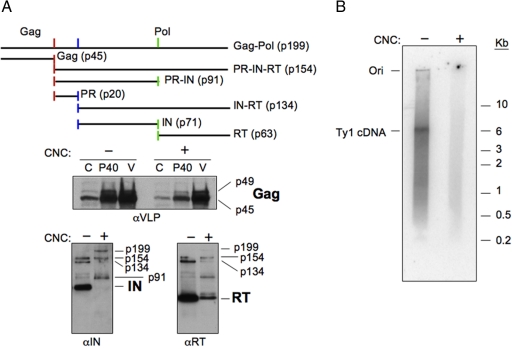
Ty1 VLPs from the CNC− and CNC+ strains were analyzed for the level of Gag, IN, and RT proteins, RT activity using endogenous VLP-associated primers and templates, and the level of Ty1 mRNA and AS RNAs. Equivalent amounts of protein were subjected to Western blot analysis using antisera for Gag (αVLP), IN (αIN), and RT (αRT) (Fig. 4A). Mature Gag-p45 was present in the CNC− and CNC+, which is a good indicator of PR activity. The level of Gag-p45 and Gag-p49 was also reduced in the CNC+ VLPs. A large amount of mature IN and RT was detected in the CNC− VLPs. In contrast, very little mature IN and much less mature RT was detected in the CNC+ VLPs. When probed with αIN, the CNC+ VLPs contained more of the PR-IN precursor (p91) and less of the IN-RT precursor (p134), suggesting that cleavage of PR-IN may be inefficient. Western blot analysis of whole-cell protein extracts from CNC− and CNC+ strains determined whether the loss of Ty1 proteins from the CNC+ VLPs could be attributed to defects in assembly or stability of the VLPs (Fig. S5). These Western blot analyses displayed the same Ty1 protein patterns as VLPs (Fig. 4A), suggesting that mature RT and IN proteins are not released from CNC+ VLPs. Consequently, the proteins essential for Ty1 reverse transcription and integration fail to accumulate in CNC+ VLPs containing a high level of Ty1AS RNAs.
Fig. 4.
Ty1AS RNAs prevent the accumulation of mature IN and RT in VLPs. (A) Western blot analysis of Ty1 Gag, IN, and RT from CNC− (YEM524) and CNC+ (YEM525) VLPs containing low and high levels of AS RNAs (Fig. S7), respectively. On the top is the Ty1 Gag-Pol protein-processing cascade. Formation of Gag-p45 from full-length Gag-p49 also occurs by a PR cleavage event near the C terminus of p49 (depicted by the red bar). The Gag-p49 precursor and Gag-p45 mature proteins were monitored during purification of VLPs from the whole-cell extract (C; 30 μg), the crude VLP pellet (P40; 15 μg), and purified VLPs (V; 5 μg). VLPs (0.5 μg) were used to detect IN and RT. Processing intermediates are indicated alongside the blots. (B) Analysis of the endogenous reaction products from CNC− and CNC+ VLPs. The products from an endogenous RT reaction using primers and templates associated with Ty1 VLPs (0.5 μg) (Fig. S6) were analyzed by agarose gel electrophoresis (12).
A key feature of Ty1 CNC is that the level of unintegrated cDNA decreases as copy number increases (15). Therefore, we analyzed the level of RT activity using endogenous primers and templates associated with VLPs (12) isolated from the CNC− and CNC+ strains. The CNC+ VLPs displayed little endogenous RT activity when compared with the CNC− VLPs (Fig. S6). The endogenous reaction from the CNC− VLPs revealed a range of products, including full-length cDNA (Fig. 4B). However, the genome size product was not detected from an endogenous reaction using the CNC+ VLPs. An equivalent amount of RNA from CNC− and CNC+ VLPs was also subjected to Northern blot analysis (Fig. S7). The CNC− and CNC+ VLPs contained approximately the same level of Ty1 mRNA, and the CNC+ VLPs contained a high level of the Ty1AS transcripts.
Discussion
Ty1AS RNAs Help Mediate CNC.
We have compared the level of Ty1 mRNA and AS RNAs with transposition in two different contexts. The most natural is when Ty1 chromosomal copy number increases as the result of transposition. Under this condition, the level of Ty1 mRNA and AS RNAs increase with copy number; however, the level of transposition decreases. These results suggest that the maximum level of endogenous transposition on a per copy basis occurs in a single-element strain. The second genomic context utilizes pGTy1 plasmids that are repressed for transcription of Ty1 mRNA, but not for AS RNAs. Reducing the level of the Ty1AS RNAs by lowering pGTy1 copy number or deleting 5′ LTR sequences eliminates CNC. Because the level of Ty1his3-AI mRNA remains unchanged in cells producing AS RNAs from additional chromosomal copies of Ty1 or a multicopy pGTy1 plasmid, CNC occurs posttranscriptionally. Also, the Ty1AS RNAs necessary to inhibit transposition map within an interval of Ty1 previously shown to be required for CNC (15).
Our results showing that Ty1AS RNAs inhibit transposition posttranscriptionally conflicts with recent work indicating that a Ty1AS RNA represses Ty1 transcription and transposition (20). Overexpressing individual Ty1AS RNAs reported by Berretta et al. (20) or characterized here (Ty1AS RNA II) confers a modest 2-fold reduction in Ty1his3-AI mobility, and does not markedly alter the level of Ty1 mRNA in S. cerevisiae or S. paradoxus. We do not understand the basis for the differences in the results presented here and elsewhere with those of Berretta et al. (20), but strain variations or different experimental conditions may be contributing factors. Nonetheless, our results define a new role for Ty1AS RNAs in modulating retrotransposition posttranscriptionally. Further studies will be required to identify all of the Ty1AS RNAs or additional factors that are necessary and sufficient for CNC.
Ty1AS RNAs Inhibit Reverse Transcription by Preventing the Accumulation of Mature Pol Proteins.
Our data suggests that Ty1AS RNAs inhibit transposition within VLPs. Surprisingly, mature IN is barely detected by Western blot analysis, and RT and Gag are also reduced in amount in CNC+ VLPs containing a high level of AS transcripts. CNC+ VLPs possess little endogenous RT activity resulting in full-length Ty1 cDNA, which supports and extends earlier results showing that Ty1 CNC affects the level of unintegrated Ty1 cDNA (15). IN and RT are required for reverse transcription in vivo and a C-terminal segment of IN attached to RT is needed for activity in vitro (5–7). Therefore, the loss of mature IN associated with the presence of Ty1AS RNAs explains the low level of endogenous RT activity and cDNA synthesis in CNC+ VLPs, and the decrease in unintegrated cDNA in cells undergoing CNC (15). The equivalent level of exogenous RT activity detected in CNC− and CNC+ VLPs is probably due to the Pol precursor proteins, which are active in an RT assay if primers and templates are provided (5, 27, 28).
These results also help explain earlier work on the low level of mature Ty1 Pol proteins in S. cerevisiae, which contains a high level of Ty1AS RNAs. Curcio and Garfinkel (11) have shown that mature IN encoded by chromosomal Ty1 elements in S. cerevisiae is undetectable, and that a pGTy1 in null mutation is not complemented by chromosomal elements. However, mature RT is detected, and a pGTy1 rt null mutation is partially complemented by chromosomal elements. Mature Gag-p45 and Gag-Pol precursors are detected in S. cerevisiae and CNC+ S. paradoxus strains, indicating that translation of POL occurs and PR is functional. Together, these results suggest that Ty1AS RNAs inhibit Ty1 retrotransposition posttranslationally within VLPs in Saccharomyces.
Model for Ty1 CNC.
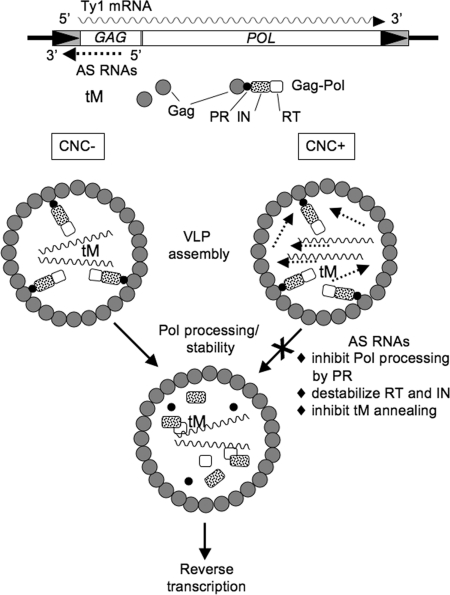
Once copackaged into a VLP, perhaps through base-pairing with Ty1 mRNA, the Ty1AS RNAs may directly inhibit processing of Pol precursors or destabilize mature IN and other proteins to a lesser extent (Fig. 5). Alternatively, Ty1 mRNA/AS RNA duplexes may inhibit Ty1 mRNA dimer formation or other mRNA interactions (3, 29), or prevent annealing of tRNA-Meti with the pbs. As a result, Ty1AS RNA-mediated interference with these RNA transactions may reduce protein processing or stability indirectly. However, mutating the Ty1 pbs or sequences possibly involved in circularization of mRNA (29) do not specifically alter the accumulation of IN (Fig. S8), suggesting that several defects in the process of retrotransposition may be needed to affect IN. Interestingly, cells lacking the MAP-kinase FUS3 have a higher level of Ty1 transposition that is correlated with an increased accumulation of Ty1 proteins (30). Because the level of Ty1AS RNAs remains unchanged in a fus3Δ mutant (Fig. S9), multiple pathways probably inhibit Ty1 protein accumulation.
Fig. 5.
Model for Ty1 CNC. Gag and Gag-Pol precursor proteins, Ty1AS RNA, Ty1 mRNA, and tRNA-Meti (tM) are shown during VLP assembly and protein maturation. In CNC- VLPs lacking Ty1AS RNAs, efficient VLP assembly, protein processing, and reverse transcription occurs. Ty1 mRNA/AS RNA base paring may be required for copackaging into CNC+ VLPs. Ty1AS RNAs may inhibit several steps in the process of retrotransposition such as blocking the cleavage of the Pol precursor protein by PR, destabilizing IN and RT, or preventing tRNA-Meti annealing with the mRNA.
Also, our results suggest that Ty1AS transcripts act stoichiometrically to inhibit transposition. One reason transposition greatly increases and CNC is suppressed in cells expressing a multicopy pGTy1 plasmid (13, 15) is that a threshold may be crossed where more Ty1 mRNA is produced than the AS RNAs. Therefore, the increased fraction of VLPs free of Ty1AS RNAs may contribute to the nonlinearity observed between the transposition rate of a given Ty1 mRNA and its concentration in transposition-induced cells (11).
In summary, our results provide evidence for a robust form of posttranslational RNAi that controls for Ty1 copy number in Saccharomyces, and could inhibit retroelement movement in other organisms. The pronounced similarities between Ty1 and HIV may also lead to the development of novel inhibitors of retroviral replication based on the properties of Ty1AS RNAs.
Materials and Methods
Strains and Genetic Techniques.
Strains are listed in Table S1. S. paradoxus strains repopulated with Ty1 or Ty1his3-AI elements were generated by induction of a pGTy1 or pGTy1his3-AI plasmid (15). Standard yeast genetic and microbiological procedures were used in this work (31).
Plasmids.
The plasmids used in this study are described in Tables S2 and S3. Additional details are presented in SI Materials and Methods or will be provided on request.
Detecting Ty1his3-AI Mobility.
The rate of Ty1his3-AI mobility was determined as described previously (9, 15). Ty1his3-AI mobility comprises Ty1HIS3 transposition and cDNA recombination events resulting in His+ cells, after splicing of the artificial intron (AI) and reverse transcription of Ty1HIS3 mRNA.
Monitoring Ty1 Insertions Upstream of Preferred Integration Targets.
Spontaneous Ty1 insertions upstream of tRNA-Gly loci were detected by PCR, as described previously (15).
VLP Characterization.
Ty1 VLPs were isolated and exogenous and endogenous RT assays were performed as described previously (32), except that YEM524 (CNC−) and YEM525 (CNC+) were grown in YEP plus galactose (2%) broth for 16–20 h at 21 °C. Equivalent amounts of protein were used in the reverse transcription and Western blot analyses. One unit of exogenous RT activity = 1 nanomole of dGTP incorporated/hr/μg protein. Western blot analyses using polyclonal antisera against Ty1 Gag (αVLP), IN (αIN; B2), and RT (αRT; B8) were performed as described previously (33). Signals were visualized by enhanced chemiluminescence (ECL; GE Healthcare).
RNA Analyses.
Total RNA was isolated using the MasterPure yeast RNA purification kit (Epicentre Biotechnologies) from strains grown to mid-to-late log phase in supplemented synthetic defined (SD) liquid media at 21 °C, or from VLPs. Equivalent amounts of the VLP preparations were used for isolating RNA, as estimated by Gag-p45 levels and protein concentrations. Contaminating DNA was removed from the RNA samples by treatment with RNase-free DNase I. Northern blot, hybridization, and phosphorimage analyses were performed as previously described (15, 34). The riboprobes were synthesized using the MAXIscript in vitro transcription kit (Ambion) and [α-32P]UTP (3,000 Ci/mmol) (PerkinElmer). Strand-specificity of the GAG and POL riboprobes was verified by Northern blot analysis using Ty1 sense and AS RNAs synthesized in vitro. All probes were added in excess. RNA size standards were obtained from Invitrogen. The VLP-fraction was determined by dividing the hybridization signal obtained from RNA extracted from VLPs by the signal obtained from total RNA with a specific probe and the same amount of RNA. RACE analyses are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Katherine Nyswaner for technical assistance; Joan Curcio, Gerry Fink, Sharon Moore, Dwight Nissley, Jeff Strathern, and Reed Wickner for helpful discussions; Jef Boeke (Johns Hopkins University School of Medicine, Baltimore, MD), Gael Cristofari (LaboRetro, Unité de Virologie Humaine, Lyon, France), Joan Curcio (Wadsworth Center, Albany, NY), Gerry Fink (Whitehead Institute, Cambridge, MA), Sharon Moore (National Cancer Institute, Frederick, MD), and Dwight Nissley (SAIC-Frederick, Frederick, MD) for reagents; and Sharon Moore, Jan Westpheling, Kristine Yoder, and Amanda Young for comments on the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908305106/DCSupplemental.
References
- 1.Girard A, Hannon GJ. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 2008;18:136–148. doi: 10.1016/j.tcb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voytas DF, Boeke JD. In: Mobile DNA II. Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Washington, DC: ASM Press; 2002. pp. 631–662. [Google Scholar]
- 3.Bolton EC, Coombes C, Eby Y, Cardell M, Boeke JD. Identification and characterization of critical cis-acting sequences within the yeast Ty1 retrotransposon. Rna. 2005;11:308–322. doi: 10.1261/rna.7860605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng YX, Moore SP, Garfinkel DJ, Rein A. The genomic RNA in Ty1 virus-like particles is dimeric. J Virol. 2000;74:10819–10821. doi: 10.1128/jvi.74.22.10819-10821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm M, Wilhelm FX. Role of integrase in reverse transcription of the Saccharomyces cerevisiae retrotransposon Ty1. Eukaryot Cell. 2005;4:1057–1065. doi: 10.1128/EC.4.6.1057-1065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm M, Wilhelm FX. Cooperation between reverse transcriptase and integrase during reverse transcription and formation of the preintegrative complex of Ty1. Eukaryot Cell. 2006;5:1760–1769. doi: 10.1128/EC.00159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm M, Boutabout M, Wilhelm FX. Expression of an active form of recombinant Ty1 reverse transcriptase in Escherichia coli: A fusion protein containing the C-terminal region of the Ty1 integrase linked to the reverse transcriptase-RNase H domain exhibits polymerase and RNase H activities. Biochem J. 2000;348:337–342. [PMC free article] [PubMed] [Google Scholar]
- 8.Elder RT, Loh EY, Davis RW. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci USA. 1983;80:2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curcio MJ, Garfinkel DJ. Single-step selection for Ty1 element retrotransposition. Proc Natl Acad Sci USA. 1991;88:936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curcio MJ, Garfinkel DJ. Heterogeneous functional Ty1 elements are abundant in the Saccharomyces cerevisiae genome. Genetics. 1994;136:1245–1259. doi: 10.1093/genetics/136.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcio MJ, Garfinkel DJ. Posttranslational control of Ty1 retrotransposition occurs at the level of protein processing. Mol Cell Biol. 1992;12:2813–2825. doi: 10.1128/mcb.12.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfinkel DJ, Boeke JD, Fink GR. Ty element transposition: Reverse transcriptase and virus-like particles. Cell. 1985;42:507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- 13.Boeke JD, Garfinkel DJ, Styles CA, Fink GR. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 14.Moore SP, et al. Analysis of a Ty1-less variant of Saccharomyces paradoxus: The gain and loss of Ty1 elements. Yeast. 2004;21:649–660. doi: 10.1002/yea.1129. [DOI] [PubMed] [Google Scholar]
- 15.Garfinkel DJ, Nyswaner K, Wang J, Cho JY. Post-transcriptional cosuppression of Ty1 retrotransposition. Genetics. 2003;165:83–99. doi: 10.1093/genetics/165.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velculescu VE, et al. Characterization of the yeast transcriptome. Cell. 1997;88:243–251. doi: 10.1016/s0092-8674(00)81845-0. [DOI] [PubMed] [Google Scholar]
- 17.Nagalakshmi U, et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hongay CF, Grisafi PL, Galitski T, Fink GR. Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell. 2006;127:735–745. doi: 10.1016/j.cell.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Martens JA, Laprade L, Winston F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature. 2004;429:571–574. doi: 10.1038/nature02538. [DOI] [PubMed] [Google Scholar]
- 20.Berretta J, Pinskaya M, Morillon A. A cryptic unstable transcript mediates transcriptional trans-silencing of the Ty1 retrotransposon in S. cerevisiae. Genes Dev. 2008;22:615–626. doi: 10.1101/gad.458008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winston F, Durbin KJ, Fink GR. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984;39:675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM, Vanguri S, Boeke JD, Gabriel A, Voytas DF. Transposable elements and genome organization: A comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence. Genome Res. 1998;8:464–478. doi: 10.1101/gr.8.5.464. [DOI] [PubMed] [Google Scholar]
- 23.Morillon A, Benard L, Springer M, Lesage P. Differential effects of chromatin and Gcn4 on the 50-fold range of expression among individual yeast Ty1 retrotransposons. Mol Cell Biol. 2002;22:2078–2088. doi: 10.1128/MCB.22.7.2078-2088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Promislow DE, Jordan IK, McDonald JF. Genomic demography: A life-history analysis of transposable element evolution. Proc Biol Sci. 1999;266:1555–1560. doi: 10.1098/rspb.1999.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeke JD, Styles CA, Fink GR. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell PH, et al. Ty1 mobilizes subtelomeric Y' elements in telomerase-negative Saccharomyces cerevisiae survivors. Mol Cell Biol. 2004;24:9887–9898. doi: 10.1128/MCB.24.22.9887-9898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawler JF, Jr, Merkulov GV, Boeke JD. A nucleocapsid functionality contained within the amino terminus of the Ty1 protease that is distinct and separable from proteolytic activity. J Virol. 2002;76:346–354. doi: 10.1128/JVI.76.1.346-354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngren SD, Boeke JD, Sanders NJ, Garfinkel DJ. Functional organization of the retrotransposon Ty from Saccharomyces cerevisiae: Ty protease is required for transposition. Mol Cell Biol. 1988;8:1421–1431. doi: 10.1128/mcb.8.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristofari G, Bampi C, Wilhelm M, Wilhelm FX, Darlix JL. A 5′-3′ long-range interaction in Ty1 RNA controls its reverse transcription and retrotransposition. EMBO J. 2002;21:4368–4379. doi: 10.1093/emboj/cdf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte D, Jr, Barber E, Banerjee M, Garfinkel DJ, Curcio MJ. Posttranslational regulation of Ty1 retrotransposition by mitogen-activated protein kinase Fus3. Mol Cell Biol. 1998;18:2502–2513. doi: 10.1128/mcb.18.5.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology. San Diego, CA: Academic; 1991. [Google Scholar]
- 32.Eichinger DJ, Boeke JD. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: Cell-free Ty1 transposition. Cell. 1988;54:955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- 33.Garfinkel DJ, Hedge AM, Youngren SD, Copeland TD. Proteolytic processing of pol-TYB proteins from the yeast retrotransposon Ty1. J Virol. 1991;65:4573–4581. doi: 10.1128/jvi.65.9.4573-4581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyswaner KM, Checkley MA, Yi M, Stephens RM, Garfinkel DJ. Chromatin-associated genes protect the yeast genome from Ty1 insertional mutagenesis. Genetics. 2008;178:197–214. doi: 10.1534/genetics.107.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.