Abstract
Sexual differentiation of the brain occurs between d 30 and 70 in the fetal lamb. The objective of this experiment was to determine if maternal fatness affects fetal steroid production and expression of their receptors which may ultimately alter endocrine systems postnatally. Fetuses were collected from ewes fed at either 100% (Control; n = 5) or 150% (Fat; n = 6) of NRC recommendations from 60 d prior to breeding until collection at 75 d of gestation. Hypothalamic and amygdala neural tissues were collected from twin male/female fetuses. Serum concentrations of testosterone were greater (P < 0.001) in male fetuses compared to female fetuses. Further, male fetuses from Fat ewes had greater (P < 0.05) serum concentrations of testosterone than male fetuses from Control ewes, but differences in testicular steroidogenic enzyme mRNA were not detected (P = 0.18). Quantity of hypothalamic mRNA for estrogen receptor (ER) β tended (P = 0.1) to be influenced by a sex by treatment interaction. Messenger RNA for ER-β was greater in female fetuses than male fetuses from Control ewes (P = 0.05). Although amount of ER-β mRNA did not differ among male fetuses (P = 0.7), amounts tended to be less (P = 0.07) in female fetuses from Fat ewes compared to those from Control ewes, and did not differ (P ≥ 0.8) from male fetuses. Hypothalamic ER-α mRNA tended (P = 0.1) to be less in fetuses from Fat ewes compared to Control fetuses but was not influenced (P = 0.3) by fetal sex or their interaction. Amount of mRNA for hypothalamic progesterone receptor tended (P = 0.06) to be greater in male fetuses than female fetuses and tended to be less (P = 0.06) in fetuses from Fat ewes than in Control fetuses, but did not differ by any sex by treatment interaction (P = 0.6). Hypothalamic RNA for the androgen receptor did not differ by sex, dam nutritional treatment, or the interaction. Likewise, amygdala RNA for the estrogen or androgen receptor did not differ (P ≥ 0.3) by sex, treatment, or their interaction. Dam fatness appears to decrease the expression of progesterone receptor, ER-α, and decrease amount of ER-β in the female fetuses while increasing circulating concentrations of testosterone in male fetuses. Altered expression of hypothalamic receptor genes by the uterine environment may affect adult responses to stress, sexual behavior and/or the pattern of gonadotropin release in response to gonadal steroids.
Keywords: Sheep - Dam obesity, Fetal steroid receptor gene expression, Sexual differentiation
1. Introduction
Sexual differentiation of the fetal sheep is initiated and controlled by gonadal steroids during sensitive periods of fetal neural development. Differentiation of the primordial gonad is initiated at approximately d 30 of gestation. Circulating concentrations of testosterone peak near d 70 in the male ovine fetus (Ford and D'Occhio, 1989). The process of defeminization and masculinization of the ovine fetal brain occurs between d 50 and 100 of gestation. During this developmental period, testosterone from the fetal gonads acts at its receptor, or via in situ aromatization to estradiol, and lays the framework necessary for the expression of male-typical sexual behaviors following puberty while permanently eliminating the positive feedback response to estradiol and the resulting estradiol-induced surge release of gonadotropin (Resko et al., 1999).
Testosterone and/or estradiol permanently affect the developing fetal brain by binding to specific receptors that alter DNA transcription. During this sensitive period, changes in steroid receptors or exposure to altered concentrations of steroid hormones have the potential to permanently affect the expression of adult sexual behavior (Morris et al., 2004; Kudwa et al., 2006), fertility (through effects on gonadotropin release; Roselli, 2007), and the response to stress (Weiser et al., 2008). Because maternal fatness influences circulating concentrations of estradiol and testosterone, (Azziz, 1989) it was hypothesized that maternal fatness would influence expression of fetal steroid receptor genes important for neural development and subsequent expression of sexual behavior and gonadotropin release.
2. Materials and methods
2.1. Animals
Animal care and use was approved by the University of Wyoming Animal Care and Use Committee. Sixty days prior to mating multiparous, white faced crossbred ewes in moderate body condition were weighed and fed differing quantities of the same diet to meet requirements for Control (100% of National Research Council recommendations; n = 5) or Fat (150% of NRC recommendations; n = 6) ewes based on metabolic BW (BW0.75) of individual ewes (NRC, 1990; Table 1). Ewes were weighed at weekly intervals with rations adjusted for weight gain. Body condition was evaluated at monthly intervals to determine change in fatness. A Body Condition Score (BSC) of 1 (emaciated) to 9 (obese) was assigned by 2 trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5) and the region around the tail head (Sanson et al., 1993).
Table 1.
Composition of diet fed to ewes 60 d prior to breeding through d 75 of gestation. Control ewes received 100% of NRC recommendations while Fat ewes received 150% NRC.
| Analyzed Composition | % |
|---|---|
| DM | 88.54 |
| NDF | 24.09 |
| ADF | 9.99 |
| CP | 17.39 |
| IVDMD | 93.92 |
At d 75 of pregnancy ewes were weighed, evaluated for body condition score, sedated with Ketamine (22.2 mg/kg body weight, i.v.) with anesthesia maintained under isofluorane inhalation (2.5%). Under anesthesia, fetal blood was collected from the umbilical vein. Following blood collection, anesthetized ewes were euthanized by heart puncture and fetuses were collected and weighed. Empty carcass weights for ewes and lambs were obtained following evisceration.
2.2. Tissue collection
Brains were removed from the fetal cranium; mid-sagitally sectioned and dissected using surface landmarks. A diencephalic block extending from the anterior margin of the optic chiasm to the mammillary bodies was dissected. This tissue contained the major nuclei of the preoptic area, anterior hypothalamus, and medial basal hypothalamus. The amygdala consisted of a block of tissue from the ventromedial temporal lobe with approximately the same rostral-caudal dimension of the hypothalamus containing entorhinal cortex as well as the major cortical, medial and basal amygdaloid nuclei. Tissues were snap frozen and stored at -80° C until RNA extraction. Only brain tissue from male/female twinned fetuses (n = 12) were utilized in this study. Testes from fetal males (n = 6) were also collected and snap frozen for analysis of steroidogenesis enzyme RNA.
2.3. RNA Isolation and cDNA synthesis
Total cellular RNA was isolated using TRI Reagent (Sigma Chemical; St. Louis, MO). Briefly, 100 mg brain tissue (hypothalamus and amygdala) was homogenized in 1 mL of Tri Reagent and incubated at room temperature for 5 min before adding 0.2 mL chloroform followed by incubation at room temperature for 10 min. The homogenate was centrifuged for 15 min at 4°C at 12,000 g. The aqueous layer was transferred with RNA was precipitated using isopropanol and centrifugation. The RNA pellet was washed with 70% ethanol and suspended in RNase free water. Concentration of RNA was determined using a NanoDrop spectrophotometer (ND-1000 Spectrophotometer, Nanodrop Technologies, Wilmington, DE) and 10 μg was purified further using RNEASY (Qiagen Inc; Santa Clara, CA) with on-column DNase digestion. Approximately 2.0 μg of RNA was mixed with 4 μL reverse transcription buffer (5X) and 1 μL of IScript reverse transcriptase (Bio-Rad Laboratories, Richmond, CA). The mixture was placed in a thermocycler for 5 min at 25° C, 30 min at 42° C, 5 min at 85° C and held at 4° C. The cDNA was diluted with 100 μL nuclease-free water and stored at -20°C until semi-quantitative real-time PCR was performed.
2.4. Semi-Quantitative Real Time PCR
Diluted cDNA (10 μL) was used as a template for semi-quantitative Real Time PCR amplification in 25 μL reactions consisting of 12.5 μL SYBR Green Supermix (Bio-Rad Laboratories), 0.5 μL H2O and 1μL each forward and reverse primer. Ovine GAPDH (housekeeping gene), androgen receptor (AR), estrogen receptor (ER)-β, ER-α, progesterone receptor (PR), P450 side chain cleavage (CPY11A), 3β-hydroxysteroid dehydrogenase (3βHSD), 17α hydroxylase (CYP17A), 17β hydroxysteroid dehydrogenase (17βHSD) primers (Table 2) were designed using Primer 3 software (Rozen and Skaletsky, 2000) to generate ∼ 100 bp amplicons. Semi-quantitative RT-PCR was performed using 40 cycles of 95° C for 30 sec and 62° C for 30 sec. Following amplification, cDNAs were melted (melting curve analysis) to ensure the quality of amplification by incubating RT-PCR products for 10 sec at each step with increase in temperature by 0.5° C from 55° C to 95° C in each cycle. Expression of AR, ER-β, and ER-α and PR RNA were quantified in hypothalamic tissue. Amygdala tissue was analyzed for expression of AR, ER-α and ER-β genes. Expression of the steroidogenesis enzyme genes was quantified in the testes tissue from male fetuses. All tissues were analyzed utilizing semi-quantitative realtime PCR and reported relative to GAPDH gene expression.
Table 2.
Forward and reverse primers used to create cDNA for semi-quantitative real-time PCR
| Target | Forward | Reverse |
|---|---|---|
| GAPDH | GATTGTCAGCAATGCCTCCT | GGTCATAAGTCCCTCCACGA |
| Estrogen Receptor α | TCCAAATGGAAAGGAACTGC | GGGTTCAAGTCTTGGGATGA |
| Estrogen Receptor β | TGATGTCCTTGACCAAGCTG | CACTTGGTCGTACAGGCTGA |
| Androgen Receptor | AATGAGTACCGCATGCACAA | AATTCCTGGGGTGTGATTTG |
| Progesterone Receptor | CCCTCAGTGCCTTAGCTCAC | AAACTGGTACACGGCCACAC |
| CYP11A | CCGCATGGGATACAATTTTC | AGGATGCCTGGGTAATTCCT |
| CYP17A | AGACAACCAAAAGGGCATTG | AGGTTGCCATCCTTGAACAG |
| 3βHSD | GCAGAAAACCAAGGAGTGGA | CACGTTCCCATCATTGTCAC |
| 17βHSD | CTGCCTCACCCATGAAATCT | CACGTAACGAGTCAGGAGCA |
2.5. Hormone analysis
Fetal blood was allowed to clot overnight at 4° C after which serum was separated by centrifugation at 1500 g for 20 min. Samples were stored at -20° C until analysis for concentrations of serum testosterone. Testosterone was quantified in duplicate according to manufacturer's directions using a commercially available solid phase RIA kit (Diagnostic Products Corp., Los Angeles, CA) in a single assay with an assay sensitivity of 0.04 ng/ml. Intra-assay variability was 2.1%.
2.6. Statistical analysis
Data were analyzed as a complete randomized design utilizing GLM procedures of SAS (Ver. 9.1, Cary, NC). Preplanned means comparisons, when appropriate, were analyzed utilizing PDIFF option of GLM; LSMEANS ± SEM are reported. Beginning and ending ewe weights and BCS were analyzed for change utilizing Paired T analysis (SAS Ver 9.1, Cary, NC).
3. Results
Body weights and body condition scores of the Control ewes did not change (P > 0.05) throughout the experiment and averaged 72.2 ± 3.3 kg and 4.7 ± 0.4, respectively by d 75 of gestation. In contrast, body weights of Fat ewe increased (P < 0.05) approximately 30% during the 60 d prior to breeding and an additional 13% from mating until tissue collection. At d 75 of gestation BW and BCS of Fat ewes averaged 102.2 ± 2.4 kg and 8.0 ± 0.2, respectively (Zhu et al., 2008). Fetal weights (374 ± 10 compared with 268 ± 12 g) and crown rump length (24.6 ± 0.2 compared with 22.1 ± 0.2 cm) were greater (P < 0.05) for fetuses from Fat than from Control fed ewes (Zhu et al., 2008).
Serum concentrations of testosterone were greater (P < 0.001) in male than in female fetuses and differed (P < 0.05) by a sex by ewe-diet interaction (Figure 1). Although serum concentrations of testosterone in female fetuses did not differ (P = 0.5) by ewe-treatment, concentrations of testosterone were greater (P < 0.05) in male fetuses from Fat ewes than in male fetuses from Control ewes.
Fig. 1.
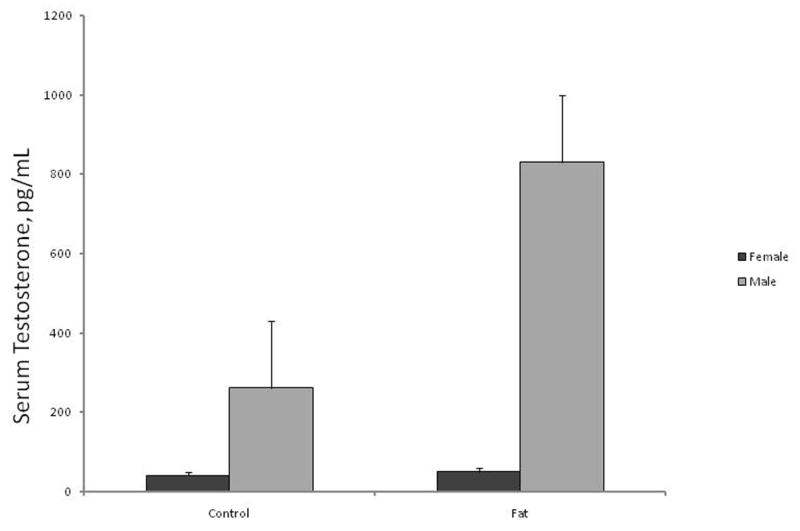
Concentrations (ng/mL) of serum testosterone in d 75 fetal lambs. Concentrations of testosterone were greater (P < 0.001) in male than female fetuses. Testosterone was increased (P < 0.05) in male fetuses from Fat ewes.
Messenger RNA for the first, and rate-limiting enzyme for the biosynthesis of steroids, CPY11A, did not differ (P = 0.18) in testes. Likewise message for other steroidogenic enzyme mRNAs (3βHSD – conversion of pregnenolone to progesterone, CYP17A- conversion of pregnenolone through the delta five pathway, or 17βHSD- conversion of testosterone to estradiol) were similar (P ≥ 0.19) in testes of fetuses from Control or Fat ewes.
Fetal mRNA for hypothalamic ER-β did not differ by ewe treatment (P = 0.28) or fetal sex (P = 0.17) but tended (P = 0.1) to be influenced by a fetal sex by treatment interaction (Figure 2). Ewe treatment did not (P = 0.7) affect amount of ER-β RNA in male fetuses, but female fetuses from Fat ewes tended (P = 0.06) to have decreased expression of ER-β as compared to female fetuses from Control ewes (Figure 2).
Fig. 2.
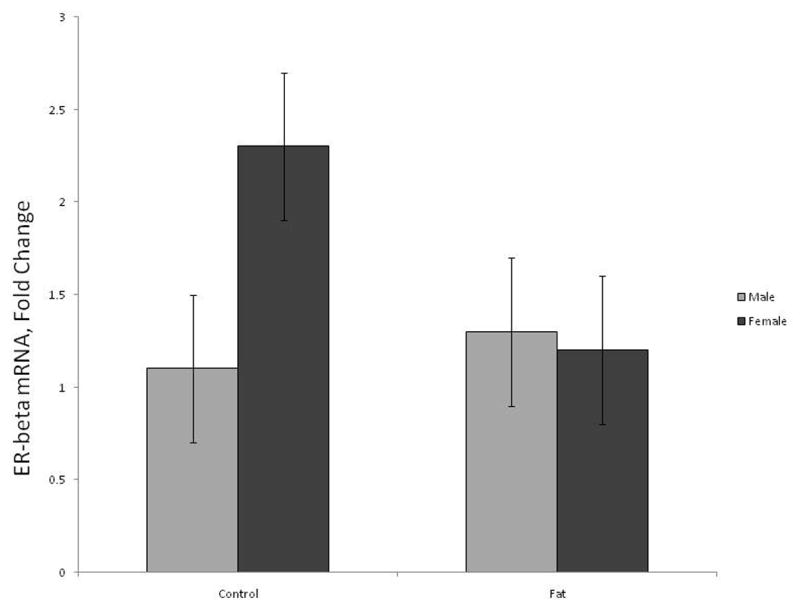
Relative expression of estrogen receptor β (ER-β) in the hypothalamic area of d 75 fetal lambs. Expression of ER-β gene tended (P = 0.1) to differ by sex by treatment interaction. Columns with differing subscripts tend to differ (P = 0.06).
Amount of ER-α in the fetal hypothalamus did not differ by fetal sex (P = 0.3) or the interaction of sex by treatment (P = 0.9). However, fetal lambs from Control ewes tended (P = 0.1) to have greater ER-α mRNA than those from Fat dams (8.4 vs. 3.3 ± 2.1 fold change).
Amount of hypothalamic PR-RNA tended (P = 0.06) to be greater in male than in female fetuses (Figure 3). In addition PR-RNA tended to be influenced (P = 0.06) by maternal treatment. Amount of PR-RNA was greater in fetuses from control ewes than from obese ewes (Figure 3). There was no ewe-treatment by fetal-sex interaction (P = 0.6).
Fig. 3.
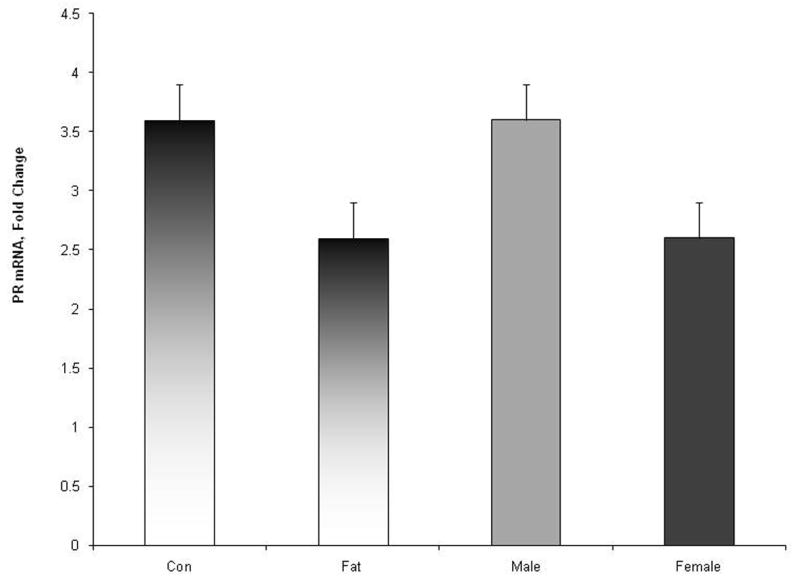
Relative expression of progesterone receptor (PR) gene in the hypothalamic area of d 75 fetal lambs. Expression of PR gene tended (P = 0.06) to be influenced by maternal diet and by fetal sex. There was no maternal treatment by fetal-sex interaction (P = 0.6).
Amount of AR in the hypothalamus was not influenced (P ≥ 0.3) by maternal treatment, fetal sex or their interaction. Amygdala expression of ER-α, ER-β, and AR was similar (P ≥ 0.3) among fetuses and did not differ due to maternal treatment, fetal-sex, or the interaction.
4. Discussion
Amount of body fatness influences circulating concentrations of reproductive steroids as lipophilic steroids are readily concentrated in adipose tissue (Azziz, 1989). Sanson et al. (1993) reported that percent lipid on an empty body basis can be accurately predicted from body condition scores. Based on Sanson et al. (1993), Fat ewes in the current study would be predicted to have approximately 10% more body lipid than Control ewes at the time of fetal collection.
Concentrations of testosterone in serum from male fetuses from Fat ewes were greater than in those from control ewes. These differences do not appear to be due to changes in testes steroidal synthesis since differences in steroid synthesizing enzymes in the fetal testes were not detected. Alternatively, amount of fatness may alter steroid storage or clearance (Azziz, 1989). Serum concentrations of testosterone in prepubertal children were positively correlated with total percent body fat (Garnett et al., 2004). Although there does not appear to be androgen synthesizing enzymes in adipose tissue (Fischer-Posovszky et al., 2007), lipophilic testosterone would likely be stored to a greater extent in the adipose of the fatter fetuses from Fat ewes. Adipose tissue has been reported to have 17-hydroxysteroid dehydrogenase activity converting androgen to testosterone (Azziz, 1989). Alternatively, increased serum concentrations of testosterone may result from adrenal production of androgens (Ayromlooi and Essman, 1978). Consequences of this increased testosterone could be alterations in neural development by increased activation of the AR or by increased availability of substrate for aromatization to estradiol. Although there are no data that suggest excess testosterone adversely affects neural development of the male fetus, proper exposure of the hypothalamus to gonadal steroids during sexual differentiation is essential for appropriate defeminization and masculinization (Schwarz and McCarthy, 2008).
Amount of mRNA is an index of gene transcription and presumably amount of protein. Although mRNA may not always be directly correlated with amount of protein (Greenbaum et al., 2003) mRNA remains an index of gene transcription and relative amounts of mRNA during the developmental period would be expected to reflect relative amounts of protein transcription. The estrogen receptor-β gene is expressed in discrete nuclei of the hypothalamus, but does not appear to be necessary for normal reproductive function (Hewitt and Korach, 2003). Although relatively normal gonadotropin synthesis and release occurs in ER-β knockout mice (Hewitt and Korach, 2003), ER-β has been implicated as an inhibitor of ER-α transcriptional activity in vivo (Gonzales et al., 2008). Estrogen receptor-β appears to have a developmental role in defeminization. ER-β knockout male mice had greater lordosis scores than their wildtype littermates (Kudwa et al., 2006) while neonatal female mice treated on d 1 to 3 with an ER-β specific agonist exhibited reduced lordosis behavior in adulthood (Kudwa et al., 2006). Furthermore, ER-β may be involved in the control of anxiety behaviors and the response to stress (Weiser et al., 2008). Changes in the expression of ER-β gene during sensitive periods of neural development, especially the ratio of ER-α: ER-β, may affect negative feedback response of hypothalamic neurons to gonadal steroids as well as altering adult sexual behavior and response to environmental stress.
The ER-α receptor is necessary for normal reproductive function (Hewitt and Korach, 2003). Female knockout mice lacking functional ER-α were anovulatory with disrupted LH regulation and uteri insensitive to estrogen (Hewitt and Korach, 2003). Male ER-α knockout mice were subfertile with decreased testes weights and reduced sperm counts (Lubahn et al., 1993). The decreased expression of hypothalamic ER-α gene in d 75 fetal lambs from Fat dams could conceivably diminish reproductive function in both adult males and females. Similar to 65 d fetal lambs (Roselli et al., 2006), sex differences in the expression of ER-α genes were not detected.
Expression of the PR gene was greater in male than female fetuses. Differences in the expression of the PR gene at d 75 may indicate that progesterone acting at its receptor may be important for the sexual differentiation of the male brain. Similar sex differences were noted by Roselli et al. (2006) in 65 d lamb fetuses. Expression of the PR gene tended to be reduced in fetuses from Fat dams. Although there was no sex by treatment interaction, the effect of maternal fatness on the expression of the PR gene would indicate a way for maternal nutrition to influence the sexual differentiation of the hypothalamus.
In conclusion, the maternal fatness appears to influence the uterine-environment resulting in an altered expression of hypothalamic ER-β, ER-α, PR genes and fetal serum concentrations of testosterone which may affect adult sexual behavior and the response to stress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayromlooi J, Essman WB. Sex differences in fetal sheep adrenal steroidogenesis. Int J Gynaecol Obstet. 1978;17:3–5. doi: 10.1002/j.1879-3479.1979.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Azziz R. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil Steril. 1989;52:703–725. doi: 10.1016/s0015-0282(16)61020-8. [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue—An update. Horm Metab Res. 2007;39:314–321. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- Ford JJ, D'Occhio MJ. Differentiation of sexual behavior in cattle, sheep and swine. J Anim Sci. 1989;67:1816–1823. doi: 10.2527/jas1989.6771816x. [DOI] [PubMed] [Google Scholar]
- Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, Cowell CT. Relationship between hormones and body composition, including bone in perpubertal children. Am J Clin Nutr. 2004;80:966–972. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor β (ERβ) modulates ERα responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrin. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117–124. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reprod. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Resko JA, Perkins A, Roselli CE, Stellflug JN, Stormshak FK. Sexual behavior of rams: male orientation and its endocrine correlates. J Reprod Fert Suppl. 1999;54:259–269. [PubMed] [Google Scholar]
- Roselli CE. Brain aromatase: Roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, Stormshak F. Expression of steroid hormone receptors in the fetal sheep brain during the critical period for sexual differentiation. Brain Res. 2006;1110:76–80. doi: 10.1016/j.brainres.2006.06.070. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci. 1993;71:1112–1116. doi: 10.2527/1993.7151112x. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculiization of the brain. J Steroid Biochem Mol Bio. 2008;109:300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signaling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol. 2008;586:2651–2664. doi: 10.1113/jphysiol.2007.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]


