Abstract
Hybrid microchannels composed of poly(dimethylsiloxane) and glass were coated with supported bilayer membranes (SBMs) by the process of vesicle fusion. The electroosmotic mobility (µeo) of zwitterionic, positively charged, and negatively charged phospholipid membranes was measured over a 4-hour time to evaluate the stability of the coatings in an electric field. Coated microchips with a simple cross design were used to separate the fluorescent dyes fluorescein and Oregon Green. Migration time reproducibility was better than 5% RSD over 70 min of continuous separations. Separation of Oregon Green and fluorescein in channels coated with zwitterionic phosphatidylcholine (PC) membranes yielded efficiencies of 611,000 and 499,000 plates/m and a resolution of 2.4 within 2 s. Both zwitterionic and negatively charged membranes were used to separate peptide substrates from their phosphorylated analogs with efficiencies of 200,000–400,000 plates/m. Notably, separations of fluorescently labeled ABL substrate peptide from its phosphorylated counterpart were achieved using a high-salt physiological buffer with near-baseline resolution in 10 s. PC-coated devices were used to successfully separate enhanced green fluorescent protein (eGFP) from a fusion protein (eGFP-Crakl) with an efficiency of 358,000 and 278,000 plates/m respectively in less than 12 s. These SBM-based coatings may enable the separation of a broad range of analytes and may be ideal in biological applications for microfluidics.
INTRODUCTION
Several recent reports have demonstrated the use of a novel semi-stable coating, supported lipid bilayer membranes (SBMs, SLBs, SPBs), for use in capillary electrophoresis (CE).1–3 SBM-coated surfaces mimic the natural environment of cells and biomolecules. Like dynamic surfactant coatings, the amphiphilic nature of SBMs allows them to spontaneously coat the surface of capillary walls. The bilayer membrane structure formed by vesicle fusion on hydrophilic surfaces is known to be stable for days. The first report of SBM CE was by Cunliffe and co-workers in 2002.1 The group fused zwitterionic 1,2-dilauroyl-sn-phosphatidylcholine (DLPC) -C12 in capillaries and performed protein separations. The µeo of coated capillaries was stable over 140 minutes of running and rinsing. Separation efficiency and reproducibility were good, protein adsorption was greatly reduced, and both cationic and anionic proteins could be separated in the same run. The same group tested capillaries coated with oligomerized 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) phospholipids in 2005.2 The separation efficiencies and reproducibility were not as good as with DLPC, although they were reportedly superior to fluorosurfactants for protein analysis. A recent report has shown that bis-SorbPC SBM coatings polymerized in capillaries can also be used for separations with charged proteins.3 The main advantage of polymerized SBMs is increased stability due to the cross linking of lipid molecules.4
SBMs have been used to coat glass and poly(dimethylsiloxane) (PDMS) microchip devices for applications other than electrophoretic separations. Enzyme kinetics were measured in PDMS microchips coated with SBMs.5 Egg-derived phosphatidylcholine (PC) was found to reduce adsorption of BSA and avidin and was used in a rapid immunoassay for cholera toxin.6 Lipids with different headgroups were used to tailor the PDMS surface for specific applications such as electrostatic repulsion of proteins with the same charge polarity as the headgroup.7 SBMs formed on PDMS were air-stable when proteins were tethered at the membrane8,9 and when cell cytoskeleton/glycocalyx-mimic strategies were incorporated.10 SBMs also appear to be an ideal semi-permanent coating for cell biology targeted-microfluidics because of their biomimetic properties.11 The microenvironment of cells in microchips is different than in conventional devices because of its high surface to volume ratio.12 While PDMS is gas permeable, allowing cells to “breathe”, contaminants from surface coatings may be toxic to cells. SBMs would provide a biocompatible environment for sensitive cells and enzymes, while making it possible to selectively enhance or prevent cell adhesion. Supported membranes patterned with peptides have been successfully proven for cell adhesion and growth in glass/PDMS hybrid microfluidic devices.13
Despite the promising potential of SBMs as a PDMS surface coating and their use in capillaries, they have not been reported for use in microchip electrophoresis. Self-assembly of phospholipids on oxidized PDMS is robust, allowing for simple fabrication with high reproducibility. Because of its high percentage composition in the cell membrane, the zwitterionic PC headgroup is naturally resistant to protein fouling14 and electrostatic interaction with a variety of biomolecules. PC SBMs should enable microchip separations with high recovery and minimal peak skew. In this report, zwitterionic phosphatidylcholine (PC), cationic ethylphosphocholine (ePC), and anionic phosphatidylglycerol (PG) SBMs were tested for use in PDMS/glass hybrid microchips to electrophoretically separate biomedically significant analytes. Hybrid microchips are often used in microfluidics since they combine the advantages of polymers with the optical properties of cover slips or slides. However generating a uniform zeta potential on these different surfaces to support electroosmotic fluid flow is difficult because glass and PDMS have different surface properties. Supported bilayer membranes form spontaneously on both materials, and therefore present a simple method to coat both PDMS and glass with a well-defined bimolecular layer. In these hybrid chips, the membranes were formed by vesicle fusion and characterized by electroosmotic mobility (µeo). The SBMs stability in an electric field was measured by monitoring the µeo over four hours. The membranes were tested for their ability to support electrophoretic separation of biologically relevant analytes with a wide range of properties. These analytes included fluorophores (fluorescein (FL) and Oregon Green carboxylate (OG)), peptides and proteins. The limits of detection, efficiency, resolution, and reproducibility were assessed for these analytes. Then PC coated-SBMs were used in the separation of a peptide substrate for ABL kinase from its phosphorylated form in a high salt extracellular buffer. Finally, a fluorescent fusion protein (eGFP-CRAKL), is separated from eGFP in a cell lysate.
EXPERIMENTAL
Materials
Egg phosphatidylcholine (PC), L-α-phosphatidyl-DL-glycerol (PG), and 1-palmitoyl-2-oleoyl-sn-glycero-3-ethylphosphocholine (ePC) were obtained from Avanti, Alabaster, AL. FL and OG were obtained from Invitrogen, Carlsbad, CA. Avidin-FITC was obtained from Sigma-Aldrich and streptavidin labeled with AlexaFluor488 was obtained from Invitrogen. eGFP was obtained from Biovision (Mountain View,CA) and GFP-Crakl fusion protein was expressed in K562 cells (human eryhtoleukemia). Phosphate buffer (PB) was prepared with potassium phosphate at the concentration indicated and adjusted to pH 7.4. Tris-ves buffer was composed of 10 mM tris and 150 mM NaCl at pH 7.4. Tris-sep buffer was composed of 25 mM tris at pH 8.4 and was used for all separations unless stated otherwise. ECB buffer was made from 135 mM NaCl, 5 mM KCL, 10 mM HEPES, 1 mM MgCl2 and 1 mM CaCl2, adjusted to pH 7.4. F-ABL is the peptide: FL-Gly-Arg-Pro-Arg-Ala-Ala-Thr-Phe-Glu while F-PKB is: FL-Gly-Arg-Pro-Arg-Ala-Ala-Thr-Phe-Glu-Gly. Both peptides were synthesized by Anaspec, Inc (San Jose, CA) with amidated C termini. The peptides were also obtained in their phosphorylated forms with the phosphate added to either the Thr (F-PKB) or Tyr (F-ABL) residues.
Methods
Vesicle Preparation
Vesicles were prepared from chloroform stocks dried in a nitrogen stream and rehydrated with a probe tip sonicator until clarity in Tris-ves buffer. They were stored at 4° C for up to two weeks before disposal. For mixed PC/PG vesicles (PCPG), 70 mol% PC and 30 mol% PG chloroform stock was prepared from the individual stocks and rehydrated as described above. For PC/ePC (ePC50) vesicles, 50 mol% PC and 50 mol% ePC chloroform stock was prepared from the individual stocks and rehydrated as described above.
Chip fabrication
PDMS chips with 30-µm × 30-µm channels were formed on silicon molds as described previously.15 Further fabrication details are presented in the Supplemental Data. Solutions containing lipid vesicles were placed in the wells immediately after device assembly while the PDMS was still hydrophilic from oxidation. This enabled to lipid solutions to spontaneously fill the channels by capillary action. The vesicles were incubated in the channels for 15 min, the channels were rinsed with Tris-sep for 10 min and fresh Tris-sep was placed in the four wells.
µeo measurements
The current monitoring method16 was used to measure the electroosmotic mobility (µeo). Further details are presented in the Supplemental Data.
Separations
Unless specified otherwise, Tris-sep buffer was used in all wells and the analytes were prepared and diluted in Tris-sep. A simple pinched-injection routine was used.17 Further details are presented in the Supplemental Data.
On-chip detection
An argon ion laser (488 nm, JDS Uniphase) was used for fluorescence excitation. The excitation light transited an excitation filter (482 ± 17.5 nm, Semrock, Rochester, NY) and was reflected from a dichroic (446 – 500 nm reflection band and a 513 – 575 nm transmission band, Semrock) onto the fluidic channel. Emitted light traveling through the dichroic filter was filtered with a second filter (536 ± 20 nm, Semrock). A photomultiplier tube (PMT) (Hamamatsu, Bridgewater,NJ) was used to detect fluorescence. The PMT output was sent to a pre-amplifier before being digitized by a USB data acquisition card (DAQ/109, Measurement Computing, Norton,MA). A CCD (Watec (Tsuruoka, Japan) LCL-902C) was used to acquire images.
RESULTS AND DISCUSSION
Formation of SBMs in Hybrid PDMS/Glass Microchannels
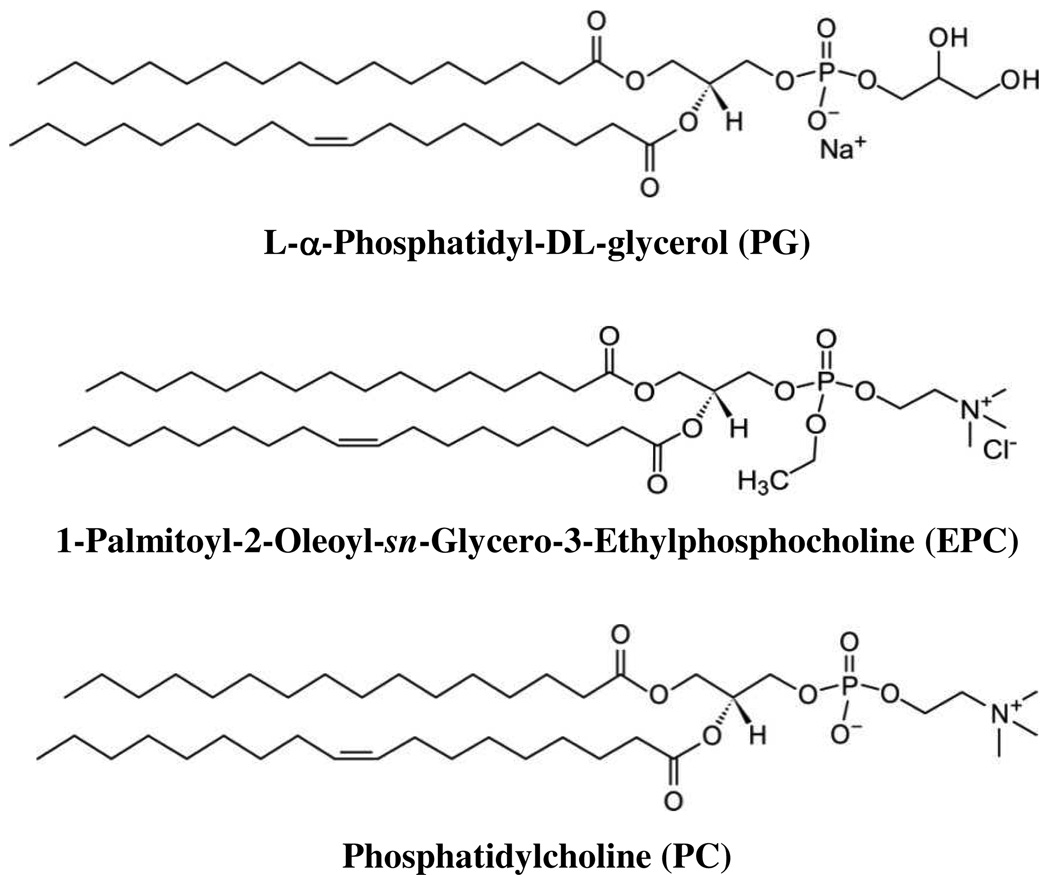
The headgroup of phospholipids can have a significant effect on SBM properties. Therefore lipids with three different headgroups of different net charge were used in this work (Fig. 1). Since SBMs can stably coat both glass and PDMS, hybrid devices composed of PDMS with imprinted microchannels and a flat glass slide were used. To form bilayer membranes in the hybrid devices, vesicles were flowed through the channel for 15 min, followed by rinsing with buffer. To determine whether a coating was obtained on the channels, the electroosmotic mobility (µeo) was measured using the current monitoring method16. PC SBM-coated channels possessed an µeo of 1.7 ± 0.2 × 10−4 cm2/Vs, which was similar to the value of 1.3 × 10−4 cm2/Vs obtained by the Aspinwall group in capillaries coated with egg-derived PC.3 The µeo of bare oxidized PDMS, previously determined to be 3.8 × 10−4 cm2/Vs15, is much higher due to negatively charged oxygen-containing moieties. For comparison, the µeo of pyrex was measured to be 4.8 × 10−4 cm2/Vs18 and the µeo of glass/PDMS hybrid chips has been reported to be 4.0 × 10−4 cm2/Vs around neutral pH19. Since the net charge of the zwitterionic PC headgroup is neutral at pH 7.4, the zeta potential value of SBM-coated PDMS on hybrid PDMS/glass microchips was likely dominated by the membrane and not the underlying substrate. Membranes made with PCPG (PG mixed with PC in a 3:7 molar ratio) had a much higher µeo of 6.6 ± 0.6 × 10−4 cm2/Vs due to the negatively charged PG headgroup. ePC50 (1:1 molar ratio of ePC and PC) membranes had an µeo of −3.5 ± 0.5 × 10−4 cm2/Vs and 100% ePC membranes produced an µeo of −5.8 ± 0.1 × 10−4 cm2/Vs. The direction and magnitude of µeo for ePC membranes is consistent with a positively charged membrane being formed in the PDMS channels, and suggests that not only the direction of µeo, but also the magnitude can be tailored in a predictable manner by controlling the net headgroup charge of the SBMs. As a control, BSA coated hybrid chips were also tested and found to have an µeo of 5.7 ± 0.3 × 10−4 cm2/Vs. This is reasonable considering that BSA has a pI of 4.7, making it negatively charged in the pH 7.4 electrophoretic buffer. The µeo values obtained with the SBM coatings suggested that good coverage of both the glass and PDMS surfaces in the microchannel was obtained.
Figure 1.
Structures of the three lipids tested in this work. Headgroup charge: PC-zwitterionic, EPC- cationic, PG- anionic. PC is the most common constituent of cell membranes and is zwitterionic. ePC is PC with the phosphoacid replaced by a methyl ester, making the net charge cationic. PG has an additional ethyl dialcohol group instead of an ammonium bridged by the phosphate, giving it a negative charge.
Stability of µeo
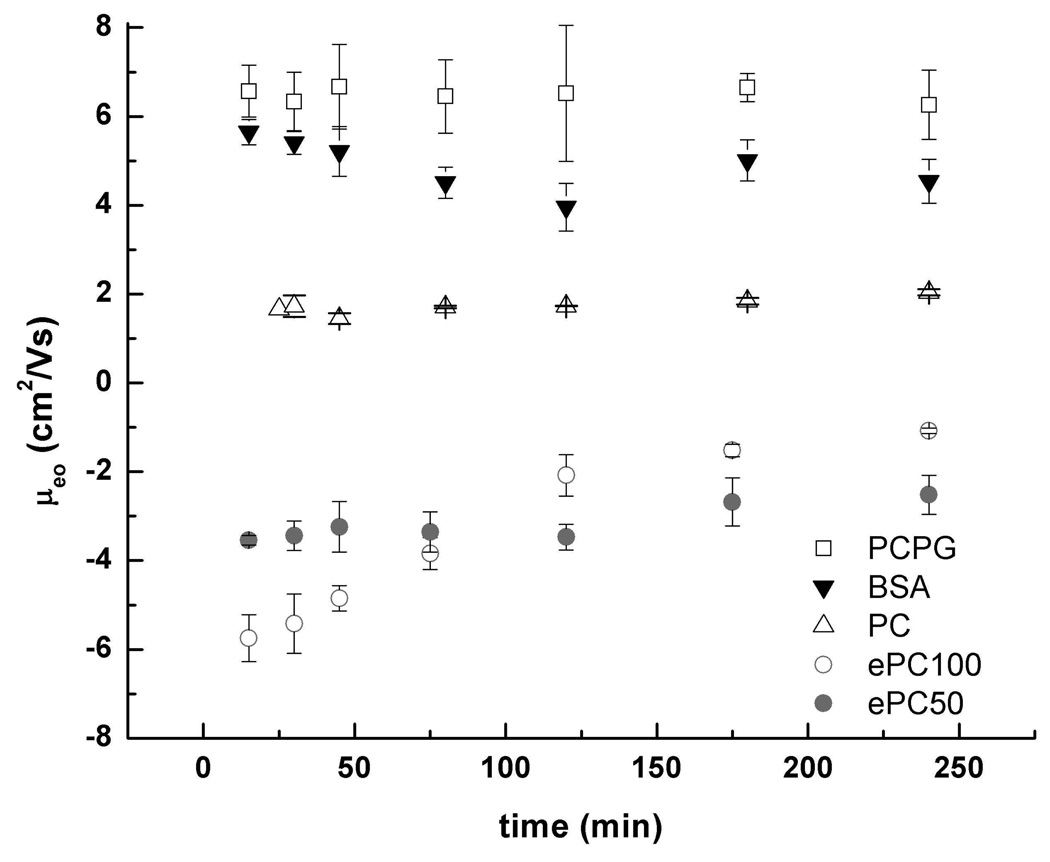
Previous reports have shown that a change in the µeo is a good indicator of supported membrane degradation since it is related to the zeta potential of the microchannel walls.20 In order to measure the stability of the SBMs in a microchip with the separation field applied, the µeo was determined at fixed time intervals over a period of 4 h and compared with that of a non-specific BSA coating (Fig. 2). Upon continuous application of 133 V/cm potential, a decrease in µeo was observed for BSA-coated microchannels (to 4.5 ± 0.5 × 10−4 cm2/Vs at 4 h). It is possible that some of the BSA coating may have been removed during application of the potential. Since there was no additional BSA in the buffer to re-coat the channels, the µeo steadily decreased to near that of native oxidized PDMS. In contrast, the µeo of PC SBM-coated channels was reproducible and stable. At times up to 4 h, no significant change was seen in the µeo value of 1.7 ± 0.2 × 10−4 cm2/Vs. The µeo of channels coated with PCPG mixed SBMs was also stable with no apparent change in µeo for up to 4 h in an electric field. This was notable because mixed lipids in a fluid bilayer can migrate in an electric field to yield an inhomogeneous lipid coating. Recent reports have demonstrated that rhodamine-labeled 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) lipids mixed with DOPC migrated when a potential was applied, rendering the membrane surface nonuniform.3 It was likely that the PCPG mixed membranes were not completely fluid under the experimental conditions.
Figure 2.
Stability of µeo for SBM-coated PDMS/glass microchips. Open triangles are PC, open squares are PCPG, closed circles are PC/ePC50, open circles are ePC100, and closed triangles are BSA. The error bars are the standard deviation of the data (n=3).
A cationic lipid, ePC, was also tested as a coating for the fluidic channels. The surface properties of channels coated with ePC membranes alone were found to be unstable in the electric field. This was surprising since previous reports have shown ePC to be more stable than PC on oxidized PDMS.6 As shown in Fig. 2, µeo for ePC100 changes from −5.8 ± 0.1 × 10−4 cm2/Vs to −1.1 ± 0.4 × 10−4 cm2/Vs within the 4 h test period. The initial rate of change is faster than that of the BSA coated surfaces. In contrast, ePC50 membranes demonstrated increased stability. µeo of −3.5 ± 0.5 cm2/Vs remained stable for 2 h after which µeo slowly changed to −2.5 ± 0.1 cm2/Vs over the last 2 h. Based on these results and the poor separation performance of ePC50 SBMs (below), it is possible that slow electromigration of ePC towards the negatively biased electrode did occur.
Electrophoretic Separations of FL and OG in SBM-Coated Microchips
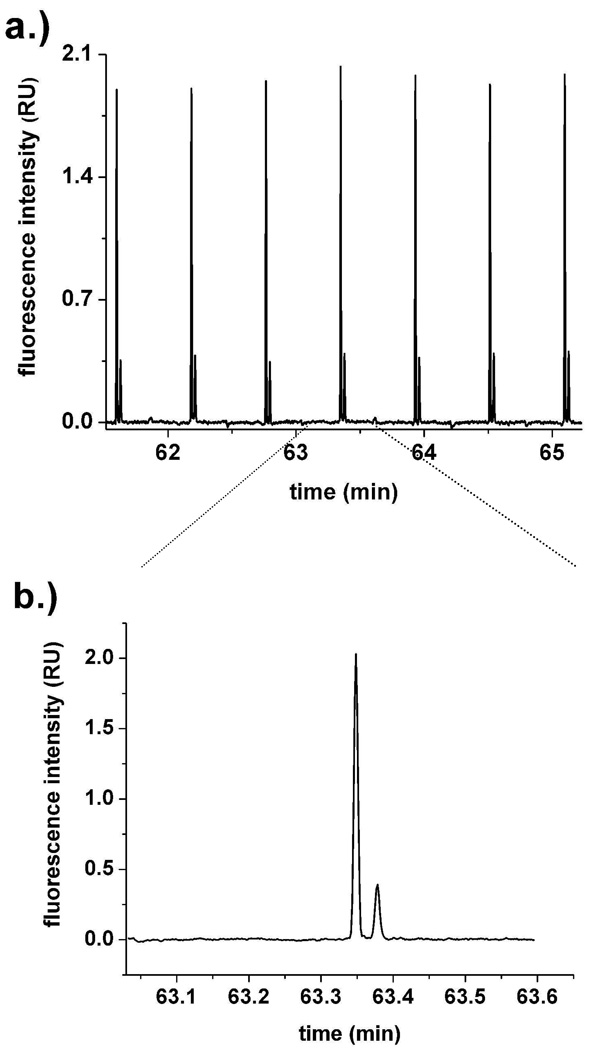
Separations of OG and FL were undertaken in order to better compare the SBM coatings’ performance with previous work and characterize variables related to reproducibility and sensitivity. OG and FL were loaded into one arm of a cross channel. A plug was then injected into the separation channel every 0.6 min and separated. PC-coated channels were first employed for a long-term study of peak reproducibility. Separations were repeated for 70 min. The average migration time obtained for OG and FL was 3.5 and 4.8 s with an RSD of 2.8 and 3.1% (n=32), respectively. When a time trace of 4 min was examined after 1 h of repeated separations (Fig. 3a), the peak heights of OG and FL were reproducible (2.1 and 4.4% RSD respectively, n=8). The separation efficiencies for 1 nM OG and FL (Table S1) were 611,000 ± 39,000 plates/m and 499,000 ± 46,000 plates/m respectively (n=3, similar results were obtained on 3 separate days, each with a different chip). The peak area variation for these traces was 5.6% RSD for OG and 2.5% RSD for FL (n=3). The peaks were well resolved with a resolution of 2.4 ± 0.1 (n=3, similar results were obtained on 3 chips) in 1.7 s.
Figure 3.
a.) Electrophoretic separations of 1 nM OG and FL in PC-coated channels. b.) Detail of Figure 3a showing OG (63.35 min) and FL (63.38 min) peaks.
For comparison, Fan and Harrison demonstrated an efficiency of 907,000 N/m for FL (50 µM) on a glass chip (30 × 30 µm channels) with a field strength of 625 V/cm 17. Chen and colleagues electrophoresed FL (10 nM) with a field strength of 149 V/cm to obtain an efficiency of 185,000 N/m with 4% RSD using PDMS channels (70 × 40 µm) coated with poly(dimethylacrylamide) with a separation length of 1.3 cm.21 The electrophoretic efficiency of FL on the SBM-coated PDMS/glass surfaces was comparable to that on glass and superior to prior results on semi permanently-coated PDMS devices. Many reports of separations on PDMS required µM-analyte concentrations to compensate for sample loss, decreased separation performance and high background signal associated with non-specific adsorption22, 23. In contrast, OG and FL at concentrations as low as 63 pM were readily separated and detected with a S/N of 3 on PC-coated channels. Since the injection volume was 30 pL, the detection limit for these two fluorophores was 1000 molecules. These detection limits are more than sufficient for the detection of many analytes in a single mammalian cell.
FL and OG (1 nM) were also separated on chips coated with PCPG and ePC50-SBMs (Table S1). The separations on PCPG-coated channels took longer (8 s) and yielded slightly lower peak efficiencies (183,000 ± 37,000 plates/m and 312,000 ± 54,000 for FL and OG, respectively) with a resolution of 1.7 ± 0.1 (n=3, similar results were obtained for two chips). The migration time reproducibility for FL and OG separations with PCPG coated devices was 1.1% and 1.5% RSD (n=3, similar results for two chips). In comparison, separations on EPC50-modified surfaces possessed a better resolution (R=2.7 ± 0.1) but were less efficient, yielding only 88,000 ± 5,000 plates/m for OG and 111,000 ± 16,000 plates/m for FL with a 1.0% (OG) and 2.0% (FL) RSD for the migration time (n=3). It was surprising that ePC retention times were longer than those for PC (2.1 s vs. 1.3 s for OG) given that the µeo of ePC50-coated channels (towards the detector) should have increased the overall velocity of the analytes. Since peak efficiencies for both analytes were reduced, it is possible that the electrostatic attraction of the dyes to the positive ePC headgroups may have caused increased surface interaction, decreasing the net analyte migration velocity.
Separation of Peptide Substrates of Kinases
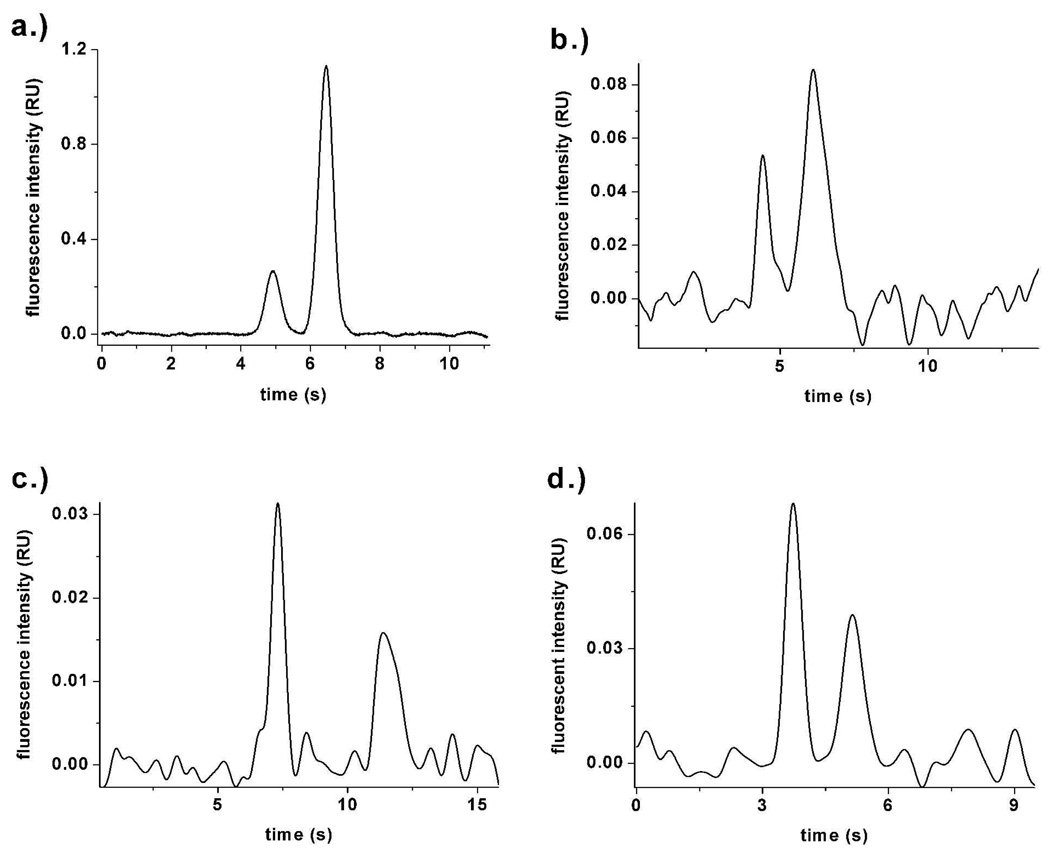
Peptide substrates are useful to probe kinase signaling pathways and may be helpful in the understanding and diagnosis of diseases such as cancer.24, 25 In this work, two fluorescent peptide substrates were tested on the SBM coated-PDMS microchips. The first, F-PKB, is a FL-labeled substrate for protein kinase B, a serine/threonine kinase that regulates cell proliferation and death. F-PKB and phosphorylated F-PKB (pF-PKB) were separated in PC and PCPG SBM-coated channels. In PC-coated channels, 239,000 ± 83,000 plates/m and 341,000 ± 28,000 plates/m were achieved for F-PKB (5 nM) and pF-PKB (10 nM), respectively, with a resolution of 1.7 ± 0.1 and a migration time RSD of 5.3% (F-PKB) and 6.3% (pF-PKB) (n=3, similar results obtained on 2 chips). In PCPG channels, a similar performance of 219,435 ± 56,000 and 327,582 ± 31,000 plates/m was obtained for F-PKB (50 nM) and pF-PKB (100 nM), respectively (Fig. 4a) with excellent migration time reproducibility, RSD=1.2% (F-PKB) and 1.7% (pF-PKB), and resolution (1.9 ± 0.1) (n=3, similar results obtained for two sets of experiments). The separation performance obtained for these protein kinase B substrates was comparable to that reported for similar peptides separated on a PDMS chip coated using two-step photografting of acrylic acid and PEG.26 However, the concentrations used here were three orders of magnitude lower.
Figure 4.
a.) Separation of 50 nM F-PKB (4.83 s) and 100 nM pF-PKB (6.29 s) in PCPG-coated microchannels. b.) Separation of 400 pM pF-ABL (4.39 s) and 800 pM F-ABL (6.12 s) in PC-coated microchannels. c.) Separation of 340 pM streptavidin-AlexaFluor488 (7.29 s) and 588 pM avidin-FITC (11.40 s) in PC-coated microchannels. d.) Separation of GFP-CRAKL (1000× diln of cell lysate, 3.74 s) and 115 pM eGFP (5.16 s) in PC-coated microchannels.
A second test substrate, F-ABL, is a substrate for the BCR-ABL kinase, the mutant fusion kinase that is the proximate cause of chronic myelogenous leukemia27 and may be of utility in monitoring the activity of BCR-ABL.28 Phosphorylated F-ABL (pF-ABL) and F-ABL were separated in PC-coated devices (Fig. 4b) with efficiencies of 365,000 ± 55,000 plates/m and 183,000 ± 34,000 plates/m, respectively. The resolution of the two analytes was 1.7 ± 0.1 and migration time RSD was 3.7% for pF-ABL and 4.5% for F-ABL (n=3, similar results for two devices). Separations on PCPG devices yielded similar efficiencies (Table S1) with better migration time reproducibility (0.1% for both analytes, n=3, similar results obtained on two devices). Although the F-ABL peptide separation has not been performed in a disposable PDMS device, similar size peptides have been separated in capillaries.29 The separation efficiency obtained for F-ABL in this report is about half that obtained on a capillary. When comparing chip-based electrophoresis with CE, although lower absolute efficiencies are obtained, it is important to consider the advantage of speed. Separation of similar sized peptides took about 100× longer30 than the times utilized on the SBM-coated microchips tested in this report. Relative to capillaries, the SBM-coated devices have unique utility for high throughput applications such as for separations of single cell contents.
To date a major challenge in the separation of the contents of single cells on microfluidic devices has been the need to exchange the high-salt physiologic cell buffer (in which the cells are maintained prior to lysis) for a low-salt conventional electrophoretic buffer.31 A switch to electrophoretic buffer is required to achieve adequate separation of the lysed cell’s contents.32 To determine whether it might be possible to separate F-ABL and pF-ABL in a high-salt, physiologic buffer, these analytes dissolved in ECB (145 mM salt, pH 7.4) were loaded into a microdevice coated with PC also possessing the same buffer (Fig. 5). The migration order of F-ABl and pF-ABL in ECB/glucose was reversed from the order observed in regular Tris-sep buffer. It is possible that this effect was due to the divalent cations (Mg2+, Ca2+) in the ECB buffer which might have neutralized the phosphate group of pF-ABL, making it less negatively charged. Both the divalent cations in ECB and its higher ionic strength may have also contributed to reduced electroosmotic flow in the PC-coated channels. The separation efficiency was 138,000 ± 13,000 plates/m for F-ABL (4 nM) and 135,000 ± 35,000 plates/m for pF-ABL (8 nM). Near-baseline resolution (1.5 ± 0.1) was achieved in under 10 s with an acceptable migration time RSD of 3.6 and 5.2% for F-ABL and pF-ABL, respectively (n=3, similar results obtained on two devices). Prior separations of peptides in the same buffer system on capillaries yielded poorer efficiencies and reproducibilities with peak tailing and unstable migration times.33 The SBM-coated chips achieve efficiencies similar to those shown previously by this group in PDMA coated capillaries for eight similar-sized peptides in an extracellular buffer (ECB), where 43,000–598,000 plates/m were achieved.34
Figure 5.
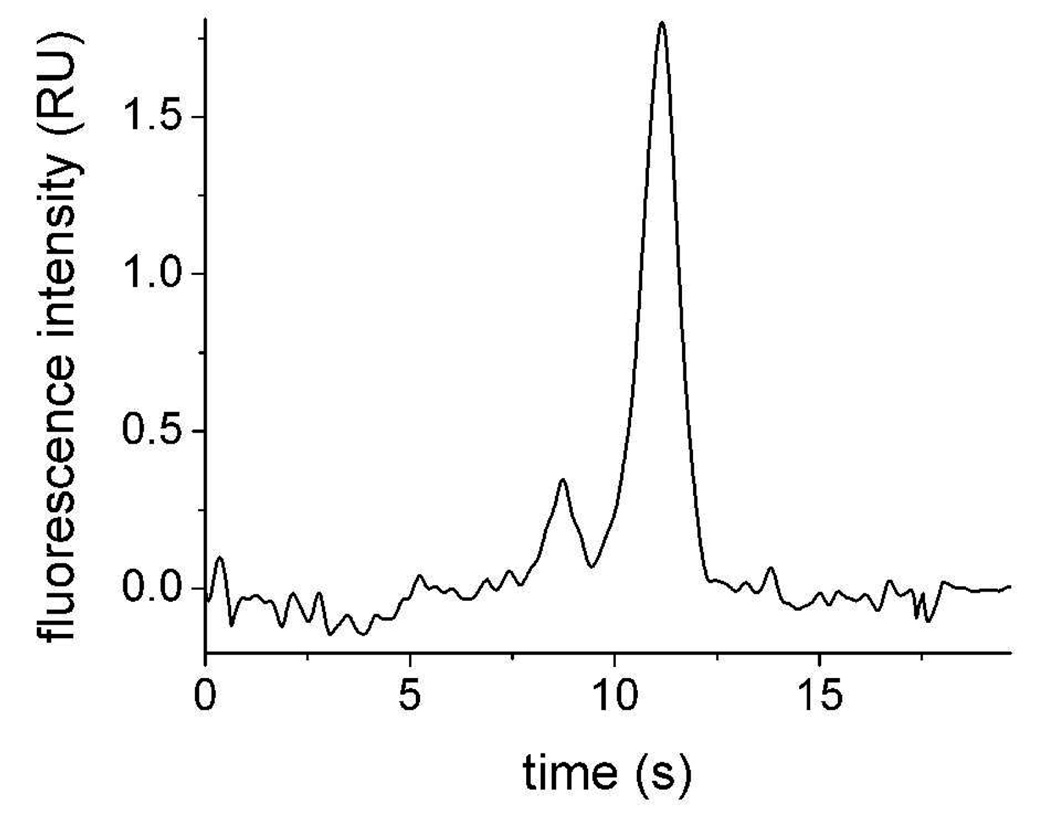
Separation of 8 nM F-ABL (8.70 s), and 16 nM pF-ABL (11.12 s) in a high-salt, physiologic buffer (ECB/glucose).
Separation of Proteins in SBM-Coated Microchannels
The separation of proteins on PDMS is especially challenging due to its hydrophobic surface which efficiently adsorbs many protein analytes. SBM-coated channels should reduce protein adsorption on the channel walls. Initially, a model protein system avidin-FITC (69 kDa, pI 10.5), known to adsorb on bare PDMS6, was separated from streptavidin-AlexaFluor488 (53 kDa), which lacks extensive glycosylation and has a more acidic pI of 5 (Fig. 4c). Both proteins were separated in PC-coated channels within 10 s with 272,000 ± 39,000 plates/m for streptavidin (340 pM) and 155,000 ± 40,000 plates/m for avidin (590 pM). Streptavidin eluted first with a higher efficiency, likely due to its more negative charge. The resolution for these two analytes was 3.1 ± 0.3 with a migration time RSD of 3.4% and 2.8% for streptavidin and avidin, respectively (n=3, similar results for two devices) (Table S2). For comparison, in silica capillaries with covalently linked tri(ethylene glycol)-terminated SAMs, efficiencies of 190,000–290,000 plates/m were obtained for protein separations.35
To test the new SBM-coated chips with an alternative protein substrate, a fusion protein (63 kDa) of eGFP and Crkl (eGFP-Crkl) was separated from eGFP on PC-coated devices. Crkl is a 39 kDa protein that is phosphorylated by BCR-ABL.36 eGFP-Crkl was expressed in a human erythroleukemia cell line (K562) and a cell lysate containing the eGFP-Crkl was utilized in these experiments. On PC-coated channels, eGFP-Crkl eluted in 3.49 ± 0.31s with an efficiency of 278,000 ± 64,000 plates/m. eGFP (115 pM), which eluted at 4.87 ± 0.32 s, possessed an efficiency of 358,000 ± 6,000 plates/m. When co-injected, the two analytes were separated with a resolution of 2.1 ± 0.3 (Fig. 4d). The higher efficiencies obtained for eGFP/eGFP-Crakl demonstrated that high quality protein separations can be obtained on the membrane-coated PDMS/glass devices. This separation performance is noteworthy as there have been limited reports of protein separations using zone electrophoresis on polymeric microchips37–39. No significant increase in the baseline was observed during repeated separations on the same PC-coated chip with the proteins suggesting that the analytes were not adsorbed onto the channel walls over time. In addition since the migration time and efficiency of eGFP-Crkl were reproducible, it is unlikely that substantial amounts of cell debris from the cell lysate adsorbed onto the SBM-coated channel walls.
Conclusion
The characterization of SBMs for microchip electrophoresis using µeo monitoring and fluorescent dyes demonstrated that these coatings are robust yet simple and thus well-suited for inexpensive and disposable polymer microchips. Since SBMs can stably coat both glass and PDMS, they allow the use of hybrid PDMS/glass devices, while many other coatings require homogenous base materials for all parts of the channel. The separations in this report illustrate the wide variety of bioanalytes, including small molecules, peptides, and proteins that can be separated on SBM-coated PDMS/glass devices. Modification of the SBM scaffold created during self-assembly should yield tailorable surface properties since a large library of lipids and lipid-tagged substrates exist. Although the efficiencies measured here were not as high as model separations in capillaries, the ability to separate analytes in physiologic buffers in less than 10 s is ideal for point of care analysis or high-throughput applications such as the analysis of contents from single cells. The high detection sensitivity achieved here (1000 molecules) should facilitiate analysis of fluorescent substrates from cells that are lysed by physical or chemical methods in PDMS microchips. Towards this goal, the demonstration of fully resolved F-ABL peptide separations in biologic buffer is an important step in eliminating the need for separate cell-compatible and separation buffers. Moreover, the separation of eGFP from the fusion protein eGFP-Crakl in a cell lysate demonstrated that the SBM-coated microchips worked well with diluted cell contents and may have future potential for larger, higher m/z protein kinase substrates that are more challenging to separate from their phosphorylated isoforms.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (CA126258 to KSP, EB004436 to NLA, CA105514 to CES). Thanks to Avanti Lipids for lipid structure images and Joey Taylor, Wei Xu, Hsuan-Hong Lai and Alex Fernandez-Villa for assistance.
REFERENCES
- 1.Cunliffe JM, Baryla NE, Lucy CA. Analytical Chemistry. 2002;74:776–783. doi: 10.1021/ac015627u. [DOI] [PubMed] [Google Scholar]
- 2.Wang CZ, Lucy CA. Analytical Chemistry. 2005;77:2015–2021. doi: 10.1021/ac0489622. [DOI] [PubMed] [Google Scholar]
- 3.Mansfield E, Ross EE, Aspinwall CA. Analytical Chemistry. 2007;79:3135–3141. doi: 10.1021/ac0618829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross EE, Bondurant B, Spratt T, Conboy JC, O'Brien DF, Saavedra SS. Langmuir. 2001;17:2305–2307. [Google Scholar]
- 5.Mao HB, Yang TL, Cremer PS. Analytical Chemistry. 2002;74:379–385. doi: 10.1021/ac010822u. [DOI] [PubMed] [Google Scholar]
- 6.Phillips KS, Cheng Q. Anal. Chem. 2005;77:327–334. doi: 10.1021/ac049356+. [DOI] [PubMed] [Google Scholar]
- 7.Phillips KS, Dong Y, Carter D, Cheng Q. Anal. Chem. 2005;77:2960–2965. doi: 10.1021/ac0500481. [DOI] [PubMed] [Google Scholar]
- 8.Holden MA, Jung SY, Yang TL, Castellana ET, Cremer PS. Journal of the American Chemical Society. 2004;126:6512–6513. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, Phillips KS, Cheng Q. Lab on a Chip. 2006;6:675–681. doi: 10.1039/b514902a. [DOI] [PubMed] [Google Scholar]
- 10.Daniel S, Albertorio F, Cremer PS. Mrs Bulletin. 2006;31:536–540. [Google Scholar]
- 11.Castellana ET, Cremer PS. Surface Science Reports. 2006;61:429–444. doi: 10.1016/j.surfrep.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker GM, Zeringue HC, Beebe DJ. Lab on a Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 13.Stroumpoulis D, Zhang HN, Rubalcava L, Gliem J, Tirrell M. Langmuir. 2007;23:3849–3856. doi: 10.1021/la062375p. [DOI] [PubMed] [Google Scholar]
- 14.Malmsten M. Journal of Colloid and Interface Science. 1995;172:106–115. [Google Scholar]
- 15.Ren XQ, Bachman M, Sims C, Li GP, Allbritton N. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2001;762:117–125. doi: 10.1016/s0378-4347(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 16.Huang XH, Gordon MJ, Zare RN. Analytical Chemistry. 1988;60:1837–1838. [Google Scholar]
- 17.Fan ZH, Harrison DJ. Analytical Chemistry. 1994;66:177–184. [Google Scholar]
- 18.Lacher NA, de Rooij NF, Verpoorte E, Lunte SM. Journal of Chromatography A. 2003;1004:225–235. doi: 10.1016/s0021-9673(03)00722-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Fanguy JC, Bledsoe JM, Henry CS. Analytical Chemistry. 2000;72:5939–5944. doi: 10.1021/ac000932l. [DOI] [PubMed] [Google Scholar]
- 20.Yassine MM, Lucy CA. Analytical Chemistry. 2004;76:2983–2990. doi: 10.1021/ac035372f. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Ren JC, Bi R, Chen D. Electrophoresis. 2004;25:914–921. doi: 10.1002/elps.200305766. [DOI] [PubMed] [Google Scholar]
- 22.Hu SW, Ren XQ, Bachman M, Sims CE, Li GP, Allbritton N. Analytical Chemistry. 2002;74:4117–4123. doi: 10.1021/ac025700w. [DOI] [PubMed] [Google Scholar]
- 23.Hu SW, Ren XQ, Bachman M, Sims CE, Li GP, Allbritton N. Electrophoresis. 2003;24:3679–3688. doi: 10.1002/elps.200305592. [DOI] [PubMed] [Google Scholar]
- 24.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 25.Weng G, Bhalla US, Iyengar R. Science. 1999;284:92–96. doi: 10.1126/science.284.5411.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu SW, Ren XQ, Bachman M, Sims CE, Li GP, Allbritton NL. Analytical Chemistry. 2004;76:1865–1870. doi: 10.1021/ac049937z. [DOI] [PubMed] [Google Scholar]
- 27.Hantschel O, Superti-Furga G. Nature Reviews Molecular Cell Biology. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 28.Goldman JM, Melo JV. N Engl J Med. 2003;349:1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 29.Dawson JF, Boland MP, Holmes CFB. Analytical Biochemistry. 1994;220:340–345. doi: 10.1006/abio.1994.1347. [DOI] [PubMed] [Google Scholar]
- 30.Gamble TN, Ramachandran C, Bateman KP. Analytical Chemistry. 1999;71:3469–3476. doi: 10.1021/ac990276t. [DOI] [PubMed] [Google Scholar]
- 31.Sims CE, Allbritton NL. Lab on a Chip. 2007;7:423–440. doi: 10.1039/b615235j. [DOI] [PubMed] [Google Scholar]
- 32.McClain MA, Culbertson CT, Jacobson SC, Allbritton NL, Sims CE, Ramsey JM. Analytical Chemistry. 2003;75:5646–5655. doi: 10.1021/ac0346510. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Wu HY, Wang Y, Sims CE, Allbritton NL. Journal of Chromatography B: Biomedical Sciences and Applications. 2001;757:79–88. doi: 10.1016/s0378-4347(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Hu SW, Li HN, Allbritton NL, Sims CE. Journal of Chromatography A. 2003;1004:61–70. doi: 10.1016/s0021-9673(03)00492-8. [DOI] [PubMed] [Google Scholar]
- 35.Meagher RJ, Seong J, Laibinis PE, Barron AE. Electrophoresis. 2004;25:405–414. doi: 10.1002/elps.200305714. [DOI] [PubMed] [Google Scholar]
- 36.Senechal K, Halpern J, Sawyers CL. Journal of Biological Chemistry. 1996;271:23255–23261. doi: 10.1074/jbc.271.38.23255. [DOI] [PubMed] [Google Scholar]
- 37.Xiao DQ, Van Le T, Wirth MJ. Analytical Chemistry. 2004;76:2055–2061. doi: 10.1021/ac035254s. [DOI] [PubMed] [Google Scholar]
- 38.Wu DP, Zhao BX, Dai ZP, Qin JH, Lin BC. Lab on a Chip. 2006;6:942–947. doi: 10.1039/b600765a. [DOI] [PubMed] [Google Scholar]
- 39.Luo YQ, Huang B, Wu H, Zare RN. Analytical Chemistry. 2006;78:4588–4592. doi: 10.1021/ac052274g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.