Abstract
A very simple and inexpensive method to detect oversulfated chondroitin sulfate (OSCS) or other high charge density polyanionic structures as contaminants in heparin products using potentiometric polyanion sensors is described. In the potentiometric measurement, a greater change in the phase boundary equilibrium potential of polymeric membranes formulated with tridodecylmethylammonium (TDMA) anion exchange sites is observed for the contaminated heparin than for the untainted heparin due to the higher charge density of OSCS or other impurities compared to that of porcine heparin. Detection of 0.5 wt% OSCS impurity is readily achieved using only 1 mg/mL of final polyion concentration. Even lower detection limits for OSCS contamination may be possible if higher final concentrations of heparin preparations can be employed in the test procedure.
Heparin is a highly-sulfated glycosaminoglycan that is widely used as an injectable anticoagulant.1-3 A variety of medical devices and diagnostic products may also contain or be coated with heparin. Recently, an acute, rapid onset of serious side effects indicative of an allergic type reaction (resulting in a large number of patient deaths) has been reported in connection with the use of certain lots of heparin. A specific contaminant, oversulfated chondroitin sulfate (OSCS), has been identified in given preparations that may have caused these adverse events.4, 5 Due to the similarity in chemical structure and anticoagulant activity that OSCS possesses relative to heparin, it is impossible for routine bioactivity assays6 to detect the presence of the OSCS species. More advanced analytical methods including nuclear magnetic resonance (NMR)5, 7 and capillary electrophoresis (CE)8 have been suggested for the detection of the OSCS contaminants.
It has been demonstrated previously that large, reproducible EMF responses toward polyanionic species can be achieved if polymeric ion-selective electrodes (ISEs) are formulated with lipophilic anion-exchangers, such as a tridodecylmethylammonium (TDMA) salt, in plasticized poly(vinyl chloride) (PVC) membranes.9-11 In the presence of relatively low concentrations of polyanions in the test solution, the surface of the polymer membrane becomes partially depleted of the small counter anion (i.e., chloride from the TDMA salt), and a gradient of the polyanion exists in aqueous layer adjacent to the polymer membrane and throughout the outermost layer of organic membrane (as an ion-pair with the TDMA species). Under such conditions, the phase boundary potential at the membrane sample/interface achieves a non-equilibrium pseudo steady-state value in proportion to the concentration of polyanion present in a background electrolyte solution.11 This potentiometric response toward the polyion is super-Nernstian, and changes significantly over a relatively narrow range of low polyanion concentrations. However, if higher concentrations of polyanion are added to a background electrolyte solution, the outer surface of the polymeric membrane achieves a relatively rapid equilibrium phase boundary potential change toward the polyion, and this equilibrium potential change can be expressed by the following equation:12
| (1) |
where ΔEMF represents the magnitude of the overall potential change after the polyanion is added, ST denotes the total concentration of the lipophilic anion exchanger (TDMA) and [R−] is the added/endogenous anionic site concentration in the membrane; aCl and apoly are the background chloride activity and polyanion (with charge z−) activity in the sample solution, respectively; kCl and kpoly are the partition coefficient between membrane and aqueous phases of chloride and polyanion, respectively; and β is the overall ion-pair formation constant in the membrane phase. Specifically, it has been shown previously12 that the magnitude of the equilibrium EMF change in response to high polyanion concentrations is related to the charge density of the polyanion since a stronger cooperative ion-pair can be formed between the extracted polyanion with higher charge density and the TDMA exchanger species in the polymeric film (greater value for β in equation 1).12 It should be noted that this equilibrium phase boundary potential change is essentially independent of polyion concentration, since when the outer surface of the ion-exchange membrane is at equilibrium, the classical Nernstian response to the polyion is observed, which given the very high charge for these species, yields very small EMF changes as function of polyion concentration (< 1 mV/decade for heparin).
Herein, we take advantage of the fundamental response principles of potentiometric polyanion sensors to demonstrate a simple and inexpensive method to detect the presence OSCS contamination in heparin products. Since OSCS has a greater charge density than heparin, a larger change in the equilibrium EMF response is observed when adequate levels of this contaminant is present compared to an untainted heparin preparation. Dextran sulfate (DS), another polysaccharide with higher charge density than porcine heparin, is also used as a model contaminant species to test the broad applicability of the proposed method to detect a variety of high charge density polyanions in commercial porcine heparin preparations.
Experimental Section
The polyanion sensitive membrane employed in the present work was formulated with 1.5 wt% tridodedylmethylammonium chloride (TDMAC) (Aldrich, St. Louis, MO), 32.5 wt% poly(vinyl chloride) (PVC) (Fluka, St. Louis, MO) and 66 wt% dioctylsebacate (DOS) (Fluka) as reported previously.10 Membrane components were dissolved in distilled tetrahydrofuran (THF) (Fisher Scientific, Pittsburgh, PA) at 100 mg per mL. To construct single-use disposable electrochemical sensors, sealed end glass capillary tubes (o.d. 0.8 – 1.1 mm) were inserted into short pieces of Tygon tubing (ca. 1 in. long, 1/16 in i.d., 1/8 in o.d.) with the sealed end protruding 3 - 4 mm beyond the end of the Tygon tubing. The protruding capillary end was dip-coated with the membrane cocktail 12 times, leaving 15 min between each dip-coating to allow for adequate solvent evaporation. The membranes were allowed to dry overnight. The sensors were soaked in 0.01 M phosphate buffered saline (PBS), pH 7.4, for approximately 1 h before the glass capillaries were carefully removed. The Tygon tubing with the sensing membrane at the tip was then filled with the same PBS, and a Ag/AgCl wire covered with heat shrink tubing on the top was inserted. A commercial miniaturized Ag/AgCl electrode (BASi, West Lafayette, IN) was used as the reference electrode.
Ten mg/mL and 50 mg/mL DS solutions were used as initial test samples, and then further diluted with 10 mg/mL and 50 mg/mL solution of pure porcine heparin (171 units/mg) (Aldrich) to obtain samples that have different DS contaminant content. DS was also obtained from Aldrich. An aliqout (400 µL) of these solutions was added to 3600 µL of PBS, (with the polyanion sensor and reference electrode in place within this well-stirred PBS solution) to yield a final polyanion concentration of 1 mg/mL and 5 mg/mL, respectively. ∆EMFs were obtained by subtracting the starting background cell potential in PBS alone from the potential value recorded 10 min after the injection of polyanion solutions OSCS was synthesized according to a previously reported procedure starting with sodium salt of chondroitin sulfate (CS) obtained from Aldrich.7 A 10 mg/mL solution (in PBS) containing the newly synthesized OSCS was mixed with pure porcine heparin to yield different levels of OSCS contaminated heparin samples and then tested with the polyanion sensors as above for DS contamination in heparin (except for 0.5 wt% OSCS in heparin at a final polyanion concentration of 1 mg/mL, the EMF change after 15 min was recorded). In addition, a 10 mg/mL reference sample of contaminated heparin known to have > 10 wt% OSCS was obtained from U.S. Pharmacopeia (USP) (Rockville, MD) and examined by the proposed method. It should be noted that the lower limit of detection for the proposed method is based on the ability to discriminate (based on EMF change) a given wt% of polyanion contaminant in the presence of a large excess of heparin.
Results and Discussion
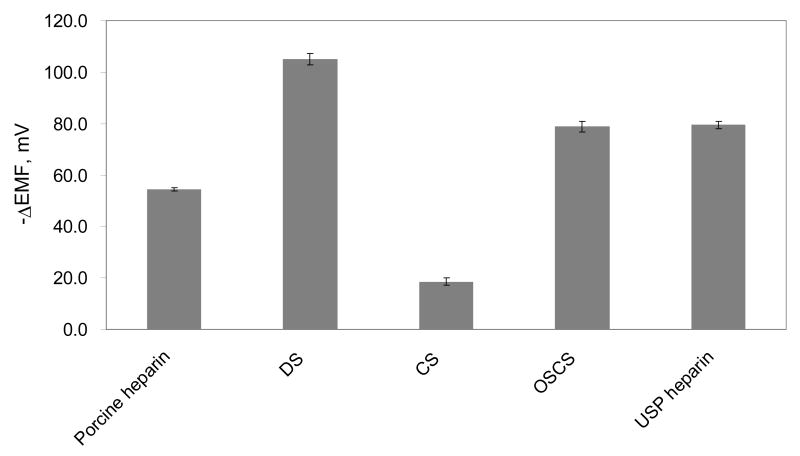
As shown in Figure 1, large differences in the equilibrium EMF responses toward porcine heparin, DS, CS, OSCS, and USP contaminated heparin are observed when added to PBS, with each species at a final total concentration of 1 mg/mL. Pure OSCS or USP contaminated heparin yield EMF changes of ca. 25 mV more negative than pure porcine heparin, clearly suggesting that the polyanion sensors can be used as a screening tool to detect the presence of OSCS in biomedical heparin preparations. Table 1 further summarizes the EMF responses toward polyanion mixtures with different ratios of OSCS to porcine heparin. The overall EMF response of the polyanion sensor is very rapid, usually within 30s at relatively high OSCS contaminant content. For low OSCS content (< 1 wt%), the potential first drops quickly to the equilibrium EMF change typically observed for pure heparin, and then drifts down slowly toward the value observed for the pure OSCS or the highly contaminated samples (see Figure 2). Indeed, when the contaminant polyanion level is lower, the amount of this species present is in a concentration range where the surface of the membrane does not achieve full equilibrium EMF response quickly.
Figure 1.
Equilibrium EMF response changes of PVC membranes doped with TDMAC toward various polyanion preparations at a final total polyanion concentration of 1 mg/mL. The standard deviations were calculated using data collected from 4 different tests with fresh sensors for each polyanion preparation reported.
Table 1. Potentiometric response of PVC membranes doped with TDMAC toward polyanion samples with different ratios of OSCS and porcine heparin a.
| OSCS wt% in polyanion preparations | Avg. b | EMF (mV)SD (mV) c |
|---|---|---|
| 100 | -78.8 | 2.2 |
| 20 | -78.8 | 1.3 |
| 10 | -78.2 | 1.8 |
| 1.0 | -74.7 | 1.5 |
| 0.75 | -72.8 | 1.8 |
| 0.50 | -72.7 | 1.1 |
| 0 | -54.4 | 0.7 |
. The final polyanion concentration is 1 mg/mL.
. EMF values were recorded 10 min after the injection of polyanion solutions
. The standard deviations were calculated using data collected from 4 sensors for each concentration.
Figure 2.
Response time trace of PVC membranes doped with TDMAC after injection of heparin/polyion preparations (at 1 mg/mL) possessing different degrees of OSCS contamination (5 wt%, 1 wt%, and 0.5 wt%). For comparison, the response to pure porcine heparin at a final polyanion concentration of 1 mg/mL is also shown.
Very similar behavior was found for samples of heparin spiked to contain DS at varying wt% (see Table 1S in Supplemental Information). Based on results obtained from DS measurements (Table 1S), a lower percentage of DS contaminant in the total polyanion preparation can be detected for 5 mg/mL final polyanion concentration than for the 1 mg/mL final polyanion concentration (Table 1S (B)). This implies that if a more concentrated contaminated heparin is used as the initial test sample, a lower weight percentage of the contaminant polyanion may be detected (i.e., an improved detection limit for contaminant wt% in the heparin). It should be noted that semi-quantitative concentration data for the contaminant can only be obtained when the total polyanion concentration in the final test solution is adjusted to be in a range where the contaminant concentration is low enough not to achieve a rapid equilibrium phase boundary potential change at the membrane/sample interface. Indeed, under these conditions the rate of EMF change of the polyanion sensor toward the equilibrium EMF response for the OSCS species is proportional to OSCS concentration.
Conclusion
The very simple methodology described here using disposable potentiometric polyanion sensors exhibits reproducible responses to high charge density polyanion contaminants such as OSCS and DS in heparin preparations. The procedure could be used as an inexpensive screening method to assess raw materials or finalized biomedical grade heparin products. The test samples can be used directly without complicated pretreatment, although predialysis of the samples vs. PBS may be necessary in rare instances if there is chance that small lipophilic anions (e.g., perchlorate, thiocyanate) or organic anions (e.g., salicylate) could be present in the given sample preparation. The presence of such anions at high concentrations would also cause significant negative EMF response of the TDMA-based polymeric membrane owing to the favorable extraction thermodynamics of such anions into the organic membrane phase of the working electrode. However, in this work, and in all previous studies in which these same membrane electrodes have been examined for response to commercial heparin preparations, no such small anion interferences have been observed. Detailed studies to develop a more quantitative test procedure for OSCS contaminant levels based on the dynamics of the observed EMF responses are now ongoing in this laboratory.
Supplementary Material
Chemical structures of heparin, OSCS, and DS. Detailed potentiometric response data of the polyanion sensors toward mixtures with different content of DS and porcine heparin at different final total polyanion concentrations. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Institutes of Health for supporting this research via grant # EB-000784.
References
- 1.Capila I, Linhardt RJ. Angew Chem Int Edn Engl. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Lepor NE. Rev Cardiovasc Med. 2007;8:S9–S17. [PubMed] [Google Scholar]
- 3.Fischer KG. Hemodial Int. 2007;11:178–189. doi: 10.1111/j.1542-4758.2007.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao GL, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, DalPan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang ZQ, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Nat Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein MD, Drongowski RA, Linhardt RJ, Langer RS. Anal Biochem. 1982;124:59–64. doi: 10.1016/0003-2697(82)90219-6. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama T, Toida T, Imanari T, Yu GY, Linhardt RJ. Carbohydr Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 8.Volpi N. Carbohydr Res. 1993;247:263–278. doi: 10.1016/0008-6215(93)84259-9. [DOI] [PubMed] [Google Scholar]
- 9.Ma SC, Yang VC, Fu B, Meyerhoff ME. Anal Chem. 1993;65:2078–2084. doi: 10.1021/ac00063a024. [DOI] [PubMed] [Google Scholar]
- 10.Fu B, Bakker E, Yun JH, Yang VC, Meyerhoff ME. Anal Chem. 1994;66:2250–2259. doi: 10.1021/ac00086a009. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhoff ME, Fu B, Bakker E, Yun JH, Yang VC. Anal Chem. 1996;68:A168–A175. doi: 10.1021/ac9618536. [DOI] [PubMed] [Google Scholar]
- 12.Fu B, Bakker E, Yang VC, Meyerhoff ME. Macromolecules. 1995;28:5834–5840. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical structures of heparin, OSCS, and DS. Detailed potentiometric response data of the polyanion sensors toward mixtures with different content of DS and porcine heparin at different final total polyanion concentrations. This material is available free of charge via the Internet at http://pubs.acs.org.