Abstract
The p75 neurotrophin receptor has been implicated in the regulation of multiple cellular functions that differ depending on the cell context. We have observed that p75NTR is strongly induced on astrocytes as well as neurons in the hippocampal CA3 region after seizures, however the function of this receptor on these glial cells has not been defined. We have employed a primary culture system to investigate the effects of neurotrophins on astrocytes. Treatment of hippocampal astrocytes with NGF caused a reduction in cell number, but did not elicit an apoptotic response, in contrast to hippocampal neurons. Instead, activation of p75NTR by NGF attenuated proliferation induced by mitogens such as EGF or serum. These studies demonstrate the cell type specificity of neurotrophin functions in the brain.
Keywords: NGF, seizures, cell cycle
Introduction
The p75 neurotrophin receptor (p75NTR) can participate in mediating multiple functions depending on the cellular context. In neurons, these functions range from facilitation of cell survival to induction of apoptosis and regulation of axonal growth. This multiplicity of function apparently depends on the ability to interact with different co-receptors to respond to different ligands (Barker 2004; Chao 2003; Hempstead 2002; Roux and Barker 2002). p75NTR can participate with Trk receptors in mediating survival responses to neurotrophins (Hempstead et al. 1991), however, this receptor can paradoxically also mediate cell death in neurons (Cotrina et al. 2000; Friedman 2000; Rabizadeh et al. 1993; Volosin et al. 2006) and oligodendrocytes (Beattie et al. 2002; Casaccia-Bonnefil et al. 1996). While neurotrophins such as NGF can induce p75NTR-mediated apoptosis (Cotrina et al. 2000; Friedman 2000), proneurotrophins are more effective ligands due to their concomitant binding to sortilin as a co-receptor with p75NTR (Nykjaer et al. 2004). The p75NTR is widely expressed in many neuronal populations in the CNS during development and after injury. However this receptor is also expressed on astrocytes, both in vitro (Hutton et al. 1992; Semkova and Krieglstein 1999) and in vivo (Rudge et al. 1994), especially after injury (Hanbury et al. 2002). Interestingly, NGF is also induced in astrocytes after injury (Goss et al. 1998; Oderfeld-Nowak and Bacia 1994) or during disease in vivo (Micera et al. 1998), and by inflammatory cytokines in vitro (Carman-Krzan et al. 1991; Friedman et al. 1996; Gadient et al. 1990). The expression of a receptor for NGF on astrocytes suggests that astrocyte-derived NGF may have an autocrine or paracrine effect on these glial cells, as well as influencing neuronal function. We have previously demonstrated that p75NTR is induced by seizures in hippocampal neurons, and mediates their apoptosis (Troy et al. 2002). In this seizure model, we have observed that p75NTR is also induced on astrocytes in the hippocampus, however it does not appear to mediate apoptosis in these cells. Our studies suggest that NGF has an inhibitory effect on astrocyte proliferation mediated by p75NTR.
Materials and Methods
Pilocarpine-Induced Seizures
Male Sprague-Dawley rats (250–275 g) were pre-treated for 0.5 hr with methyl-scopolamine (1 mg/kg, subcutaneous, Sigma) and then treated with pilocarpine hydrochloride (350 mg/kg, i. p., Sigma). After 1 hr of status epilepticus, rats were treated with diazepam (10 mg/kg, Abbott Labs) and phenytoin (50 mg/kg, Sigma) to stop seizure activity. Additional diazepam was administered as necessary to prevent further seizures. Control animals received all the same treatments except they were injected with saline instead of pilocarpine. During recovery the animals were treated with Hartman’s solution (130 mM NaCl, 4 mM KCl, 3 mM CaCl, 28 mM lactate; 1 ml/100 g) injected subcutaneously twice daily until the animals were capable of eating and drinking freely. Seven days later animals were anesthetized with ketamine hydrochloride (30 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with saline followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde for 1 hour, cryoprotected in 30% sucrose for 3 days, then snap frozen and kept at −80°C. All animal studies were conducted using the NIH guidelines for the ethical treatment of animals with approval of the Rutgers Animal Care and Facilities Committee.
Immunocytochemistry
Brain sections (12 μm) were cut on a cryostat (Leica) and mounted onto charged slides. Sections were blocked in PBS/10% goat serum and permeabilized with PBS/0.3%Triton X-100, then exposed to anti-GFAP (Roche) and anti-p75NTR (9651, generously provided by Moses Chao, NYU, or 07-476, Upstate, Lake Placid, NY) overnight at 4°C in PBS/0.3% triton. Slides were then washed 3x in PBS, exposed for 1 hr at room temp to secondary antibodies coupled to Alexa 488 and 594 fluorophores. Sections were coverslipped with anti-fading medium (ProLong Gold, Invitrogen, Oregon, USA) and analyzed by fluorescence microscopy (Nikon).
Astrocyte cultures
Pregnant rats were sacrificed by exposure to CO2 and soaked in 80% ethanol for 10 minutes. Embryonic day 21 (E21) fetuses were removed under sterile conditions and kept in PBS on ice. Hippocampi were dissected, dissociated by trituration, and plated on poly-D-lysine-coated flasks in NM15 medium (Eagle’s MEM with earle’s salts and 2mM L-glutamine, 15% heat-inactivated fetal bovine serum, 6mg/ml glucose, 0.5 μg/ml penicillin and 0.5 U/ml streptomycin). Astrocytes were grown to confluence. Flasks were shaken at 450 rpm for 10 minutes to remove neurons and microglia, fresh medium was added, and the flasks were returned to the incubator for at least 2 hr. The flasks were then shaken overnight at 225 rpm to remove type 2 astrocyes and oligodendrocytes. The confluent astrocytes were exposed to cytosine arabinboside (0.1mM) for 3 days to eliminate any remaining non-astrocyte cell populations. Finally, the astrocytes were trypsinized and replated onto poly-D-lysine-coated dishes at subconfluent density.
Western blot
After 48 hours of serum starvation, astrocytes were treated with EGF (100ng/ml), NGF (100ng/ml) or both. Regulation of p75 and TrkA expression was examined after 24 hr of treatment, and induction of P-Erk and P-Akt was examined after 20 min of treatment. Cells were then harvested using lysis buffer containing 120mM Tris pH 6.8, 2% SDS, and 10% glycerol. Equal amount of protein samples were run on SDS gels and transferred to nitrocellulose membrane. The blots were blocked in 5% non-fat milk in TBST for 1 hour, and incubated with primary antibody (1: 1000 in milk) overnight at 4°C. The primary antibodies were anti-phospho-ERK, anti-ERK, anti-phospho-Akt, anti-Akt (Cell Signaling Technologies), anti-TrkA (generously provided by Louis Reichardt, UCSF), anti-p75 (9651, generously provided by Moses Chao). The blots were washed with TBST, and incubated with secondary antibody for 1 hour at room temperature. The membranes were developed using either the ECL kit (Pierce) or Odyssey infrared imaging system (LICOR Bioscience).
Reverse transcription–polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen). One μg of RNA was reverse transcribed at 42°C for 2 h in a 20 μl reaction mixture using SuperScript™ II Reverse Transcriptase (Invitrogen Carlsbad, CA). PCR reactions were performed in a 25μl volume with Go Taq Green Master Mix (Promega) and 0.8 μM of TrkA primers (forward: 5′-GGCTGAGGTCTCTGTCCAAG-3′, reverse: 5′-AGGAGGGCAGAAAGGAAGAG-3′) or β-actin (forward: 5′-TCATGAAGTGTGACGTTGACATCCGT-3′, reverse: 5′-CTTAGAAGCATTTGCGGTGCACGATG-3′). PCR products were resolved on 0.9% agarose gels and stained with ethidium bromide.
Cell counts
Astrocytes were plated in NM15 overnight, washed with PBS twice and changed to serum free medium consisting of a 1:1 mixture of Eagle’s MEM and Ham’s F12 supplemented with glucose (6 mg/ml), putrescine (60 μM), progesterone (20 nM), transferrin (100 μg/ml), selenium (30 nM), penicillin (0.5 U/ml) and streptomycin (0.5 μg/ml). After 48 hours of serum starvation, fresh NM15 or SFM containing EGF (100ng/ml) was added back to the cells with or without NGF (100ng/ml) for 48 and 72 hours. When cells were treated with anti-p75 (9651, 1:1000) or K252a (200 nM), these were added 15 min before NGF. At the end of each time point, cells were lysed and intact cell nuclei were counted with a hemacytometer, a quantification method we have used previously (Farinelli et al. 1998; Friedman 2000; Volosin et al. 2006). Nuclei of dead or dying cells are irregularly shaped or disintegrated. In contrast, nuclei of healthy cells are phase bright and have clearly defined limiting membranes. Cell counts were performed in triplicate wells.
Apoptosis assay
Astrocyte apoptosis was analyzed using Annexin V-FITC apoptosis detection kit I™ (BD Pharmingen) as recommended by the manufacturer. Briefly, after treatment, astrocytes were trypsinized and washed in PBS buffer. 1×105 cells were incubated with 5 μl annexin V-FITC and 5 μl propidium iodide for 15 minutes in the dark. 400 μl binding buffer was added at the end of staining, and cells were then analyzed by FACScan flow cytometry within one hour of staining.
Astrocyte proliferation
Astrocytes were plated at 5 × 105 cells/well in 12-well plates in NM15. After one day, cells were washed with PBS twice and changed to serum free medium for 48 hours. After 2 days serum starvation, mitogens were added with or without NGF and BrdU (10 μM) for 24 hours. Cells were then fixed in 4% PFA for 30 minutes, washed 3X with PBS, blocked in 10% goat serum/0.3% triton for 30 minutes, and incubated with anti-BrdU (Upstate, 1:500) overnight at 4°C. Cells were then washed with TBS, and incubated with secondary antibody (goat anti mouse, Alexa 594) for 1 hour in the dark at room temperature. After three washes with TBS, Hoechst 33342 dye or DAPI (1 μg/ml, Sigma) was added and cells were visualized by flurorescence microscopy.
To identify a potent mitogen for astrocytes, after serum starvation astrocytes were changed to fresh SFM and treated with CNTF (40ng/ml), EGF (100ng/ml) or PDGF (10ng/ml) for 48 hours. BrdU (10 μM) was added during the last 24 hours of treatment. Cells were fixed for immunostaining.
The proliferation rate was quantified as follows: three 10X fields were counted per well. Two wells were used per treatment and each experiment was performed in triplicate. The results are expressed as a percentage of BrdU positive cells/DAPI normalized to control values. Statistical significance was determined by ANOVA followed by Newmann-Keuls post hoc analysis.
Results
p75NTR is induced on hippocampal astrocytes in vivo after seizure and in vitro
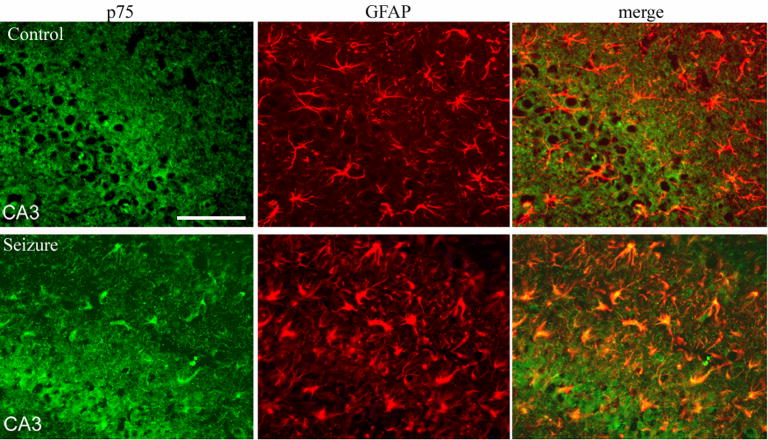
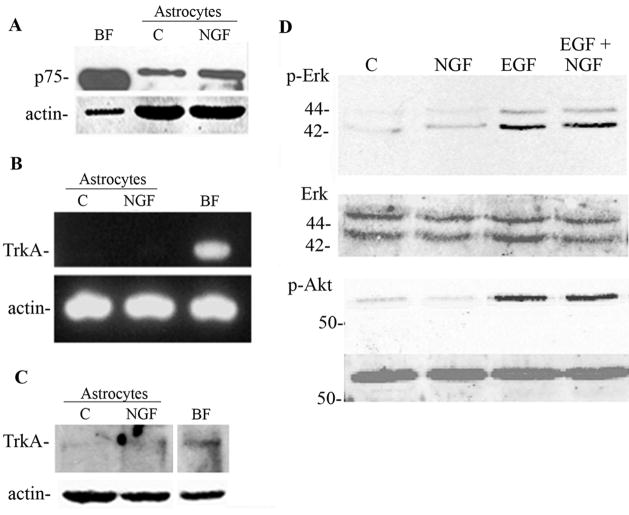
Pilocarpine-induced seizures have been previously shown to elicit expression of p75NTR on hippocampal neurons (Roux et al. 1999) and mediate their apoptosis (Troy et al. 2002). We have observed that these seizures resulted in the induction of p75NTR on astrocytes in the hippocampus as well (Figure 1). The induction of p75NTR was detected on mature astrocytes expressing GFAP, but was not co-localized with markers of progenitors such as nestin or vimentin. Since the role of p75NTR in astrocyte function is unknown, we employed a primary culture system to investigate the possible effects of neurotrophins on astrocyte function. NGF is known to be produced by astrocytes under inflammatory conditions both in vivo (Oderfeld-Nowak and Bacia 1994) and in vitro (Friedman et al. 1996), therefore we investigated which NGF receptors were expressed on astrocytes in vitro. NGF is known to bind two different receptors, the p75NTR and the TrkA receptor tyrosine kinase. Western blot analysis demonstrated that p75NTR was expressed at low levels and was upregulated by NGF treatment (figure 2A). In contrast, neither TrkA mRNA (figure 2B) nor protein (figure 2C) was detected in the hippocampal astrocytes. When Trk receptors are activated they elicit phosphorylation of downstream signaling proteins such as Erk and Akt (Huang and Reichardt 2003; Kaplan 1995). When cultured astrocytes were treated with NGF, no phosphorylation was detected of either Erk or Akt (figure 2D), confirming the absence of TrkA in these cells. In contrast, EGF, a known growth factor for astrocytes, elicited robust Erk and Akt phosphorylation (figure 2D).
Figure 1.
p75NTR is induced in hippocampal astrocytes after seizure. Expression of p75NTR (green) and GFAP (red) after seizures (bottom row) compared with saline-injected controls (top row). p75NTR is expressed in numerous astrocytes after seizure. Size bar equals 100 μm.
Figure 2.
Hippocampal astrocytes express p75NTR but not TrkA. A, p75 was detected by Western blot in astrocytes and was upregulated after 24 hr treatment with NGF (100ng/ml). Quantification of 3 independent experiments showed a 3.01±0.98 fold increase. Cultured basal forebrain (BF) neurons were used as a positive control. The blot was stripped and re-probed for actin. B, C Astrocytes were analyzed by semi-quantitative PCR for TrkA receptor mRNA, and by Western blot for TrkA protein. Neither mRNA (B) nor protein (C) for TrkA was detected in astrocytes. Cultured neurons from basal forebrain (BF) were positive controls for TrkA expression. D, NGF does not activate TrkA signaling pathways in astrocytes. Astrocytes were treated with NGF (100ng/ml), EGF (100ng/ml), or both for 10 min and analyzed by Western blot for phospho- Erk and phospho-Akt. EGF served as a positive control for Erk and Akt activation. Blots were stripped and re-probed for Erk and Akt, respectively.
NGF decreases cell number but does not cause death of astrocytes
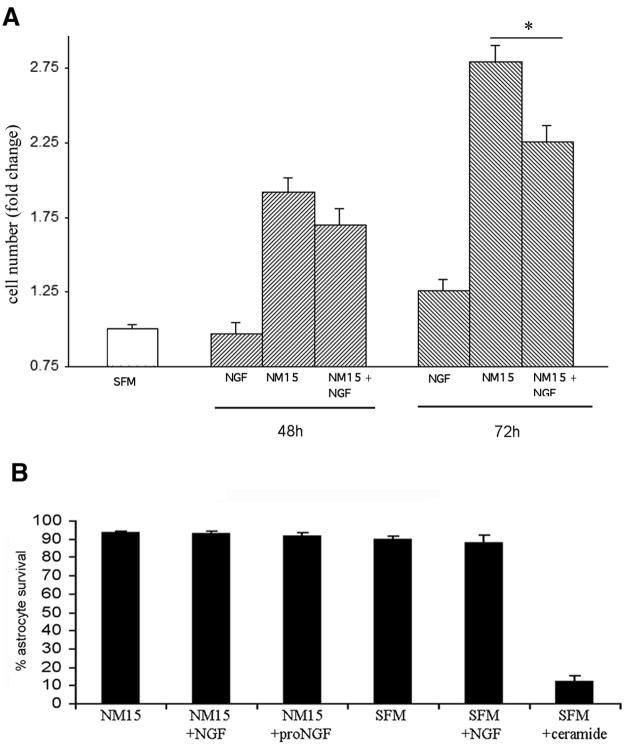
To identify possible functional effects of NGF on astrocytes, the cells were exposed to NGF over time in culture. The cells were first synchronized by serum withdrawal for 48 hr, and were switched back to serum-containing media in the presence or absence of NGF at a dose known to bind p75NTR (100 ng/ml). Cell counts demonstrated that the presence of NGF decreased the number of astrocytes over time in culture (figure 3A). Since p75NTR is known to mediate apoptosis of neurons (Bamji et al. 1998; Frade et al. 1996; Friedman 2000) and oligodendrocytes (Casaccia-Bonnefil et al. 1996) in response to NGF, we investigated whether the decreased cell number was due to induction of apoptosis by NGF. Astrocytes were analyzed by fluorescence-activated cell sorting (FACS) for labeling with Annexin V and uptake of propidium iodide as indicators of cell death. Treatment with NGF in either serum-free or serum-containing media did not induce astrocyte cell death (figure 3B). Moreover, treatment with proNGF, a more potent ligand for p75NTR (Lee et al. 2001; Volosin et al. 2006), also did not induce apoptosis, while ceramide, as a positive control, elicited significant astrocyte cell death (figure 3B).
Figure 3.
A. NGF decreases astrocyte number. Synchronized astrocyte cultures were treated with NM15 alone or with NGF (100ng/ml). At the end of each time point cells were lysed and intact cell nuclei were counted with a hemacytometer. Statistical differences between NM15 and NM15+NGF *p<0.05.
B. NGF does not induce apoptosis in astrocytes. Astrocytes were grown in NM15 or serum-free medium (SFM) and treated with NGF (100 ng/ml) or proNGF (1 ng/ml) for 24 hr. Apoptosis was analyzed by flow cytometry using annexin V-based detection of phosphatidylserine combined with propidium iodide labeling. No increase in the number of apoptotic astrocytes was detected after NGF treatment. The results were plotted as fluorescence intensity of annexin V as a function of fluorescence intensity of PI. Ceramide treatment served as positive control for apoptosis. Graph shows quantification of astrocyte survival from three experiments.
NGF attenuates BrdU incorporation induced by mitogens
Since NGF caused a decrease in cell number, even when the astrocytes were grown in the presence of serum, yet did not elicit cell death, this suggested that NGF might have an inhibitory effect on astrocyte proliferation. To monitor proliferation in culture in a more defined environment, astrocytes were maintained in serum-free medium and exposed to specific potential mitogens in the presence of bromodeoxyuridine (BrdU) for 24 hr. Of the potential mitogens tested in this system, EGF proved to be the most potent (figure 4A, B). Since p75NTR was expressed on the cultured astrocytes (figure 4B), we assessed whether NGF might have an inhibitory effect on the astrocyte cell cycle. Cultured cells were exposed to EGF or serum in the absence or presence of NGF. The presence of NGF caused a modest, but significant, attenuation of BrdU incorporation induced either by EGF or serum (figure 4C), suggesting that the decrease in cell number observed with NGF treatment was due to an inhibition of astrocyte proliferation.
Figure 4.
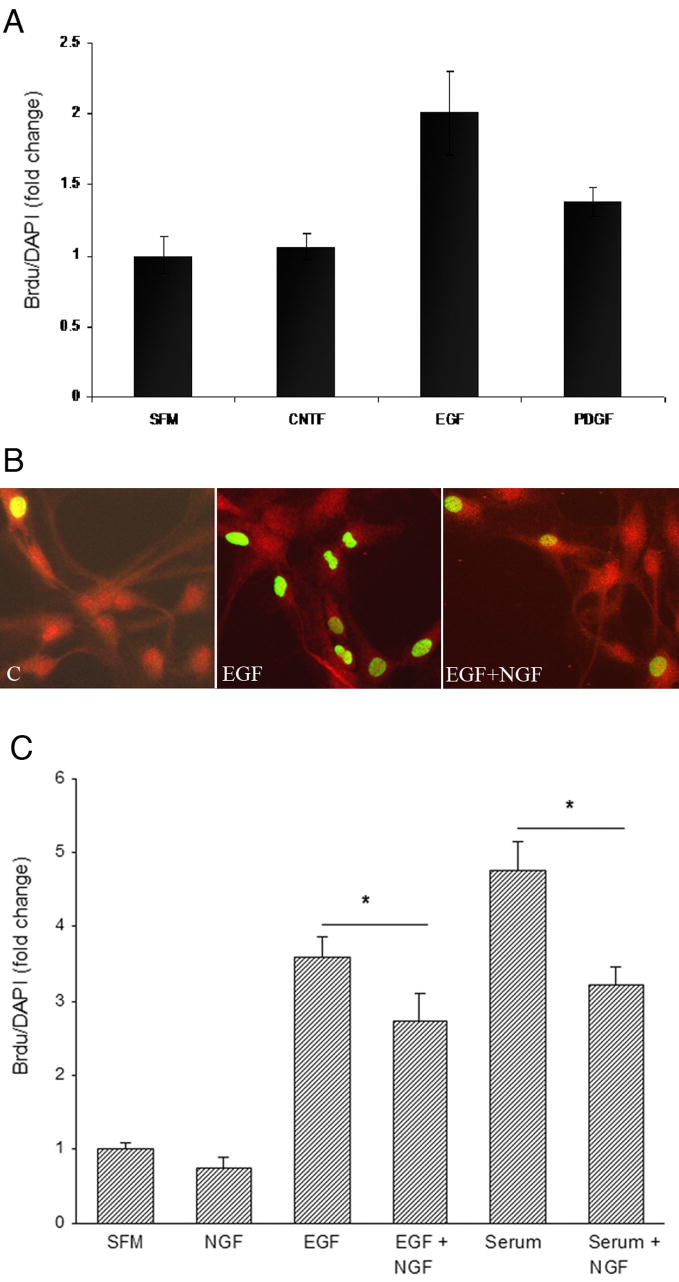
NGF inhibits BrdU incorporation. A, Astrocytes were stimulated with different mitogens: CNTF (40ng/ml), EGF (100ng/ml) or PDGF (10 ng/ml) and provided with BrdU (10 μM) for 24 hr. Astrocytes were fixed and labeled for BrdU and DAPI. The proliferation rate was quantified as the ratio of BrdU positive cells to DAPI positive cells normalized to SFM without any added mitogens. B, EGF increased the number of astrocytes incorporating BrdU, which was inhibited in the presence of NGF. Astrocytes were labeled for p75NTR and BrdU. Control or EGF-treated astrocytes were labeled with anti-p75 and BrdU. All the astrocytes were p75+. C, Quantification of BrdU incorporation in astrocytes stimulated with serum (NM15) or EGF with or without the addition of NGF. *p<0.05.
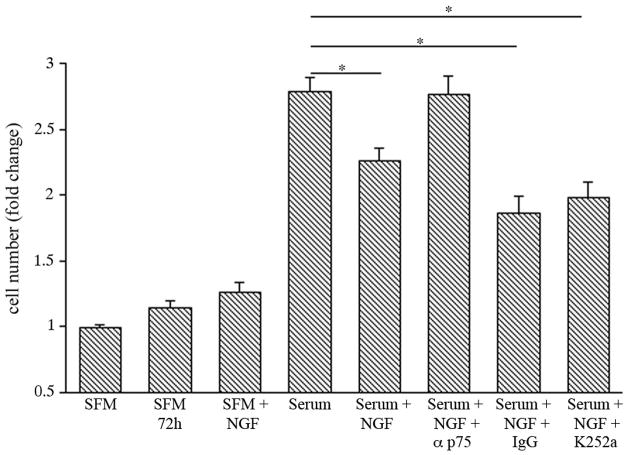
Exposure of the astrocytes to serum+NGF in the presence of a blocking antibody to p75NTR reversed the NGF–induced decrease in cell number. In contrast, the presence of K252a, a selective inhibitor of Trk tyrosine kinases (Tapley et al. 1992), did not reverse the NGF effect confirming that the effect of NGF was mediated via p75NTR and not Trk signaling (Figure 5).
Figure 5.
p75NTR mediates the effect of NGF on astrocyte number. Synchronized astrocyte cultures were treated as indicated for 72 hr. Cells were lysed and intact cell nuclei were counted with a hemacytometer. NGF significantly inhibited proliferation compared to serum alone. The presence of anti-p75, but not control IgG or K252a, reveresed this effect *p<0.05.
Discussion
The p75NTR can mediate multiple cellular functions depending on the cell type and physiological circumstances. We, and others, have previously shown that p75NTR mediates neuronal apoptosis in the brain after seizures (Troy et al. 2002; Volosin et al. 2006) and other types of insults (Harrington et al. 2004). Interestingly, we noted that p75NTR was also induced on astrocytes in the hippocampus after seizures, therefore we employed an in vitro system to investigate the potential function of p75NTR in astrocytes. In contrast to hippocampal neurons, where the stimulation of this receptor by NGF elicited apoptosis (Friedman 2000), NGF did not induce death of astrocytes, but attenuated the incorporation of BrdU, suggesting that NGF may regulate astrocyte proliferation.
There are several physiological situations in the CNS where regulation of glial proliferation is critical. During development progenitor cells must withdraw from the cell cycle and differentiate appropriately, and after injury astrocytes proliferate to form a glial scar. Since p75NTR is expressed in multiple cell types during development, one possible role may be in regulating withdrawal from the cell cycle and initiation of a differentiation program. Several p75NTR–binding proteins have been implicated in cell cycle regulation. Schwann cell factor-1 (SC-1) has been shown to induce cell cycle withdrawal in Schwann cells by repressing transcription of cyclin E (Chittka et al. 2004). In PC12 cells, another p75NTR binding protein, Bex1, was suggested to provide a link between cell proliferation and neurotrophin-induced differentiation (Vilar et al. 2006).
In the normal adult brain astrocytes are quiescent, but after injury these cells become activated and produce numerous cytokines and growth factors. The astrogliosis that occurs after many types of injury and in neuropathological disorders leads to the formation of the glial scar, and may have a profound negative influence on the ability of neurons to regenerate and recover function. Cell cycle inhibition has been shown to attenuate glial scarring (Byrnes and Faden 2007; Cernak et al. 2005) and limit the damage to neuronal function (Zhu et al. 2007). One key growth factor that stimulates astrogliosis is EGF (Liu et al. 2006). The EGF receptor is expressed in developing astrocytes, but not in quiescent astrocytes in the mature brain, however it is re-induced following injury. One of the consequences of astrocyte activation by EGF is the production of NGF (Liu et al. 2006). The glial production of growth factors has traditionally been considered important for their ability to influence neurons, to support neuronal survival, or, as recently demonstrated for proNGF, to induce neuronal apoptosis via p75NTR (Domeniconi et al. 2007; Friedman 2005). The potential effects of NGF on astrocytes themselves have not been investigated extensively, however p75NTR upregulation coincides with activation of astrocytes that occurs after injury and disease. Since EGF induces astrocyte proliferation, the subsequent induction of NGF may serve as a negative modulator to restrict this proliferation and limit the degree of glial scarring.
It is interesting to note that NGF, acting via the p75NTR, has been shown to have a negative regulatory effect on the proliferation of a variety of tumor cells (Khwaja et al. 2006) including C6 astrocytoma cells (Kimura et al. 2002; Weis et al. 2002). Although these are tumor cells, this particular response is similar to what we have observed for primary astrocytes. These observations, together with our current study, suggest that p75NTR may mediate an anti-proliferative response. During development, this receptor may participate in regulating the transition from a dividing to a differentiating population of cells. After injury, and possibly in tumors, activation of p75NTR may serve as a brake on excessive or inappropriate proliferation.
Acknowledgments
The authors would like to thank Richard Farias for excellent technical assistance. This work was supported by NIH grant NS045556.
References
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140(4):911–23. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: Novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF Induces p75-Mediated Death of Oligodendrocytes following Spinal Cord Injury. Neuron. 2002;36(3):375–86. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Faden AI. Role of Cell Cycle Proteins in CNS Injury. Neurochem Res. 2007 doi: 10.1007/s11064-007-9312-2. [DOI] [PubMed] [Google Scholar]
- Carman-Krzan M, Vigé X, Wise BC. Regulation by interleukin-1 of nerve growth factor secretion and nerve growth factor mRNA expression in rat primary astroglial cultures. J Neurochem. 1991;56:636–643. doi: 10.1111/j.1471-4159.1991.tb08197.x. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI. Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle. 2005;4(9):1286–93. doi: 10.4161/cc.4.9.1996. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nature Reviews Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chittka A, Arevalo J, Rodriguez-Guzman M, Perez P, Chao M, M S. The p75NTR-interacting protein SC1 inhibits cell cycle progression by transcriptional repression of cyclin E. J Cell Biol. 2004;164(7):985–996. doi: 10.1083/jcb.200301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Gonzalez-Hoyuela M, Barbas JA, Rodriguez-Tebar A. Programmed Cell Death in the Developing Somites Is Promoted by Nerve Growth Factor via Its p75(NTR) Receptor. Dev Biol. 2000;228(2):326–336. doi: 10.1006/dbio.2000.9948. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34(2):271–9. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18(14):5112–5123. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383(6596):166–8. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20(17):6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ. Interactions of interleukin-1 with neurotrophic factors in the central nervous system: beneficial or detrimental? Mol Neurobiol. 2005;32(2):133–44. doi: 10.1385/MN:32:2:133. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Thakur S, Seidman L, Rabson AB. Regulation of nerve growth factor mRNA by interleukin-1 in rat hippocampal astrocytes is mediated by NFkB. J Biol Chem. 1996;271(49):31115–31120. doi: 10.1074/jbc.271.49.31115. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Cron KC, Otten U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor (NGF) release from cultured rat astrocytes. Neurosci Lett. 1990;117(3):335–40. doi: 10.1016/0304-3940(90)90687-5. [DOI] [PubMed] [Google Scholar]
- Goss JR, O’Malley ME, Zou L, Styren SD, Kochanek PM, DeKoskey ST. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149:301–309. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- Hanbury R, Charles V, Chen EY, Leventhal L, Rosenstein JM, Mufson EJ, Kordower JH. Excitotoxic and metabolic damage to the rodent striatum: role of the P75 neurotrophin receptor and glial progenitors. J Comp Neurol. 2002;444(4):291–305. doi: 10.1002/cne.10104. [DOI] [PubMed] [Google Scholar]
- Harrington A, Leiner B, Blechschmitt C, Arevalo J, Lee R, Morl K, Meyer M, Hempstead B, Yoon S, Giehl K. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci, USA. 2004;101(16):6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12(3):260–7. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Chao MV. High affinity NGF binding requires co-expression of the trk proto-oncogene and the low affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hutton LA, deVellis J, Perez-Polo JR. Expression of p75NGFR TrkA, and TrkB mRNA in rat C6 glioma and type I astrocyte cultures. J Neurosci Res. 1992;32(3):375–83. doi: 10.1002/jnr.490320309. [DOI] [PubMed] [Google Scholar]
- Kaplan DR. Signal transduction by the trk/nerve growth factor receptor. Perspectives in Developmental Neurobiology 1995 [Google Scholar]
- Khwaja F, Tabassum A, Allen J, Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 2006;341(4):1184–92. doi: 10.1016/j.bbrc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- Kimura S, Yoshino A, Katayama Y, Watanabe T, Fukushima T. Growth control of C6 glioma in vivo by nerve growth factor. J Neurooncol. 2002;59(3):199–205. doi: 10.1023/a:1019919019497. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26(28):7532–40. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micera A, Vigneti E, Aloe L. Changes of NGF presence in nonneuronal cells in response to experimental allergic encephalomyelitis in Lewis rats. Exp Neurol. 1998;154(1):41–6. doi: 10.1006/exnr.1998.6864. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng K, Jansen P, Madsen P, Nielsen M, Jacobsen C, Kliemannel M, Schwarz E, Willnow T, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Oderfeld-Nowak B, Bacia A. Expression of astroglial nerve growth factor in damaged brain. Acta Neurobiol Exp. 1994;54(2):73–80. [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong L, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–358. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Roux P, Barker P. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67(3):203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19(16):6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Li Y, Pasnikowski EM, Mattson K, Pan L, Yancopoulos GD, Wiegand SJ, Lindsay RM, Ip NY. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994;6:693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Semkova I, Krieglstein J. Ciliary neurotrophic factor enhances the expression of NGF and p75 low-affinity NGF receptor in astrocytes. Brain Res. 1999;838(1–2):184–92. doi: 10.1016/s0006-8993(99)01728-x. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7(2):371–81. [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons: Role of caspases. J Biol Chem. 2002;277(37):34295–302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Vilar M, Murillo-Carretero M, Mira H, Magnusson K, Besset V, Ibanez CF. Bex1, a novel interactor of the p75 neurotrophin receptor, links neurotrophin signaling to the cell cycle. Embo J. 2006;25(6):1219–30. doi: 10.1038/sj.emboj.7601017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26(29):7756–66. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis C, Wiesenhofer B, Humpel C. Nerve growth factor plays a divergent role in mediating growth of rat C6 glioma cells via binding to the p75 neurotrophin receptor. J Neurooncol. 2002;56(1):59–67. doi: 10.1023/a:1014410519935. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhang Q, Yu Z, Zhang L, Tian D, Zhu S, Bu B, Xie M, Wang W. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia. 2007;55(5):546–58. doi: 10.1002/glia.20476. [DOI] [PubMed] [Google Scholar]