Abstract
We previously reported that reactive oxygen species (ROS) generated during hypoxia decrease hERG current density and protein expression in HEK cells stably expressing hERG protein. In the present study, we investigated the molecular mechanisms involved in hypoxia induced downregulation of hERG protein. Culturing cells at low temperatures and addition of chemical chaperones during hypoxia restored hERG expression and currents to normoxic levels while antiarrhythmic drugs, which selectively block hERG channels, had no effect on hERG protein levels. Pulse chase studies showed that hypoxia blocks maturation of the core glycosylated form in the endoplasmic reticulum (ER) to the fully glycosylated form on the cell surface. Co-immunoprecipitation experiments revealed that hypoxia inhibited interaction of hERG with Hsp90 chaperone required for maturation, which was restored in the presence of ROS scavengers. These results demonstrate that ROS generated during hypoxia prevents maturation of the hERG protein by inhibiting Hsp90 interaction resulting in decreased protein expression and currents.
Keywords: hERG, hypoxia, chaperones, Hsp 90, ROS, low temperatures
INTRODUCTION
Hypoxic inhibition of K+ channel activity is central to the homeostatic mechanisms that underlie diverse physiological and pathological process such as adaptation to high altitude, pulmonary hypertension and stroke [1, 2]. Human ether–a-go-go-related gene (hERG) encodes the α subunit of a voltage gated potassium channel that underlies a delayed rectifier current (IKr) [3]. Congenital or drug–induced disruptions of the hERG channel cause long QT syndrome type 2 (LQT2), a cardiac disorder that predisposes affected individuals to ventricular arrhythmias and sudden cardiac arrest [4]. Although loss of channel function can be caused by multiple mechanisms including abnormal protein trafficking, generation of nonfunctional channels and altered channel gating, defective trafficking of hERG protein to the cell surface has been shown to be the most common mechanism of hERG channel dysfunction in LQT2 [5–8]. Cultivation of cells at low temperature or in the presence of chemical chaperones like glycerol and DMSO has improved the folding and membrane localization of trafficking defective hERG mutant proteins [9,10]. In addition, drugs that bind with high affinity to hERG channels can also act as chemical chaperones to promote proper folding, permitting trafficking to the plasma membrane [11–13]. HERG protein is synthesized in the endoplasmic reticulum (ER), exported to the Golgi apparatus for complex glycosylation and eventually inserted into the cell surface membrane [10]. HERG maturation and trafficking of the protein to the cell surface is regulated by Hsp90, a molecular chaperone that protects client proteins from misfolding and degradation [14].
We have recently demonstrated that recombinant hERG channels are reversibly inhibited by prolonged hypoxia in a time and stimulus dependent manner, and that ROS generated by mitochondria mediate the effects of low O2 [15]. More importantly, we showed that hypoxia downregulated hERG protein via translational control rather than by transcriptional or degradational changes [15]. In the present study, we show that hypoxia affects folding and maturation of hERG protein which can be corrected by culturing cells at low temperature but not by antiarrhythmic drug treatment. ROS generated during hypoxia inhibit hERG maturation by inhibiting interaction with the chaperone Hsp90. We therefore conclude that regulation of hERG mRNA translation by hypoxia may be a cellular quality control mechanism to prevent accumulation of misfolded proteins that could not bind to Hsp90 in the ER.
Materials and methods
Cell cultures and exposure to hypoxia
HEK293 cells stably transfected with wild type hERG were maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin/geneticin at 37°C. Cells cultured in 6 well culture plates were subjected to hypoxia (1%O2) in a hypoxic chamber.
Western blot analysis
Cells were solubilized at 4°C in lysis buffer (50mM Tris[pH 7.5], 150mM NaCl, 1mM EDTA, 1% Triton X-100) containing a protease inhibitor mix. Protein concentrations were determined and equal amounts of proteins were separated on a 6% SDS-PAGE gel and transferred to polyvinylidene difluoride membrane (PVDF). Membranes were blocked in 5% milk overnight and immunoblotted with hERG antibody (Alomone Labs), followed by horseradish peroxidase conjugated secondary antibody. ECL was used to develop the blots (GE Healthcare). Image densities were quantified using Scion Image software (NIH) by integrating pixel densities of individual protein bands.
Metabolic labeling and immunoprecipitation
Cells starved in methionine/cysteine-free medium for 30 minutes were pulse-labeled for 60 min in 100µci of [35S]-methionine/cysteine (EXPRESS [35S] protein labeling mix, Dupont, NEN) containing medium and chased for 6hrs with excess cold methionine and cysteine. To crosslink, cells were washed once with phosphate-buffered saline (PBS) and then incubated for 15 minutes at room temperature with 2mM dithiobis(succinimidyl propionate) (DSP, Pierce) [14]. The crosslinker was quenched by addition of 1M Tris (pH 7.5). Cells were harvested in 1ml cold PBS, pelleted and lysed in 0.1% NP40, 50mM Tris (pH7.5), 150mM NaCl and a protease inhibitor cocktail mix. Insoluble material was pelleted and radiolabeled protein complexes were isolated in the supernatant by immunoprecipitation with anti-hERG, anti-Hsp90 and anti-Hsp70 (Santa Cruz) antibodies. Immunocomplexes precipitated by addition of Protein A/G agarose beads (Santa Cruz) for 2hrs at 4°C were eluted with SDS sample buffer and analysed by SDS- PAGE gels.
Measurements of hERG K+ currents
hERG currents were recorded at room temperature by patch clamp as described by Ficker et al [14]. Currents were elicited from a holding potential of –90mV with depolarizing pulses to +100mV. The current density was analyzed on return to −50mV after maximal activation of hERG currents in a 0.5sec pre-pulse. Capacitance was determined using a 10mV step from −100 to −90mV. Currents given are the mean of the peaks from 80, 90 and 100mV. Maximal tail current amplitudes were normalized to cell capacitance to compute hERG current densities.
RESULTS
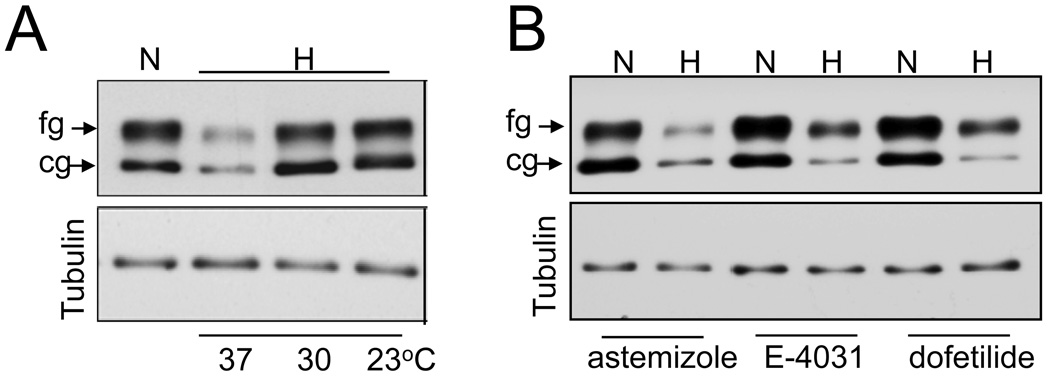
As shown previously, exposure to 24 hrs of hypoxia at 37°C resulted in about 80% reduction in both, core glycosylated 135kDa immature form (cg) located in the endoplasmic reticulum and the fully glycosylated mature 155kDa form (fg) forms located in the plasma membrane (Fig 1A). Low temperature culturing of cells has been reported to increase the surface expression of many hERG mutants. Exposure of cells to hypoxia at lower temperatures (30°C and 23°C), fully restored the expression of the cg form of hERG to normoxic levels (Fig 1A). The effect on the fg form was temperature dependent, with 65% and 80% recovery at 30°C and 23°C respectively. PO2 of the culture medium at lower temperature (23°C) was comparable with that seen at 37°C (25±2mm Hg vs 28±3mm Hg) under hypoxic conditions suggesting that the effect was not due to PO2 differences. Chemical chaperones such as glycerol and DMSO have been shown to improve protein trafficking. Exposing cells to 24 hrs of hypoxia in the presence of 5% glycerol or 2% DMSO restored hypoxia induced hERG downregulation, albeit to lesser extent than that obtained with low temperature incubation (data not shown). In contrast, antiarrhythmic drugs like dofetilide (3µM), astemizole (5µM) and E-4031 (5µM), which selectively block hERG channels and correct trafficking of some LQT2 mutants, increased the expression of the fg form modestly under normoxic conditions but most importantly failed to inhibit hypoxia induced hERG downregulation (Fig 1B).
Fig 1. Effect of temperature and antiarrhythmic drugs on hERG expression during hypoxic exposure.
A. Immunoblot analysis of HEK293/hERG cell lysates exposed to 24hrs of normoxia (N), and hypoxia (H) at 37°C, 30°C, 23°C. B) Cells treated with antiarryhthmic drugs astemizole (5µM), dofitilde (3µM) and E-4031 (5µM) were exposed to hypoxia at 37°C for 24 hrs and cell lysates analyzed for hERG expression by immnoblots. Tubulin protein expression was used as a loading control.
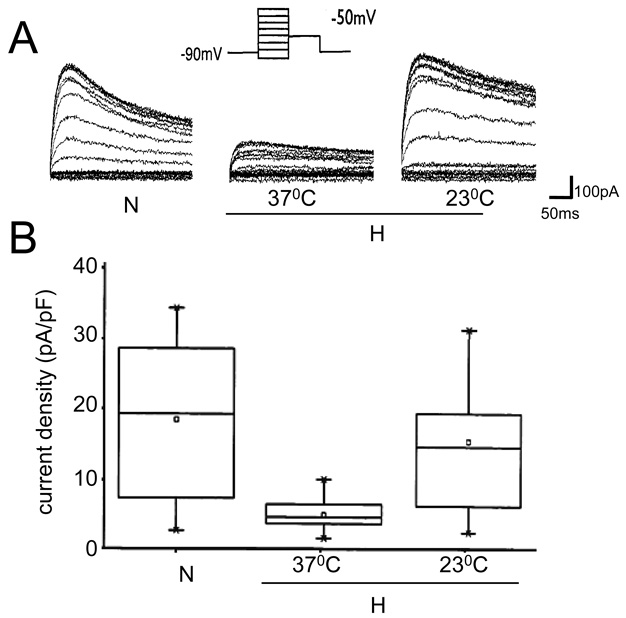
We have previously reported that hypoxia induced down-regulation of hERG resulted in attenuation of hERG current density [15]. To assess whether the restoration of hERG expression at low temperatures during hypoxia is also reflected in functional channels, we measured hERG currents in cells exposed to hypoxia at 23°C and 37°C for 24hrs. As shown in Fig 2A, hERG current amplitude reduced significantly in cells exposed to hypoxia at 37°C compared to cells under normoxic conditions as reported previously. When the cells were exposed to hypoxia at 23°C, hERG current amplitude markedly increased. The reduction in current densities observed in cells exposed to hypoxia at 37°C (5.03 ± 0.71; n=12) compared to controls (18.45 ± 2.71; n=15) is abolished when the cells are exposed to hypoxia at 23°C (16.76 ± 2.93; n= 11), reflecting the stable protein expression observed under these conditions (Fig 2B).
Fig 2. hERG current measurements in cells exposed to hypoxia at different temperatures.
A. Representative traces of hERG currents recorded from a control cell and a cell exposed to hypoxia at 37°C or 23°C. B) Current densities derived from cells exposed to normoxia (n=15), hypoxia at 23°C (n=11) and at 37°C (n=12), as described in methods. To give a measure of data dispersion, data are presented in box charts, whiskers marking the 5th and 95th percentile and the box determining the 25th and 75th percentile. Arithmetic means are represented by open squares.
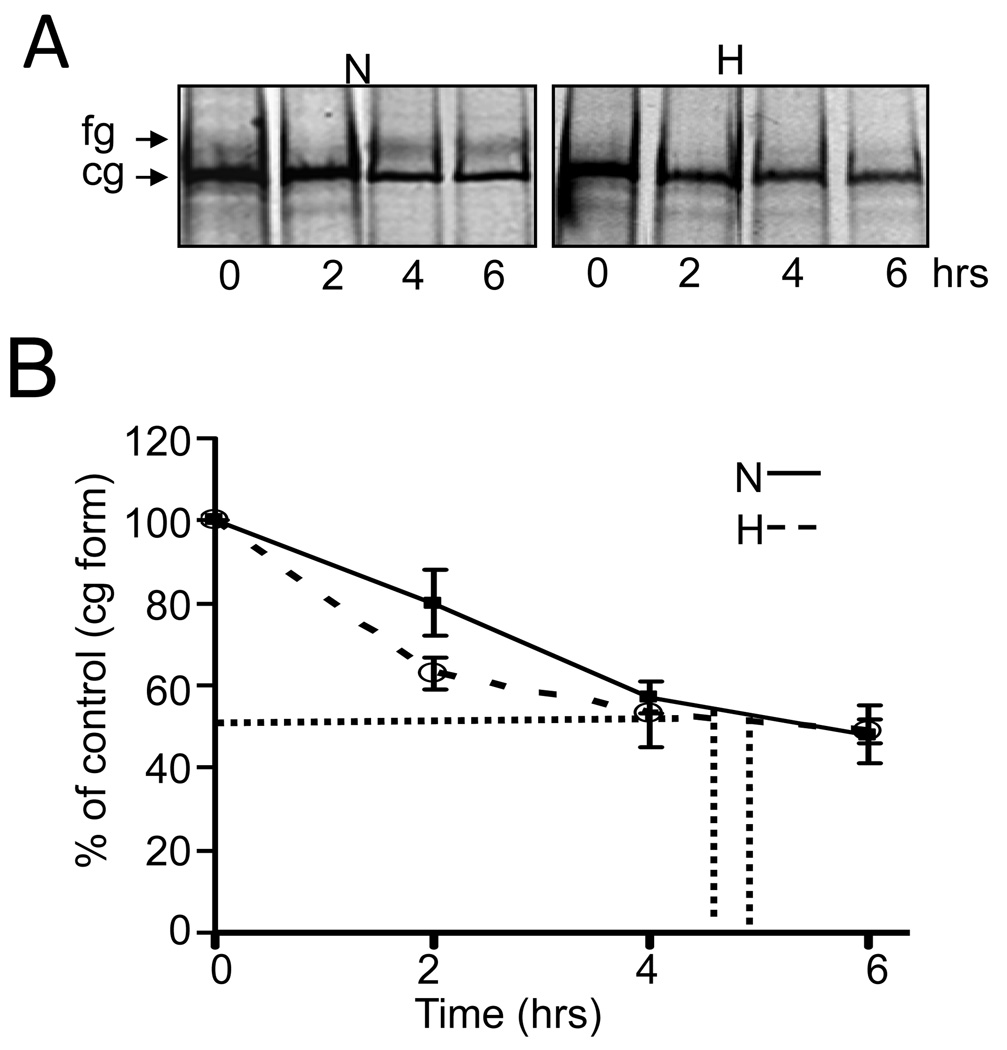
The temperature effect on hERG stability during hypoxia suggest that proper folding of hERG in the ER could be compromised. Since misfolding in the ER is known to cause defects in maturation and trafficking of hERG mutants, we studied the effects of hypoxia on the conversion of the newly synthesized ER restricted cg form into the mature cell surface fg form using pulse-chase experiments. HEK293-hERG cells were metabolically labeled with 35S methionine and 35S cysteine for 1hr and chased with unlabeled methionine / cysteine for 2–6hrs under normoxia or hypoxia. As shown in Fig 3A, under normoxic conditions, within the first 6hrs, about 30% of the initially synthesized 135 kDa form was converted to the mature glycosylated 155 kDa channel protein, in contrast to only small amounts (<5%) of the fg form produced in hypoxia exposed cells. Analyses of the degradation kinetics of the cg form by densitometric analysis (Fig 3B) showed no significant differences in the turnover rate between normoxia (5hrs) and hypoxia (4.5hrs). Taken together these results suggest that hypoxia inhibits maturation of the hERG cg form to the fg form, which does not result in an increased degradation rate nor in accumulation of the ER form.
Fig 3. Hypoxia inhibits maturation of cg to fg form but has no effect on the degradation rate of the hERG protein.
A: HEK/hERG cells were pulse-labeled with 35S methionine/cysteine for 1hr and then chased in isotope-free medium for 2, 4 and 6hrs under normoxia or hypoxia. Radiolabeled hERG was isolated by immunoprecipitation before and after the chase period indicated and analyzed by SDS-PAGE and fluorography. Shown is a typical fluorogram.
B: The densitometric signal intensities for the cg form from the above experiment were plotted as function of chase time, from which the half life of hERG protein was calculated (n=3).
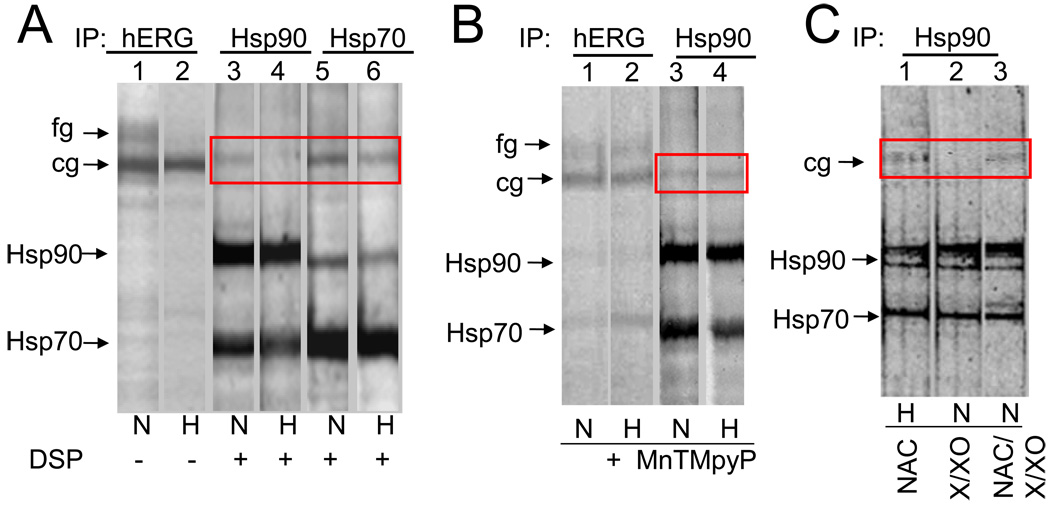
Because the trafficking block observed with hypoxia was reminiscent of effects induced by geldanamycin and radicicol, (two specific inhibitors of Hsp90) and since Hsp90 and Hsp70 are required for the biochemical maturation of hERG [14], we tested whether hypoxia modified the association of hERG channels with these chaperones using co-immunoprecipitation experiments. As shown in Fig 4A, after a 6hr pulse, hERG antibody immunoprecipitated both cg and fg forms of hERG under normoxic conditions whereas only cg form was observed during hypoxia (lane 1 vs lane2) confirming our pulse chase data that hypoxia inhibits maturation. Since Hsp90 interacts transiently with proteins, hERG-Hsp90 complexes could be isolated only after crosslinking with chemical crosslinker DSP. Hsp90 and Hsp70 were isolated in complexes with the cg but not with the fg form of the hERG protein (lane 1 to lane 3 and 5). Most importantly, hERG co-immunoprecipitated with Hsp90 only in normoxia but not in hypoxia (lane 3 vs lane 4, Fig 4A) exposed cells. The formation of the Hsp70/hERG complex, on the other hand, was unaffected by hypoxia treatment (lane 5 and 6). It could be that hypoxia inhibits Hsp90 binding to hERG by reducing steady state expression levels of endogenous Hsp90 and/or Hsp70. However neither Hsp90 nor Hsp70 protein levels were significantly altered by hypoxia in HEK/hERG cells as determined by immunoblots (data not shown).
Fig 4. Hypoxia blocks interaction of Hsp90 but not hsp70 with the cg form of hERG which is restored in the presence of ROS inhibitors.
A & B: HEK/hERG cells were pulse labeled with 35S-methionine for 6 hrs under normoxia or hypoxia in absence (A) or presence of MnTMPyP (B). Cross-linked radiolabeled hERG/chaperone complexes were immunoprecipitated with anti-hERG (lane 1 & 2), anti-Hsp90 (lane 3 & 4) and anti-Hsp70 (lane 5 & 6) antibodies and analyzed by 6% SDS-PAGE gels. The cg form of hERG co-immunoprecipitated with Hsp90 is shown in the box. C. Analysis of hERG binding to Hsp90 in cells treated with anti-oxidant N-acetyl cysteine (NAC; 500µm) (lane 1), during hypoxia and with xanthine/xanthine oxidase X/XO (400µM/5units/ml), a superoxide generating system, with or without NAC (lanes 2 & 3), during normoxia.
We have previously shown that hypoxia downregulates hERG protein via increased generation of ROS in the mitochondria [15]. We therefore investigated whether ROS, by modulating the interaction of hERG with Hsp90, mediates hypoxia-induced hERG downregulation. Treatment with MnTMPyP, a ROS scavenger, during hypoxia resulted in both cg and fg forms being immunoprecipitated similar to that observed during normoxia (lanes 1 and 2, Fig 4B). More importantly, addition of MnTMPyP during hypoxia restored Hsp90 interaction with cg form of hERG (lane 3 vs lane 4, Fig 4B). Similar results were obtained when cells were treated with a chemically different antioxidant, N-acetyl-L-cysteine (NAC, 1mM), a glutathione precursor during hypoxia (lane 1, Fig 3C). To further confirm the role of ROS in Hsp90 binding to hERG, normoxic cells were exposed to xanthine/xanthine oxidase (X/XO; 500µm/5milliunits/ml), which generates O2−. As shown in lane 2 Fig 3C, no hERG was detected in Hsp90 immunoprecipitates and addition of NAC prevented X/XO-induced inhibition of Hsp90 binding (lane 3, Fig 3C).
DISCUSSION
The work described here documents a previously unrecognized mechanism for hypoxic regulation of the hERG K+ channel. This is the first study to demonstrate that prolonged hypoxic exposure leads to hERG protein misfolding, resulting in maturation inhibition. We propose that this mechanism underlies the hypoxia-induced decrease in hERG protein expression and currents previously observed [15]. Hypoxia prevented maturation of hERG protein by inhibiting newly synthesized hERG from binding to Hsp90 chaperone complexes. The modulation of Hsp90 interaction with hERG during hypoxia is mediated by ROS generated during prolonged hypoxic exposure.
We have previously shown that hypoxia induced downregulation is not due to increased degradation nor transcriptional changes. Misfolding in the ER leading to defective trafficking of hERG protein to the plasma membrane which is increasingly being recognized as an important mechanism of hERG channel dysfunction in LQT2 [5–8], seem to contribute to hypoxia induced hERG downregulation. HERG channel blockers and chemical chaperones, as well as low temperature, have been shown to correct hERG defective protein trafficking observed with LQT2-causing mutants [9–13]. HERG channel blockers bind to aromatic residues within the pore of the assembled tetrameric channel, implying that only proteins that are able to assemble into tetramers can be stabilized by these drugs. Lower incubation temperatures or chemical chaperones, on the other hand, can stabilize a wide variety of mutants, including some that cannot be rescued by specific drugs. These treatments therefore are likely to stablize co-translational folding, in which the different functional domains of a protein fold sequentially and independently during translation. Our observations that lower incubation temperatures or chemical chaperones but not channel blockers restored expression of hERG during hypoxia suggest that loss of hERG function during hypoxia could be a result of a co-translational disruption of folding occurring early in the folding process of nascent molecules, resulting in protein that never reaches the state of tetramer assembly.
Our pulse chase studies show that hypoxia inhibits progression of the cg form into the fg form of hERG due to a direct consequence of the interference of Hsp90 binding to nascent hERG molecules. The complete absence of Hsp90 binding to hERG during hypoxia observed in our studies is in contrast to what is observed with trafficking-deficient LQT2 mutants (hERG R752W and G601S), in which the interactions of Hsp90 and Hsp70 are increased for both mutants [14]. This may explain why the cg form of hERG is decreased during hypoxia but results in ER retention in the case of mutants. Future experiments will be necessary to determine whether chaperones like calnexin and co-chaperones like FKBP38 [16], which promote hERG folding, also contribute to hypoxia induced defective trafficking of hERG. Maturation defect of hERG observed during hypoxia did not result in accelerated turnover of the protein nor in ER retention, but rather resulted in a dramatic decrease in the synthesis of the protein as shown in our previous studies. The decrease in translation may be an important adaptive response of cells, triggered by the hERG protein that cannot bind to Hsp90 and fold successfully. The reduction in synthesis would reduce the load of misfolded proteins in the ER, prolonging survival in a stressful hypoxic environment.
Hypoxia could modulate hERG maturation by affecting the redox state of the cell through altering the level of reactive oxygen species (ROS) which are reported to be important regulators of cellular responses [17]. In line with this idea, ROS have been shown to modulate the kinetics of hERG channels [18–21]. Our finding that ROS scavengers restore hERG interaction with Hsp90 could account for the stabilization of hERG protein by antioxidants during hypoxia. Two mechanisms could account for how ROS regulates hERG protein interaction with Hsp90. ROS could directly oxidize hERG which contains numerous residues both in the PAS domain and elsewhere in the N-terminus that are potentially susceptible to redox modification [22]. In this context, hERG is structurally different from other potassium channels (Kv1.5, Kv2.1) that apparently lack such specialized domains as PAS and therefore do not constitute a target for Hsp90. Alternatively, ROS could interact with a conserved thiol pair of Hsp90 rendering it nonfunctional, thereby interfering with chaperone binding to hERG.
Our observations provide new insights into a potential mechanism underlying inhibition of delayed rectifier K+ channels, leading to membrane depolarization by prolonged hypoxia. In summary, ROS generated during hypoxia inhibit Hsp90 interactions, preventing proper folding and maturation, resulting in translational inhibition of hERG protein production. Our findings add hERG to the list of other PAS containing proteins like HIF-1α [23] for which hypoxia modulates Hsp90 interactions, and suggests that molecular chaperones, especially Hsp90, are integral components of the hypoxic response pathway.
Acknowledgements
This work is supported by grants from National Institutes of Health, Heart, Lung and Blood Institute HL-090554, HL-086493 and HL-717189 to Eckhard Ficker. We thank Dr. Dorothy Hanck for help with the patch clamp experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kemp PJ, Peers C. Oxygen sensing by ion channels. Essays Biochem. 2007;43:77–90. doi: 10.1042/BSE0430077. Review. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2005;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti MC, Jiang C, Curran ME, Keating MY. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 4.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinetti MC, Curran ME, Spector PS, Keating MY. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human syndrome. long QT Intracellular transport and functional defects. J. Biol. Chem. 1998;273:21061–21066. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CL, Delisle BP, Anson JA, Kilby ML, Will DJ, Tester Q, Gong Z, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 8.Ficker E, Dennis AT, Obejero-Paz CA, Castaldo P, Taglialatela M, Brown AM. Retention in the endoplasmic reticulum as a mechanism of dominant-negative current suppression in human long QT syndrome. J Mol. Cell. Cardiol. 2000;32:2327–2337. doi: 10.1006/jmcc.2000.1263. [DOI] [PubMed] [Google Scholar]
- 9.Chen MX, Sandow SL, Doceul V, Chen YH, Harper H, Hamilton B, Meadows HJ, Trezise DJ, Clare JJ. Improved functional expression of recombinant human ether-a-go-go (hERG) K+ channels by cultivation at reduced temperature. BMC Biotechnol. 2007;7:93–103. doi: 10.1186/1472-6750-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J. Biol. Chem. 1999;274:31123–31126. doi: 10.1074/jbc.274.44.31123. [DOI] [PubMed] [Google Scholar]
- 11.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb. Exp. Pharmacol. 2006;171:349–355. doi: 10.1007/3-540-29715-4_14. Review. [DOI] [PubMed] [Google Scholar]
- 12.Gong Q, Jones MA, Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J. Biol. Chem. 2006;281:4069–4074. doi: 10.1074/jbc.M511765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas D, Kiehn J, Katus HA, Karle CA. Defective protein trafficking in hERG-associated hereditary long QT syndrome (LQT2): molecular mechanisms and restoration of intracellular protein processing. Cardiovasc. Res. 2003;60:235–241. doi: 10.1016/j.cardiores.2003.08.002. Review. [DOI] [PubMed] [Google Scholar]
- 14.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 2003;92:87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 15.Nanduri J, Wang N, Bergson P, Yuan G, Ficker E, Prabhakar NR. Mitochondrial reactive oxygen species mediate hypoxic down-regulation of hERG channel protein. Biochem. Biophys. Res. Commun. 2008;373:309–314. doi: 10.1016/j.bbrc.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 promotes HERG trafficking. J. Biol. Chem. 2007;282:23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 17.Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R. Novel aspects of oxidative stress in cardiovascular diseases. Circ J. 2009;73:201–207. doi: 10.1253/circj.cj-08-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berube J, Caouette D, Daleau P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 2001;297:96–102. [PubMed] [Google Scholar]
- 19.Taglialatela M, Castaldo P, Pannaccione S, Iossa A, Fresi A, Ficker EL. Annunziato Regulation of the human ether-a-gogo related gene (HERG) K+ channels by reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11698–11703. doi: 10.1073/pnas.94.21.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz O, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol. Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J. Biol. Chem. 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- 22.Morais Cabral JH, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell. 1998;95:649–655. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 23.Katschinski DM, Le L, Schindler SG, Thomas T, Voss AK, Wenger RH. Interaction of the PAS B domain with HSP90 accelerates hypoxia-inducible factor-1alpha stabilization. Cell. Physiol. Biochem. 2004;14:351–360. doi: 10.1159/000080345. [DOI] [PubMed] [Google Scholar]