Abstract
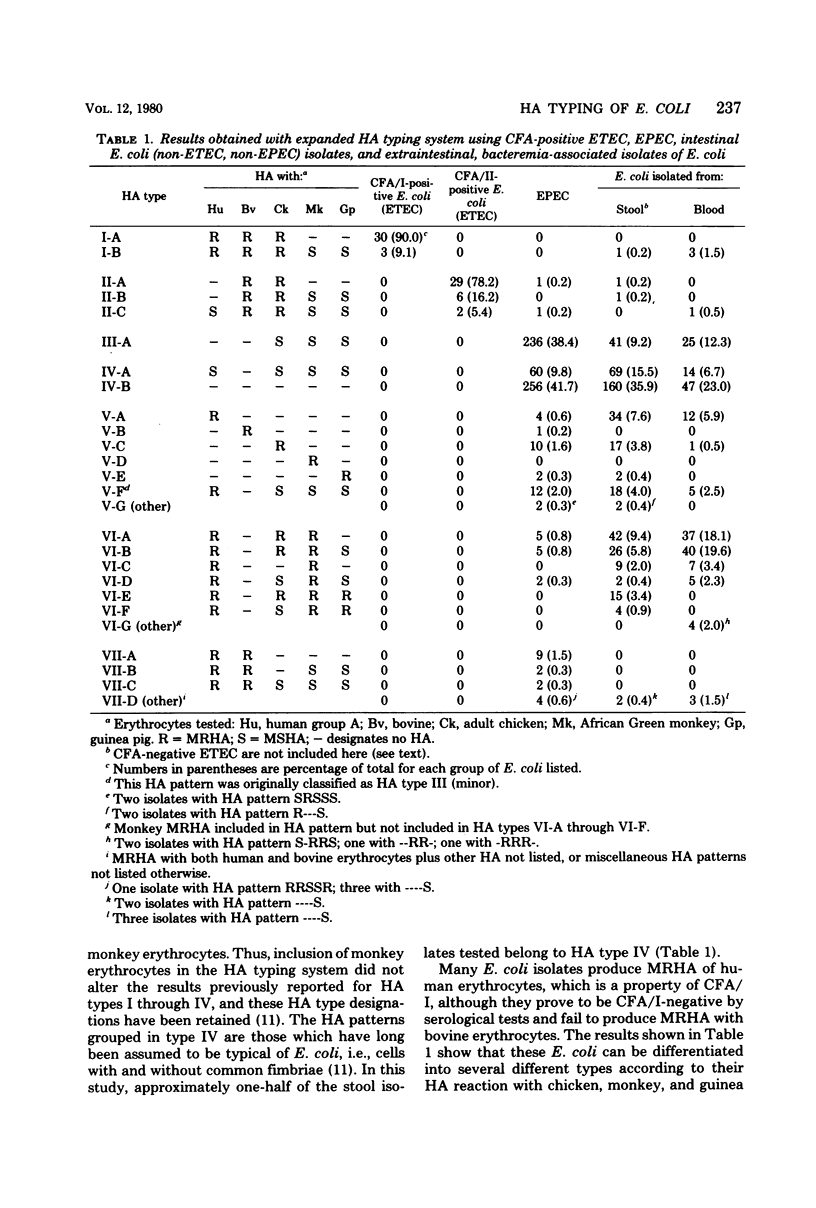
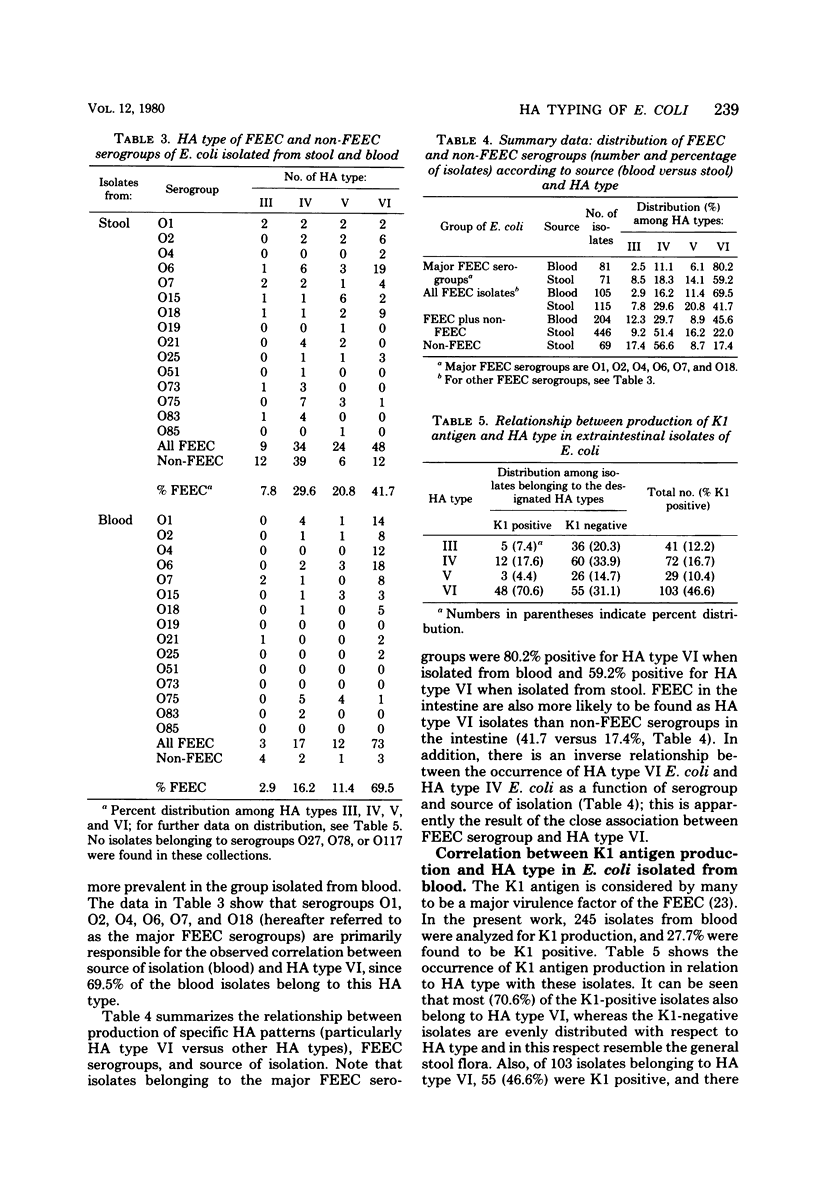
A hemagglutination (HA) typing system has been developed for demonstrating and characterizing the mannose-sensitive and mannose-resistant hemagglutinins produced by Escherichia coli isolated from human sources. HA typing is performed by testing CFA agar-grown E. coli cells for HA with human, bovine, adult chicken, African Green monkey, and guinea pig erythrocytes in the presence and absence of mannose. Seven major HA types, designated HA type I through HA type VII, have been defined according to the HA patterns produced by 1,334 test cultures consisting of 33 colonization factor antigen I (CFA/I)-positive enterotoxigenic E. coli (ETEC), 37 CFA/II-positive ETEC, 614 isolates belonging to the classical enteropathogenic E. coli, or EPEC, serogroups, 446 non-ETEC, non-EPEC stool isolates, and 204 bacteremia-associated E. coli. Facultatively enteropathogenic E. coli (FEEC) serogroups, which are the causative agents of extraintestinal infections but also sporadic cases of enteritis, comprised 38% of the stool isolates and 91% of the blood isolates examined. Previous observations concerning the HA patterns of CFA-positive ETEC and the EPEC were confirmed. A significant correlation was found between FEEC serogroups and the production of mannose-resistant HA with human, monkey, and usually chicken erythrocytes (the HA patterns designated HA type VI). A large majority (80.2%) of the FEEC strains belonging to the most frequently isolated serogroups from cases of bacteremia (O1, O2, O4, O6, O7, and O18) produced type VI HA patterns. Stool isolates belonging to these same serogroups were 59.2% positive for HA type VI patterns. In contrast, only 17.4% of the non-FEEC stool isolates and 1.9% of the EPEC isolates belong to HA type VI. Of the blood isolates, the HA type VI phenotype was two times more prevalent among K1-positive E. coli than among K1-negative E. coli, 70.6 versus 31.1%. These results suggest that surface-associated hemagglutinins of E. coli, many of which are known to be fimbriae, should be considered in addition to serotype (O:K:H antigenicity) in the description of isolates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEPPELLINI R., LANDY M. Suppression of blood group agglutinability of human erythrocytes by certain bacterial polysaccharides. J Exp Med. 1963 Mar 1;117:321–338. doi: 10.1084/jem.117.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirók E., Madár J., Budai J., Stverteczky Z., Kertész A., Dombi I., Gyengési L. The role in sporadic enteritis of facultatively enteropathogenic Escherichia coli serogroups. Acta Microbiol Acad Sci Hung. 1976;23(4):359–369. [PubMed] [Google Scholar]
- Czirók E., Milch H., Madár J., Semjén G. Characterization of Escherichia coli serogroups causing meningitis, sepsis and enteritis. I. Serological properties and animal pathogenicity of O18, O78 and O83 isolates. Acta Microbiol Acad Sci Hung. 1977;24(2):115–126. [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, DuPont H. L. Virulence factors of enterotoxigenic Escherichia coli. J Infect Dis. 1977 Aug;136 (Suppl):S118–S123. doi: 10.1093/infdis/136.supplement.s118. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B., Hanson L. A., Jodal U., Lidin-Janson G., Robbins J. B. Frequency of E. coli K antigens in urinary-tract infections in children. Lancet. 1977 Mar 26;1(8013):663–666. doi: 10.1016/s0140-6736(77)92111-0. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Orskov F. Virulence factors of the bacterial cell surface. J Infect Dis. 1978 May;137(5):630–633. doi: 10.1093/infdis/137.5.630. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. K-1 antigen of Escherichia coli: epidemiology and serum sensitivity of pathogenic strains. Infect Immun. 1978 Oct;22(1):219–224. doi: 10.1128/iai.22.1.219-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. Virulence of Escherichia coli K1 in adults. J Infect Dis. 1979 Jan;139(1):106–108. doi: 10.1093/infdis/139.1.106. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Normann B., Edebo L. Influence of O and K antigens on the surface properties of Escherichia coli in relation to phagocytosis. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):85–91. doi: 10.1111/j.1699-0463.1979.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R., Young L. S. Phagocytic resistance of Escherichia coli K-1 isolates and relationship to virulence. J Clin Microbiol. 1978 Dec;8(6):748–755. doi: 10.1128/jcm.8.6.748-755.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



