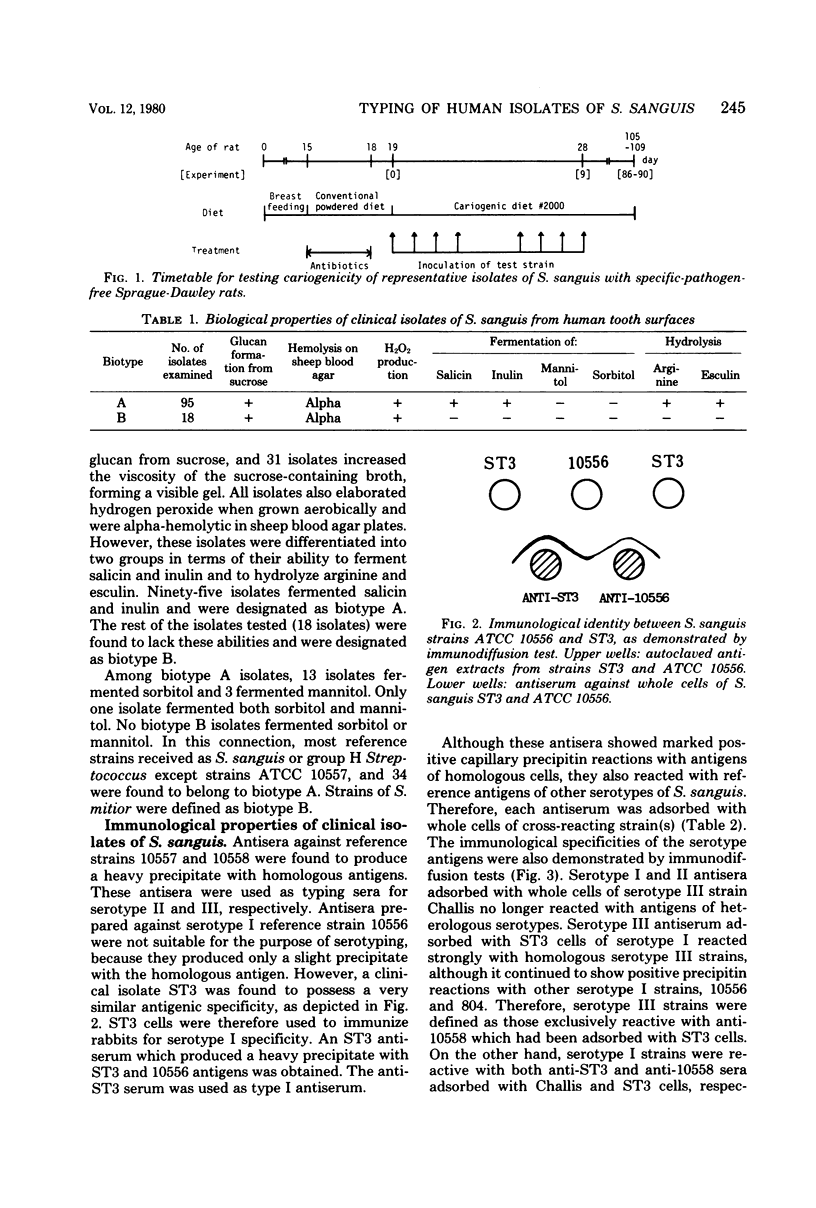
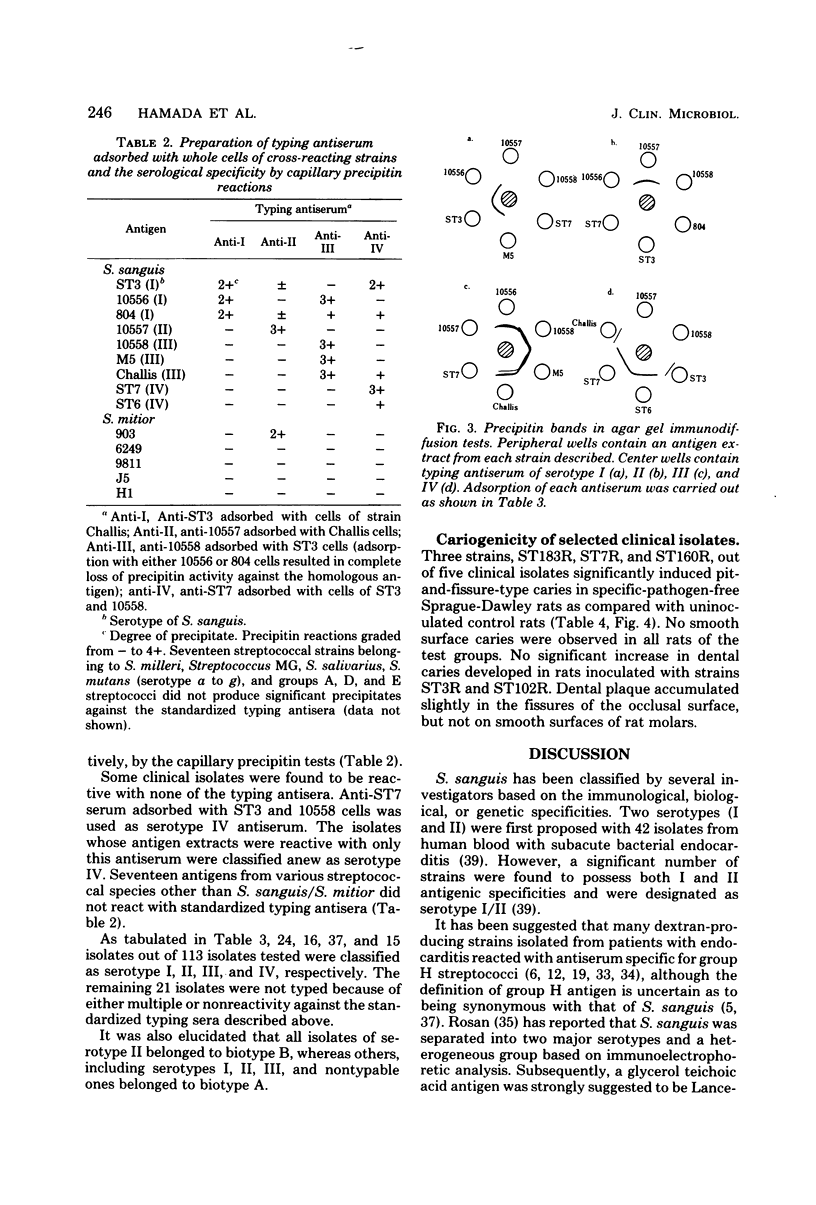
Abstract
A total of 113 pure cultures of Streptococcus sanguis were obtained from dental plaque samples of 64 subjects. All isolates synthesized glucan from sucrose, elaborated peroxide, and were alpha-hemolytic. Two biotypes and four serotypes were differentiated within the species. Biotype A (95 isolates) fermented salicin and inulin and hydrolyzed arginine and esculin, whereas biotype B (18 isolates) did not possess these activities. The isolates were serotyped with autoclaved extracts against whole-cell antiserum to strains ATCC 10556 or ST3 (serotype I), ATCC 10557 (serotype II), ATCC 10558 (serotype III), and ST7 (serotype IV), by the capillary precipitin test. Serotypes I, II, III, and IV were found to consist of 24, 16, 37, and 15 isolates. Type IV was demonstrated anew in this study. The remaining 21 isolates were not typed because of either multiple reactions or nonreactivity against the standardized typing sera. All isolates of serotype II belonged to biotype B, which resembles Streptococcus mitior physiologically. Five isolates representing four serotypes and an untypable strain were examined for their cariogenicity against specific-pathogen-free Sprague-Dawley rats fed high sucrose diet no. 2000. Organisms of each isolate were established in the mouths of the rats, but only three isolates induced weak caries that were restricted to pits and fissures of occlusal surfaces of the teeth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A. DNA base sequence homologies among strains of Streptococcus sanguis. J Gen Microbiol. 1975 Nov;91(1):92–98. doi: 10.1099/00221287-91-1-92. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- FARMER E. D. Serological subdivisions among the Lancefield group H streptococci. J Gen Microbiol. 1954 Oct;11(2):131–138. doi: 10.1099/00221287-11-2-131. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsum U., Holmberg K. Identification of Streptococcus sanguis by defined immunofluorescence. Caries Res. 1974;8(2):105–112. doi: 10.1159/000260098. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Ooshima T., Sobue S., Kotani S. Epidemiological survey of Streptococcus mutans among Japanese children. Identification and serological typing of the isolated strains. Jpn J Microbiol. 1976 Feb;20(1):33–44. doi: 10.1111/j.1348-0421.1976.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Ooshima T., Torii M., Imanishi H., Masuda N., Sobue S., Kotani S. Dental caries induction in experimental animals by clinical strains of Streptococcus mutans isolated from Japanese children. Microbiol Immunol. 1978;22(6):301–314. doi: 10.1111/j.1348-0421.1978.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Tai S., Slade H. D. Selective adsorption of heterophile polyglycerophosphate antigen from antigen extracts of Streptococcus mutans and other gram-positive bacteria. Infect Immun. 1976 Oct;14(4):903–910. doi: 10.1128/iai.14.4.903-910.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S. D., Eriksen J. Characterization of a new group specific antigen of Streptococcus sanguis. Immunochemistry. 1978 Nov;15(10-11):761–765. doi: 10.1016/0161-5890(78)90106-2. [DOI] [PubMed] [Google Scholar]
- Henriksen S. D., Henrichsen J. Further studies of twitching Streptococcus sanguis isolated from the human throat. Isolation of strains with a new antigen. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):428–432. doi: 10.1111/j.1699-0463.1976.tb01962.x. [DOI] [PubMed] [Google Scholar]
- KEYES P. H., JORDAN H. V. PERIODONTAL LESIONS IN THE SYRIAN HAMSTER. III. FINDINGS RELATED TO AN INFECTIOUS AND TRANSMISSIBLE COMPONENT. Arch Oral Biol. 1964 Jul-Aug;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., NICKERSON J. F., PERRY W. I., WALKER A. P. Peroxide and peroxidogenic bacteria in human saliva. J Bacteriol. 1957 Jun;73(6):727–735. doi: 10.1128/jb.73.6.727-735.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B., Carlsson J. Various types of streptococci and experimental caries in hamsters. Arch Oral Biol. 1970 Jan;15(1):25–32. doi: 10.1016/0003-9969(70)90142-1. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Tsutsumi N., Sobue S., Hamada S. Longitudinal survey of the distribution of various serotypes of Streptococcus mutans in infants. J Clin Microbiol. 1979 Oct;10(4):497–502. doi: 10.1128/jcm.10.4.497-502.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Kiziuta Z., White J. C. Synthesis of a Polysaccharide from Sucrose by Streptococcus S.B.E. J Bacteriol. 1946 Jun;51(6):711–716. doi: 10.1128/jb.51.6.711-716.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTERFIELD J. S. Classification of the streptococci of subacute bacterial endocarditis. J Gen Microbiol. 1950 Jan;4(1):92–101. doi: 10.1099/00221287-4-1-92. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Ranke E., Ranke B., Ahrens G., Heeschen W. Plaqueflora und Zahnkaries. 1. Vorkommen alpha-hämolysierender und vergrünender Streptokokken. Dtsch Zahnarztl Z. 1967 Jul;22(7):883–890. [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Lai C. H., Listgarten M. A. Streptococcus sanguis: a model in the application in immunochemical analysis for the in situ localization of bacteria in dental plaque. J Dent Res. 1976 Jan;55:A124–A141. doi: 10.1177/002203457605500105011. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Washburn M. R., White J. C., Niven C. F., Jr Streptococcus S.B.E.: Immunological Characteristics. J Bacteriol. 1946 Jun;51(6):723–729. doi: 10.1128/jb.51.6.723-729.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. C., Niven C. F., Jr Streptococcus S.B.E.: A Streptococcus Associated with Subacute Bacterial Endocarditis. J Bacteriol. 1946 Jun;51(6):717–722. doi: 10.1128/jb.51.6.717-722.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]



