Abstract
This study evaluated the antimicrobial activity of lauric acid (LA) and its liposomal derivatives against Propionibacterium acnes (P. acnes), the bacterium that promotes inflammatory acne. First, the antimicrobial study of three free fatty acids (lauric acid, palmitic acid and oleic acid) demonstrated that LA gives the strongest bactericidal activity against P. acnes. However, a setback of using LA as a potential treatment for inflammatory acne is its poor water solubility. Then the LA was incorporated into a liposome formulation to aid its delivery to P. acnes. It's demonstrated that the antimicrobial activity of LA was not only well maintained in its liposomal derivatives but also enhanced at low LA concentration. In addition, the antimicrobial activity of LA-loaded liposomes (LipoLA) mainly depended on the LA loading concentration per single liposomes. Further study found that the LipoLA could fuse with the membranes of P. acnes and release the carried LA directly into the bacterial membranes, thereby killing the bacteria effectively. Since LA is a natural compound that is the main acid in coconut oil and also resides in human breast milk and liposomes have been successfully and widely applied as a drug delivery vehicle in the clinic, the LipoLA developed in this work holds great potential of becoming an innate, safe and effective therapeutic medication for acne vulgaris and other P. acnes associated diseases.
Keywords: Acne vulgaris, Antimicrobial, Free fatty acid, Lauric acid, Drug delivery, Liposome
1. Introduction
Acne vulgaris (commonly called acne) is a skin disease that is most common during adolescence, afflicting more than 85% of teenagers and over 40 million people in the United States alone [1, 2]. Acne is inflammatory and associates with the immune response to Propionibacterium acnes (P. acnes), a Gram-positive bacterium that colonizes sebum-rich follicles [3]. The entire genome analysis of P. acnes has revealed numerous genes that regulate products involved in degrading host molecules and triggering inflammation [4]. It has been reported that P. acnes releases chemoattractants that attract the immune system cells such as neutrophils, monocytes and lymphocytes [5, 6]. Previous studies have also found that P. acnes stimulates the production of pro-inflammatory cytokines such as interleukins-1β, −8, −12, and tumor necrosis factor-α [7]. Besides acne, the overgrowth of P. acnes in human is also associated with many other diseases such as endocarditis and toxic shock syndrome [8].
Antimcriobial agents and antibiotics have been used epicutaneously to treat acne for several decades and are still widely prescribed for acne patients. The oxidizing agent benzoyl peroxide (BPO) has been one of the most frequently used epicutaneous medications to decrease P. acnes population in patients suffering from mild to moderate acne [9]. However, several side effects of BPO have been reported including erythema, scaling, burning, and flare [10]. In contrast, we recently demonstrated that lauric acid (LA), one of the typical free fatty acids found in the human sebum, shows stronger antimicrobial activity than BPO while not inducing any cytotoxicity to human sebocytes [11]. Nevertheless, LA is poorly water soluble and a solvent such as dimethylsulfoxide (DMSO) is required to dissolve LA into topical dosage forms. DMSO is a penetration enhancer that improves the transport rate through the skin barrier; however, its irritative and toxic side effects have been reported [12]. Furthermore, the conventional dosage forms such as cream, gel, and ointment have some major limitations, for example, they do not penetrate through the pilosebaceous unit efficiently and the effective concentration of drug is not sustained [12].
Liposomes have been extensively studied as a drug carrier since the early 1980s [13]. They have shown great potential to act as a topical delivery system for carrying drugs and skin care products. Liposomes can transport drugs to target sides and maintain a higher drug concentration than conventional dosage forms. As a result, the therapeutic effectiveness of liposomal drugs can be enhanced for several folds [14]. Because of the similarity in lipid composition to the epidermis, liposomes can also enhance dermal and transdermal drug delivery while reducing systemic absorption [15]. The study on liposomes for targeting drugs into the pilosebaceous units has suggested that liposomes are potent drug delivery systems for treating hair follicle-associated disorders such as acne [13]. In fact, Lieb et al. has proved that liposomes deliver much higher drug concentrations to the pilosebaceous unit than conventional drug formulations [16]. During the past few decades, liposomes have been used as carriers to enhance clinical efficacy for a large number of drugs. Pevaryl Lipogel as the first topical liposomal drug in the market was launched in 1988 [13]. In addition, antiacne drug-loaded liposomes such as tretinoin, clindamycin, salicylic acid, and tea tree oil-loaded liposomes have been recently reported [17, 18].
Here we use liposomes to encapsulate LA and deliver it to P. acnes without using any solvents such as DMSO. LA is an amphiphilic molecule consisting of a hydrophobic hydrocarbon chain and a hydrophilic carboxylic acid headgroup. This structure makes it a good candidate drug to be incorporated into the bilayered wall of liposomes that provides an amphiphilic environment. The present study focuses on the preparation, characterization, antimicrobial activity, and drug delivery mechanism of LA-loaded liposomes (LipoLA) against P. acnes bacteria.
2. Materials and methods
2.1. Materials
Hydrogenated L-a-Phosphatidylcholine (Egg PC), cholesterol, C6-NBD Phytosphing, and 1,2-Dimyristoyl-sn-Glycero-3-Phosphoethanolamine-N-Lissamine Rhodamine B Sulfonyl (DMPE-RhB) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Lauric acic (LA), palmitic acid (PA), oleic acid (OA), Trifluoroacetic acid, and 18-crown-6 were obtained from Sigma Aldrich (St Louis, MO). KHCO3 was from Fisher Scientific (Pittsburgh, PA). 3,4-difluorophenacyl bromide was purchased from Maybridge (Cambridge, UK). Brucella broth, Gas-Pak, and agar were purchased from BD (Sparks, MD). Defibrinated sheep blood and hemin and vitamin K solutions were from Remel (Lenexa, KS). Reinforced clostridium medium was from Oxoid (Hampshire, UK).
2.2. Preparation of bacteria and antimicrobial activity of FFAs against P. acnes
P. acnes (ATCC 6919) (American Type Culture Collection, Manassas, VA) was cultured on Brucella broth, supplemented with 5% (v/v) defibrinated sheep blood, vitamin K (5 μg/mL), and hemin (50 μg/mL), under anaerobic condition using Gas-Pak at 37°C. Single colonies were inoculated in reinforced clostridium and cultured at 37°C until reaching around OD600=1.0 (logarithmic growth phase) under anaerobic condition. The bacteria were harvested by centrifugation at 5,000 × g for 10 minutes, washed with PBS, and suspended to appropriate amount of PBS for the experiments.
The antimicrobial activities of LA, PA, and OA against P. acnes were investigated. P. acnes (1×107 CFU/mL) was incubated with LA, PA, or OA (0–100 μg/mL) in 5% DMSO in PBS at 37°C for 5 hours under anaerobic condition, and 5% DMSO in PBS was used as a control. After incubation, the samples were diluted 1:10 to 1:106 in PBS, and 5 μL of dilutions was spotted on Brucella Broth agar plates supplemented with 5% defibrinated sheep blood and hemin and vitamin K. Agar plates were incubated at 37°C under anaerobic condition for 3 days, and CFU (colony forming units) of P. acnes was quantified.
2.3 Preparation and characterization of LA-loaded liposomes
Large unilamellar LA-loaded liposomes (LipoLA) were prepared by the well-known vesicle extrusion technique [19]. Briefly, liposomes composed of 4.5 mg of Egg PC, cholesterol and LA (9:1:0, 8.5:1:0.5, 8:1:1, 7:1:2 and 5:1:4, weight ratio, respectively) were dissolved in 3 mL of chloroform, which was then evaporated by blowing argon gas for 15 minutes. The resultant lipid films were then stored over night under high vacuum to remove any traces of chloroform. The dried lipid films were then rehydrated with 3 mL of deionized water or sterile PBS buffer (1X, pH = 7.4). The suspensions of lipid were vortexed for 5 minutes and then sonicated for 10 minutes in a bath sonicator (Fisher Scientific FS30D) to produce multilamellar vesicles (MLVs). A Ti-probe (Branson 450 sonifier) was used to sonicate the MLVs for 1–2 minutes at 20 W to produce small unilamellar vesicles (SUVs). The resulting SUVs were then extruded through a 100 nm pore-sized polycarbonate membrane for 11 times to form the final products of LipoLA.
The size and zeta potential of the LipoLA were measured using the Malvern Zetasizer ZS (Malvern Instruments, UK). The mean diameters of LipoLA were determined through dynamic light scattering (DLS) and the zeta potential was determined from electrophoretic mobility measurements. All characterization measurements were repeated three times at 25°C.
2.4. LA loading yield efficiency studies
After preparation, LipoLA, which has an initial LA input of 0, 25, 50, 100, and 200 μg/mL, respectively, was dried by rotavapor (Buchi, Model R-124, Switzerland). Subsequently, the samples were dissolved in methanol and derivatized with phenacyl ester following a published protocol [20]. Briefly, the samples (10 μL) were mixed with 10 μL of KHCO3 (40 mM in aqueous solution). The solutions were then mixed with Vortex mixer and dried overnight in a vacuum oven (Isoemp model 280A). Consequently, the samples were mixed with 20 μL of Phenacyl-8 (a solution of 0.1 M phenacyl bromide and 0.005 M 18-crown-6 in acetonitrile) and agitated at 80°C for 30 min using a thermomixer (Eppendorf). Samples (20 μL) were mixed with a solution (220 μL) composing of 2 mL of 0.1 M trifluoroacetic acid and 8 mL of acetonitrile. The resulting solutions were assayed by reversed phase high performance liquid chromatography (HPLC) using Agilent 1100 series LC-MSD-Trap-SL system with an electrospray ionization source equipped with a low-flow nebulizer in positive mode (Rev B.01.03, Agilent technologies). Samples were injected into a Discovery HS C18 column (3 μm, 2.1 mm ID × 5 cm, Sigma Aldrich) with an injection volume of 25 μL. The mobile phase composed of methanol and water. The concentration gradient was 80:20 v/v at the beginning, 90:10 v/v at 10 min, and 80:20 v/v at 20 min. The flow rate was 0.2 mL/min. Derivatized LA was detected by UV/VIS detector at 254 nm, and the detector temperature was 20 °C.
2.5. Antimicrobial activity of LipoLA
To determine the antimicrobial activity of LipoLA, two sets of samples were investigated. (1) LipoLA with a constant liposome concentration and a LA loading concentration of 0, 12, 33, 80, and 102 μg/mL, respectively. In this set, for all five samples, the liposome concentration was constant while the LA concentration per liposome was different. (2) LipoLA with a LA loading concentration of 25.5, 51, and 102 μg/mL, respectively. However, the 25.5 μg/mL LipoLA and the 51 μg/mL LipoLA were obtained by diluting the 102 μg/mL LipoLA solution with PBS fourfold and twofold, respectively. In this set, for all three samples, the liposome concentration was different while the LA concentration per liposome was constant. PBS (1X, pH=7.4) and empty liposomes (without LA) were used as negative controls. All samples were incubated with P. acnes (1×107 CFU/mL) at 37°C for 5 hours under anaerobic condition. After serial dilution and spotting the dilutions on the agar plate, CFU of P. acnes was quantified as previously described in section 2.2.
2.6. LipoLA-Bacteria fusion studies
Förster resonance energy transfer (FRET) [21] was preformed to investigate the interaction mechanism between LipoLA and P. acnes. To prepare a FRET-pair labeled LipoLA, a florescent donor (C6NBD, 0.1 mol %) and a fluorescent quencher (DMPE-RhB, 0.5 mol %) were simultaneously incorporated into the LipoLA (102 μg/mL LA) by mixing the donor and acceptor with eggPC, cholesterol, and LA before the preparation of LipoLA. The resulting LipoLA was then diluted with PBS twofold to prepare LipoLA (51 μg/mL LA). Subsequently, the diluted LipoLA solutions were mixed with P. acnes (8×107, 1.2×108, 1.6×108, and 3.2×108 CFU/mL, respectively). The total volume of the final solution (LipoLA + P. acnes) was 1 mL. After 10 min incubation at room temperature, samples were centrifuged at 13,500 rpm for 1 min to remove the excess amount of LipoLA and were resuspended in PBS (1 mL). Consequently, emission spectra in the region of 500–700 nm were obtained by exciting the sample at 470 nm using fluorescent spectrophotometer (Infinite M200, TECAN, Switzerland). Solution of LipoLA (51 μg/mL LA) without incubating with P. acnes was used as a negative control. Fluorescence emission of each sample was subtracted with background signal, which was the emission of P. acnes itself at the corresponding concentration.
3. Results and discussion
3.1. Antimicrobial activity of free fatty acids (FFAs) against P. acnes
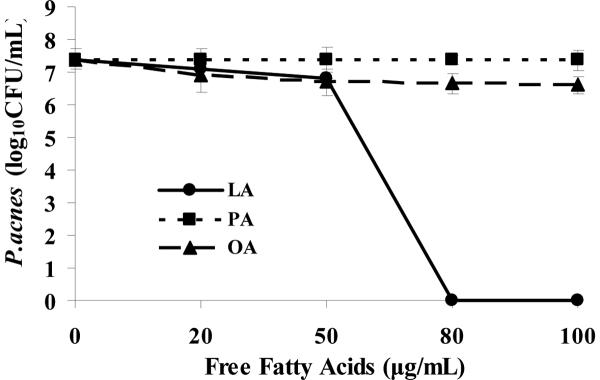
After incubating LA, PA, and OA (0–100 μg/mL) with P. acnes (1×107 CFU/mL) for 5 hours, PA and OA did not show significant inhibitory effects against bacteria. In contrast, LA completely killed P. acnes at 80 μg/mL. This result indicates that among the three typical free fatty acids found in the human sebum, LA showed the strongest antimicrobial activity against P. acnes (Figure 1). From the previous observations by Skrivanova et al. and Bergsson et al, it is likely that LA kills Gram-positive bacteria by separating their inner and outer membranes, resulting in cytoplasmic disorganization of the bacteria [22, 23]. However, this mechanism might not be applied to PA or OA, accounting for the low antimicrobial activity against P. acnes of these two FFAs.
Figure 1.
Antimicrobial effects of free fatty acids on P. acnes. P. acnes (1×107 CFU/mL) was incubated with 0∓100 μg/mL of lauric acid (LA), palmitic acid (PA), or oleic acid (OA) in 5% DMSO in PBS for 5 hours under anaerobic condition. After incubation, P. acnes suspension was diluted 1:10 –1:106 with PBS, and 5 μL of the dilutions were spotted on a Brucella Broth agar plate supplemented with 5% defibrinated sheep blood, vitamin K and hemin. After liquid in the P. acnes suspension was absorbed into the agar, the plate was incubated under anaerobic condition for 3 days before quantifying CFU of P. acnes. Data represents mean ± SD of three individual experiments.
LA is composed of a long hydrophobic carbon chain which contributes to its hydrophobic properties. It is generally difficult to dissolve LA into water. To dissolve LA into topical dosage forms such as cream and gel, a solvent such as dimethylsulfoxide (DMSO) is typically required in the preparation step. For example, in this study, we added 5% DMSO to the PBS buffer in order to dissolve LA for the antimicrobial activity studies. However, the addition of DMSO could cause irritation and toxicity to the skin [12]. It would be desirable to develop a LA delivery system without involving any solvents. One possible way is to neutralize LA with a base such as sodium hydroxide to produce laurate salt, which has higher water solubility. However the laurate salt may not have the same antimicrobial activity as LA. Another possible solution is to load LA into liposomes and then deliver it to P. acnes in its liposomal formulation. This method will be systematically investigated in this study.
3.2. Preparation and characterization of LA-loaded liposomes
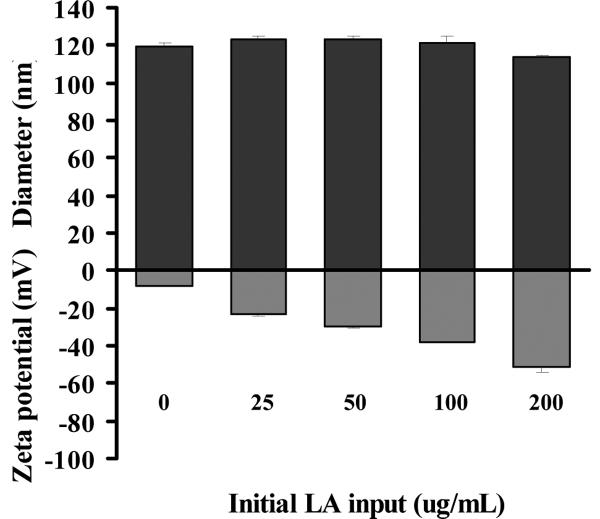
Liposome size is an important parameter in using liposomes as drug carriers in regards to encapsulation and adsorption efficiency [24]. Small liposomes (diameter, 30~200 nm) are usually unstable; they are prone to fuse with cell membranes, bacterium membranes or other artificial membranes to reduce their high surface tension [25, 26]. It has also been demonstrated that smaller liposomes with diameters near 100 nm have improved penetration through skin as compared to larger ones [13]. So LipoLA with a diameter of ~100 nm was prepared for the experiments to evaluate their potential antimicrobial activity. The vesicle size of LipoLA was characterized through two parameters: z-average mean size and polydispersity index, both of which were calculated from cumulant analysis of dynamics light scattering measurements. As shown in Figure 2, the z-average mean size of LipoLA was in the range of 113.6 ± 1.6 nm and 123.6 ± 1.5 nm when the initial LA input varied from 0~200 μg/mL. These results suggest that the increase of LA concentration has no significant effect on the liposome size. We also observed that LipoLA prepared by vesicle extrusion technique has monomodal size distribution with a polysidersity index less than 0.15, which is the characteristic of relatively narrow distribution of particle sizes.
Figure 2.
Characterization of lauric acid-loaded liposomes (LipoLA). LA at various concentrations ranging from 0~200 μg/mL was mixed with other lipid components to prepare LipoLA. The size (diameter, nm) and surface zeta potential (mV) of the LipoLA were determined by dynamic light scattering. Data represents mean ± SD of three individual experiments.
Liposome surface charge is another important factor to determine the interaction of liposomes with biological substances, and it can be characterized by the surface zeta-potential. Binding or uptake of a compound to the phospholipid membrane of a liposome can cause changes in the electrostatic potential profile across the membrane and surface, and the changes can be evaluated through the measurement of zeta potential [27]. The zeta potential of LipoLA in deionized water determined by electrophoretic mobility measurements are summarized in Figure 2. The zeta-potentials of LipoLA formulated with 0, 25, 50, 100, and 200 μg/mL initial LA input were −8.4 ± 0.1, −23.3 ± 0.9, −29.3 ± 1.2, −37.7 ± 0.4 and −51.1 ±3.3 mV, respectively. These results showed that the liposomes with and without LA were negatively charged and an increase of LA concentration led to a decrease of the zeta-potential. Comparable results were obtained for the corresponding LipoLA formulations in PBS buffer (pH = 7.4). Due to the high ionic strength of the media, the variation of zeta-potential of different LipoLAs was significantly reduced. Briefly, the zeta-potentials of LipoLA formulated with 0, 25, 50, 100, and 200 μg/mL initial LA input were −3.1 ± 0.4, −6.9 ± 0.4, −10.1 ± 0.7, −14.6 ± 1.6 and −22.5 ± 2.1 mV, respectively, in PBS buffer (pH = 7.4). Free fatty-acids have a pKa value of approximately 5, thus at near physiological pH of 7.4 the carboxyl group of the free fatty acids will deprotonate and attribute to the negatively charged surface of the liposome. This explains the observation of an increase of LA concentration in liposome leading to a decrease of the zeta-potential, and confirms that LA is indeed incorporated into the liposomes.
3.3. Quantification of LA loading yield in LipoLA formulations
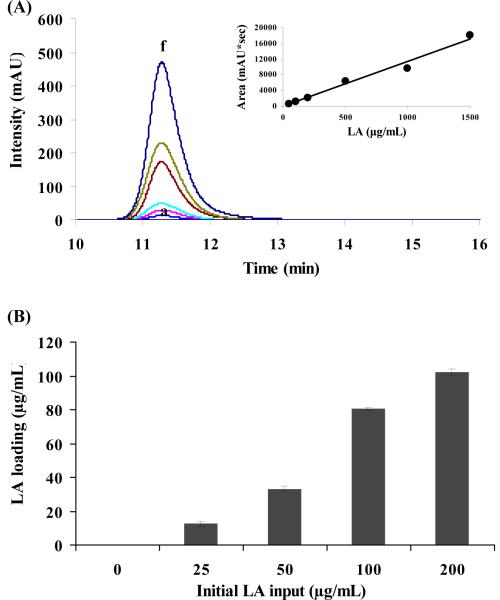
The loading yield of LA in LipoLA formulations was evaluated by HPLC. When using the HPLC technique to separate LA from the other components in LipoLA, LA must be derivatized with phenacyl ester first because the functional group of LA is a carboxylic group which has negligible UV absorbance. After being derivatized with phenacyl bromide, derivatized LA could be detected by the UV/VIS detector at 254 nm. We first measured the UV absorbance intensity of a series of derivatized LA samples ranging from 0∓1500 μg/mL to genterate a standard curve (Figure 3A). Then the loading concentration of LA in all LipoLA formulations was quantified by comparing with the standard curve. As shown in Figure 3B, for the LipoLA formulations with an initial LA input concentration of 0, 25, 50, 100, and 200 μg/mL, their LA loading yield was 0, 12, 33, 80, and 102 μg/mL, respectively. The corresponding drug encapsulation efficiency was 50%, 66%, 80%, and 51% respectively. These results showed that the encapsulation efficiency of LA increased as the weight ratio of LA increased until it reached 100 μg/mL of the initial LA input. At 200 μg/mL of the initial LA input, however, encapsulation efficiency dropped. This might be because the LA loading reaches a saturation point at the range of 100∓200 μg/mL initial input. These results also indicated that some LA was lost during the LipoLA preparation steps which could be sonication and extrusion steps. Using needle sonicator, the samples were in a close contact with the needle, so some LA might adhere to the needle and the glass vials. In addition, LA may stick with extrusion membrane or apparatus itself resulting in the loss of LA.
Figure 3.
Quantification of LA loading in LipoLA by HPLC. (A) UV absorption intensity of derivatized LA at the concentrations of a-f: 50, 100, 200, 500, 1000, and 1500 μg/mL. Inset: the corresponding linear calibration standard curve. (B) The loading of LA in the LipoLA formulations with an initial LA input of 0, 25, 50, 100, and 200 μg/mL was 0, 12, 33, 80, and 102 μg/mL, respectively. Data represents mean ± SD of three individual experiments.
3.4. Antimicrobial activity of LipoLA
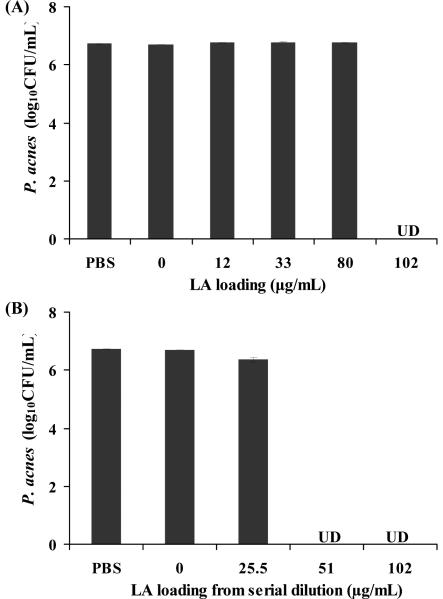
Five samples of LipoLA (0, 12, 33, 80, and 102 μg/mL LA) were incubated with P. acnes (1×107 CFU/mL) for 5 hours under anaerobic condition in order to determine their antimicrobial activity. The results showed that LipoLA with 102 μg/mL LA completely killed the bacteria (Figure 4A). In these five samples, the LA loading concentration was different but the molar concentration of the liposome in each sample was kept constant. To further investigate the system, LipoLA with 102 μg/mL LA was diluted twofold and fourfold to obtain 51 and 25.5 μg/mL LipoLA, respectively, and their antimicrobial activities were determined. Surprisingly, experimental results showed that P. acnes was completely killed by 51 μg/mL LipoLA (Figure 4B). One possible way to explain this phenomenon is the following. By diluting 102 μg/mL LipoLA to 51 μg/mL LipoLA, the amount of LA encapsulated inside each liposome of these two samples was identical although the amount of liposome per volume of 51 μg/mL LipoLA was lower. Due to the small size of P. acnes (1~2 μm, measured by dynamic light scattering), only a few LipoLA (~120 nm) could interact with each P. acnes bacterium, and for 51 μg/mL sample, the amount of liposome was enough to interact with all bacteria in the solution, thus the fully killing effect was observed. On the other hand, fully killing effect of 80 μg/mL LipoLA in the previous set of experiments (Figure 4A) was not observed because the amount of LA in each liposome was not high enough to kill bacteria even though the amount of liposome was enough to interact with all bacteria. From these results, it was obvious that the antimicrobial activity of LipoLA was determined mostly by the amount of LA loaded in each liposome, not the liposome concentration because one colony of P.acnes could interact only with a few liposomes.
Figure 4.
Antimicrobial activity of LipoLA against P. acnes. Two sets of LipoLA were incubated with P. acnes (1×107 CFU/mL), respectively, for 5 hours under anaerobic condition to test their antimicrobial activity. (A) LipoLA with a LA loading concentration of 0, 12, 33, 80, and 102 μg/mL, respectively. In this set, the LipoLA concentration of each sample was constant while the LA concentration per liposome was different. The results showed that 102 μg/mL LipoLA completely killed P. acnes. (B) LipoLA with a LA loading concentration of 25.5, 51, and 102 μg/mL, respectively. The 25.5 μg/mL LipoLA and the 51 μg/mL LipoLA were obtained by diluting the 102 μg/mL LipoLA solution with PBS fourfold and twofold, respectively. In this set, the LipoLA concentration of each sample was different while the LA concentration per liposome was constant. The results showed that both 51 μg/mL LipoLA and 102 μg/mL LipoLA completely killed bacteria. Incubation with PBS buffer and empty liposome solution (without LA) served as negative controls. Data represents mean ± SD of three individual experiments. UD: undetectable.
These studies confirmed that by loading LA into liposomes to form LipoLA, the antimicrobial activity of LA has been well maintained. Moreover, serial dilution data (Figure 4B) showed that LipoLA is even more effective than free LA (Figure 1), in which 50 μg/mL free LA did not produce significant bacteria killing. A major reason caused this antimicrobial activity enhancement might be that each LipoLA carries a lethal dosage of LA. Thus, even only one or few LipoLA interacts with P. acnes; the amount of LA is high enough to kill the bacterium effectively.
3.5. Mechanism of the interaction between LipoLA and P. acnes
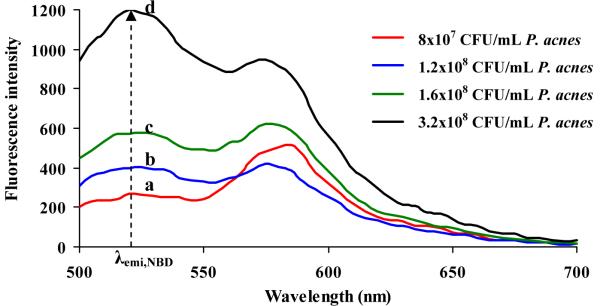
In order to further understand the interaction mechanism between LipoLA and the bacteria, we labeled LipoLA with a FRET pair of chromophores and monitored the FRET signal changes upon mixing LipoLA with the bacteria at various conditions. FRET is a widely used technique that precisely measures the distance of two subjects at the molecular level based on an energy transfer mechanism of two chromophores [21, 28]. Typically, the two subjects are labeled with a donor chromophore and an acceptor chromophore, respectively. When excited, the donor chromophore can transfer energy to the acceptor chromophore in close proximity (<10 nm) through a nonradiative long-range dipole-dipole coupling mechanism. Here we incorporated a fluorescence donor (C6NBD: excitation/emission= 470 nm/520 nm) and a fluorescence acceptor (DMPE-RhB: excitation/emission= 550 nm/580 nm) into the lipid membranes of LipoLA. By controlling the molar ratio between the donor and the acceptor, we prepared the fluorescent LipoLA in which the fluorescence emission from the donor was completely quenched by the acceptor. We hypothesized that if the LipoLA fuses with bacterial membranes, the spread of the donor and acceptor chromophores within the bacterial membranes will alleviate or eliminate the FRET efficiency, resulting in fluorescence recovery of the donor. To this end, we mixed the FRET-pair labeled fluorescent LipoLA with several concentrations of P. acnes, followed by removing the excess LipoLA. The samples were then excited at the wavelength of 470 nm. As shown in Figure 5, the rise in the emission peak of C6NBD at 520 nm was detected when the concentration of P. acnes increased indicating spatial separation between C6NBD and DMPE-RhB increased and the efficiency of energy transfer decreased. The changes of the emission peaks of DMPE-RhB, however, were neglected in our studies for the reason that the DMPE-RhB could be excited by not only the FRET from C6NBD but also excitation wavelength at 470 nm. This made it difficult to draw a conclusion based on the emission changes of DMPE-RhB. LipoLA at the same concentration while not mixing with bacteria was used as a control for this study. When the control sample was excited at 470 nm, no emission at 520 nm was detected but the emission peak at 580 nm was observed.
Figure 5.
FRET measurements of the fusion between LipoLA and P. acnes. A florescent donor (C6NBD) and a fluorescent acceptor (DMPE-RhB) were simultaneously incorporated into the LipoLA (51 μg/mL LA) with a proper molar ratio that the acceptor completely quenched the fluorescence emission from the donor. The FRET-pair labeled LipoLA was incubated with P. acnes at a concentration of a-d: 8×107, 1.2×108, 1.6×108, and 3.2×108 CFU/mL) for 10 minutes. After removing the excess LipoLA, all samples were excited at 470 nm. A rise in emission intensity of C6NBD (donor) at 520 nm was observed with the increasing bacterial concentration, indicating the occurrence of fusion between LipoLA and bacteria which caused the spatial separation of C6NBD and DMPE-RhB.
These observations indicate that the interaction mechanism between LipoLA and P. acnes was fusion; not adsorption or aggregation. That is, LipoLA fused with the membranes of P. acnes and released the carried LA into the bacterial membranes. This finding together with the antimicrobial activity of LipoLA is consistent with the mechanism of LA killing Gram-positive bacteria by separating the inner and outer membranes of the bacteria. This fusion mechanism is also consistent with previous reports of liposomal drug delivery to Gram-positive bacteria such as Staphylococcus aureus and Stenotrophomonas maltophilia [29].
4. Conclusions
We evaluated the antimicrobial property of lauric acid (LA), oleic acid (OA), and palmitic acid (PA) against P. acnes in vitro. Among the three free fatty acids, LA showed the strongest bactericidal activity. To resolve the poor water solubility issue of LA, we successfully incorporated LA into liposomes to form LipoLA. The size of the LipoLA was near 120nm regardless of the loading concentration of LA and the negative surface charge of the LipoLA reflected the amount of LA in the liposome membrane. We further demonstrated that after loading LA into liposomes, its antimicrobial activity against P. acnes was well maintained and even enhanced at low LA concentration. The LipoLA showed complete killing of P. acne at the LA concentration of 51 ug/mL, while free LA dissolved in PBS buffer containing 5 % DMSO near the same concentration had negligible bactericidal effects on P. acnes. We also found that the antimicrobial activity of LipoLA mainly depended on the LA loading concentration per single liposomes. Further study disclosed that the LipoLA could fuse with the membranes of P. acnes and release the carried LA directly into the bacterial membranes. We conclude that liposomal encapsulation of LA at optimal formulation can result in an increased availability of the LA for efficient elimination of P. acnes. Since liposomes have been widely used in cosmetics products and also approved for use in the clinic as a drug delivery vehicle, and LA is the main acid in coconut oil and naturally resides in human breast milk, the LipoLA developed in this work has the potential of becoming an innate, safe and effective therapeutic medication for acne vulgaris and other P. acnes associated diseases.
Acknowledgements
LZ acknowledges a financial support from the University of California-San Diego (faculty startup fund) and the Hellman Faculty Fellowship. CH acknowledges financial supports from NIH (R01-AI067395-01, R21-R022754-01 and R21-I58002-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fried RG, Wechsler A. Psychological problems in the acne patient. Dermatol Ther. 2006;19:237–40. doi: 10.1111/j.1529-8019.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- [2].Taglietti M, Hawkins CN, Rao J. Novel topical drug delivery systems and their potential use in acne vulgaris. Skin Therapy Lett. 2008;13:6–8. [PubMed] [Google Scholar]
- [3].Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–9. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [4].Bruggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–3. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- [5].Burkhart CG, Burkhart CN, Lehmann PF. Acne: a review of immunologic and microbiologic factors. Postgrad Med J. 1999;75:328–31. doi: 10.1136/pgmj.75.884.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Webster GF, Leyden JJ. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation. 1980;4:261–9. doi: 10.1007/BF00915027. [DOI] [PubMed] [Google Scholar]
- [7].Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–8. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- [8].Nakatsuji T, Rasochova L, Huang CM. Vaccine therapy for P. acnes-associated diseases. Infect Disord Drug Targets. 2008;8:160–5. doi: 10.2174/1871526510808030160. [DOI] [PubMed] [Google Scholar]
- [9].Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17–24. doi: 10.1016/j.det.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [10].Castro GA, Ferreira LA. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin Drug Deliv. 2008;5:665–79. doi: 10.1517/17425247.5.6.665. [DOI] [PubMed] [Google Scholar]
- [11].Nakatsuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL, et al. Antimicrobial Property of Lauric Acid Against Propionibacterium Acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.93. doi:10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmid MH, Korting HC. Therapeutic progress with topical liposome drugs for skin disease. Adv Drug Delivery Rev. 1996;18:335–42. [Google Scholar]
- [13].Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258:141–51. doi: 10.1016/s0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- [14].Honzak L, Sentjurc M. Development of liposome encapsulated clindamycin for treatment of acne vulgaris. Pflugers Arch. 2000;440:R44–5. [PubMed] [Google Scholar]
- [15].Mezei M, Gulasekharam V. Liposomes-a selective drug delivery system for the topical route of administration: gel dosage form. J Pharm Pharmacol. 1982;34:473–4. doi: 10.1111/j.2042-7158.1982.tb04767.x. [DOI] [PubMed] [Google Scholar]
- [16].Lieb LM, Ramachandran C, Egbaria K, Weiner N. Topical delivery enhancement with multilamellar liposomes into pilosebaceous units: I. In vitro evaluation using fluorescent techniques with the hamster ear model. J Invest Dermatol. 1992;99:108–13. doi: 10.1111/1523-1747.ep12611886. [DOI] [PubMed] [Google Scholar]
- [17].Bhalerao SS, Raje Harshal A. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev Ind Pharm. 2003;29:451–67. doi: 10.1081/ddc-120018380. [DOI] [PubMed] [Google Scholar]
- [18].Biju SS, Ahuja A, Khar RK. Tea tree oil concentration in follicular casts after topical delivery: determination by high-performance thin layer chromatography using a perfused bovine udder model. J Pharm Sci. 2005;94:240–5. doi: 10.1002/jps.20250. [DOI] [PubMed] [Google Scholar]
- [19].Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986;858:161–8. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- [20].Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase HPLC: Chromatographic mobility as a function of physico-chemical properties. Int J Molecular Sci. 2007;8:1111–24. [Google Scholar]
- [21].Ha T. Single-molecule fluorescence resonance energy transfer. Methods. 2001;25:78–86. doi: 10.1006/meth.2001.1217. [DOI] [PubMed] [Google Scholar]
- [22].Bergsson G, Arnfinnsson J, Steingrimsson O, Thormar H. Killing of Gram-positive cocci by fatty acids and monoglycerides. Apmis. 2001;109:670–8. doi: 10.1034/j.1600-0463.2001.d01-131.x. [DOI] [PubMed] [Google Scholar]
- [23].Skrivanova E, Marounek M, Dlouha G, Kanka J. Susceptibility of Clostridium perfringens to C-C fatty acids. Lett Appl Microbiol. 2005;41:77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- [24].Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv Drug Deliv Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- [25].Haluska CK, Riske KA, Marchi-Artzner V, Lehn J-M, Lipowsky R, Dimova R. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci USA. 2006;103:15841–6. doi: 10.1073/pnas.0602766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang L, Granick S. How to stabilize phospholipid liposomes (using nanoparticles) Nano Lett. 2006;6:694–8. doi: 10.1021/nl052455y. [DOI] [PubMed] [Google Scholar]
- [27].Carrion FJ, Delamaza A, Parra JL. The influence of ionic-strength and lipid bilayer charge on the stability of liposomes. J Colloid Inter Sci. 1994;164:78–87. [Google Scholar]
- [28].Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, et al. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7:3065–70. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- [29].Beaulac C, Sachetelli S, Lagace J. In-vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against gram-negative and gram-positive bacteria. J Antimicrob Chemother. 1998;41:35–41. doi: 10.1093/jac/41.1.35. [DOI] [PubMed] [Google Scholar]