Abstract
We evaluated the effect of cacao procyanidins (CP) on plasma lipid levels in high cholesterol-fed rats. Animals were divided into 4 groups, and each group was fed on either a normal diet, high cholesterol diet (HCD) containing 1% cholesterol (HCD without CP), HCD with 0.5% (HCD with 0.5% CP) or 1.0% CP (HCD with 1.0% CP) for 4 weeks. Plasma cholesterol level was significantly higher in the HCD without CP group than the normal diet group (p<0.01). Supplementation of CP significantly decreased plasma cholesterol (p<0.01) to levels similar to those of the normal diet group. The liver cholesterol and triglyceride levels in all HCD groups were significantly higher (p<0.01), but 1.0% CP feeding significantly reduced this increase. Fecal excretion of neutral sterol and triglyceride was significantly increased in all HCD groups (p<0.01), and the excreted amounts tended to be higher in the HCD with CP groups. The procyanidins dose-dependently reduced micellar solubility of cholesterol and this activity increased with increasing molecular weight. These results suggest that one of the mechanisms of CP to lower plasma cholesterol is inhibition of intestinal absorption of cholesterol.
Keywords: Cacao, procyanidins, cholesterol, rat, micellar
Introduction
Cacao bean, the seed of Theobroma cacao, is known to be rich in polyphenols, such as the procyanidin monomers ((+)-catechin and (−)-epicatechin) and oligomers (B-type procyanidins that are linked by C4–C8 bonds) [1–3]. Recent epidemiological evidence suggests that the ingestion of monomers of procyanidins prevents coronary heart diseases [4, 5]. In addition, several reports have indicated that the susceptibility of low-density lipoprotein (LDL) to oxidation was significantly decreased in humans [6–8] and high cholesterolemic rabbits [9] by dietary supplementation with cocoa or its procyanidins fraction. Moreover, many reports support the possibility that procyanidins in cacao can prevent cardiovascular disease by improving blood flow rate [10], improving platelet function [11, 12], changing inflammatory responses in endothelial cells of blood vessels [13, 14], and so on. On the other hand, many reports attribute the decrease in plasma cholesterol levels in experimental animals to the ingestion of tea [15], citrus fruit [16], grape [17] and wine flavanoids [18].
In this report, we evaluated the plasma cholesterol lowering activity of cacao procyanidins (CP) in rats fed a high cholesterol diet.
Materials and Methods
Ethics
The protocol of this study was approved by the Animal Care and Use Committee of Meiji Seika Food and Health Laboratories. All animals received humane care under the guidelines of this institution.
Animals and diets
Nine-week-old male Sprague-Dawley rats were obtained from Crea Japan Inc. (Tokyo, Japan). The rats were housed individually and kept in a room at regulated temperature 23–25°C and controlled lighting (12-h light and dark cycle). CP was prepared from cacao liquor by the method described in a previous report [19]. Briefly, cacao liquor was defatted with n-hexane, and then the residue was extracted with acetone. The n-butanol soluble fraction of the extract was applied to a Diaion HP2MG column (Mitsubishi Kasei Co. Ltd., Tokyo, Japan). The fraction eluted with 80% ethanol was collected, freeze dried, and then used. The composition of the CP is as follows: total polyphenols, 79.3%; (+)-catechin, 2.5%; (−)-epicatechin, 5.9%; procyanidins B2, 4.0%; procyanidins C1, 2.6%; and cinnamtannin A2, 3.2%.
Experimental procedure
The animals were fed on a basal diet (MF, Oriental Yeast, Co. Ltd., Tokyo, Japan) for 4 days. Forty rats were divided into four groups, and each group was fed on experimental diets for 4 weeks: either a normal diet (AIN-93 diet), high cholesterol diet (HCD) (containing 1% cholesterol and 15% fat), HCD with 0.5% CP, or HCD with 1.0% CP. The dose of CP (0.5% and 1.0%) was selected for testing because 1.0% CP-containing diet showed significant reduction of atherosclerotic lesion formation in previous animal model studies. Blood was taken from the tail vein before and at the end of each week during the feeding period, and the plasma cholesterol level was measured. To determine the excretion of neutral sterol and triglyceride, feces were collected at 2 and 4 weeks of the experiment. At the end of the experiment, each animal was anesthetized with ethyl ether, a blood sample was taken from the abdominal vein, and the liver was removed and stored at −80°C until analysis.
Analysis
Cholesterol in plasma was measured enzymatically using the Cholesterol C-test Wako (Wako Pure Chemical Industries, Tokyo, Japan). Other plasma components were also measured using commercially available kits (Wako Pure Chemical Industries). Liver and feces lipids were extracted with methanol and chloroform according to the method described in Folch et al. [20]. Triglyceride in liver and feces and cholesterol in liver were measured using commercially available kits as mentioned above. Neutral sterol in feces was determined by the method of Sautier et al. with a kit (Wako Pure Chemical Industries).
In vitro study
The effect of CP on micellar solubility was measured by the method of Ikeda et al. with slight modification [21]. Briefly, a micellar solution containing 6.6 mM sodium taurocholate, 0.6 mM egg yolk phosphatidylcholine, 0.5 mM cholesterol, 132 mM NaCl, and 15 mM sodium phosphate at pH 7.4 was prepared by sonication and kept at 37°C. Exactly 100 µl of various concentrations of CP, or solutions of monomeric, dimeric, trimeric, or tetrameric procyanidins was added to 5 ml of micellar solution, and the mixtures were incubated for 1 h at 37°C and centrifuged at 1000 × g for 10 min. The cholesterol concentration of the supernatant, which was still soluble in the micellular mixture, was measured using a commercially available kit. Procyanidin monomer and oligomer fractions were prepared using a previously reported method [22]. Briefly, CP was injected onto a semipreparative HPLC column (Supelcosil LC-Si), eluted with a dichloromethane – methanol – formic acid – water linear gradient, and collected.
Statistical analysis
The data are expressed as the mean and standard deviation. Analyses were performed using the SPSS statistical software package (SPSS Inc., Chicago, IL). Comparisons of the multi-groups in the in vivo and in vitro studies were made using ANOVA followed by Tukey’s multiple comparison test.
Results
Animal study
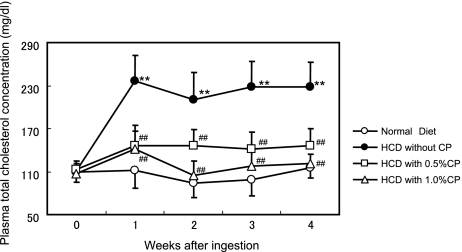
Body weight and food intake were significantly higher in all HCD groups than in the normal diet group, and were not influenced by CP treatment (Table 1). The total cholesterol concentration in plasma was markedly increased throughout the experimental period by ingestion of the HCD (Fig. 1). In contrast, this increase was strikingly inhibited by CP supplementation. The plasma total cholesterol levels were similar in the CP groups and the normal diet group. Table 2 shows free cholesterol, cholesterol ester, triglyceride, phospholipids, and free fatty acid levels at the end of the experiment. A significant difference between the normal diet group and the HCD without CP group was seen in free cholesterol but not in the other plasma lipid levels.
Table 1.
Body Weight and Food Intake and Food Efficiency of Rats Fed the Experimental Diet
| body weight (g) | food intake (g/day) | |
|---|---|---|
| normal diet | 448.9 ± 18.2 | 21.2 ± 1.4 |
| HCD | ||
| without CP | 518.2 ± 29.5** | 22.4 ± 1.9* |
| with 0.5% CP | 526.5 ± 54.8* | 22.2 ± 1.4* |
| with 1.0% CP | 517.6 ± 36.1** | 22.8 ± 1.5* |
Values were mean and standard deviation.
Significanlty different from normal diet; * p<0.05, ** p<0.01
Fig. 1.
Changes in levels of plasma total cholesterol in rats fed on the experimental diet. Values are presented as the mean and standard deviation. Open circle; normal diet, closed circle; high cholesterol diet, open square; 0.5% CP-containing HCD diet, open triangle; 0.5% CP-containing HCD diet. Significantly different from the Normal Diet, ** p<0.01. Significantly different from the HCD without CP, ## p<0.01.
Table 2.
Plasma Lipid Concentration of Rats Fed the Experimental Diet
| free cholesterol (mg/dl) | cholesterol ester (mg/dl) | tryglyceride (mg/dl) | phospholipid (mg/dl) | free fatty acid (mEq/l) | |
|---|---|---|---|---|---|
| normal diet | 10.9 ± 2.3 | 56.8 ± 5.4 | 75.7 ± 22.2 | 127.0 ± 15.3 | 0.71 ± 0.09 |
| HCD | |||||
| without CP | 19.7 ± 11.2** | 61.5 ± 12.6 | 78.7 ± 31.3 | 114.4 ± 10.6 | 0.77 ± 0.10 |
| with 0.5% CP | 17.2 ± 7.6** | 64.1 ± 16.3 | 81.3 ± 22.3 | 118.6 ± 21.5 | 0.74 ± 0.10 |
| with 1.0% CP | 24.8 ± 12.5** | 45.5 ± 19.5 | 71.7 ± 15.3 | 122.9 ± 24.0 | 0.78 ± 0.13 |
Values were mean and standard deviation. Significanlty different from normal diet, ** p<0.01
Liver weight, and cholesterol and triglyceride levels in liver are shown in Table 3. Liver weight was significantly different between the normal diet group and all HCD groups and was not affected by CP. Cholesterol and triglyceride levels in liver were markedly increased by the HCD and significantly lower in the rats fed on the HCD with 1% CP than in those fed on the HCD without CP.
Table 3.
Weight, Levels of Cholesterol and Fat in the Liver of Rats Fed the Experimental Diet
| weight (g) | cholesterol (mg/liver) | triglyceride (g/liver) | |
|---|---|---|---|
| normal diet | 11.7 ± 1.0 | 9.2 ± 1.9 | 0.63 ± 0.10 |
| HCD | |||
| without CP | 26.8 ± 3.9** | 404.7 ± 73.7** | 7.69 ± 1.86** |
| with 0.5% CP | 26.6 ± 2.6** | 304.9 ± 49.7** | 6.18 ± 0.86** |
| with 1.0% CP | 24.9 ± 4.0** | 285.1 ± 86.3**## | 5.89 ± 1.84**## |
Values were mean and standard deviation.
Significanlty different from normal diet; * p<0.05, ** p<0.01
Significanlty different from HCD without CP; ## p<0.01
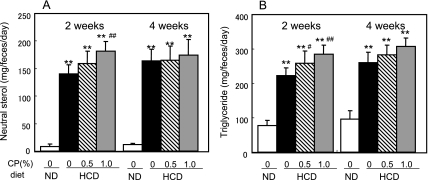
Fecal excretion levels of neutral sterol and triglyceride were markedly increased by ingestion of the HCD (Fig. 2). After 2 weeks of feeding, neutral sterol excretion was significantly higher in rats fed on the HCD with 1% CP than rats fed on the HCD without CP. At 4 weeks, this difference persisted but was not significant. Similarly, increase in triglyceride excretion in both CP groups was significant at 2 weeks but not significant at 4 weeks.
Fig. 2.
Excretion of neutral sterol (A) and triglyceride (B) in feces of rats fed on the experimental diet. Values are presented as the mean and standard deviation. Significantly different from the Normal Diet (ND), ** p<0.01. Significantly different from the HCD without CP, ## p<0.01.
In vitro study
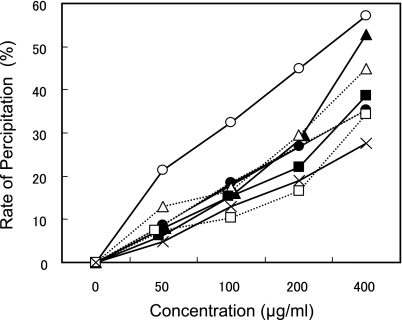
The effect of catechins on micellar solubility of cholesterol in vitro is shown in Fig. 3. The addition of CP to a mixed micellar solution caused precipitation of cholesterol and decreased cholesterol concentration in the supernatant. Each of the procyanidins increased the precipitation rate in a dose-dependent manner. The effect of procyanidins on precipitation of micellar cholesterol increased with the size of the oligomer as follows: tetramer > trimer > CP (procyanidins mixture) > dimer > monomer.
Fig. 3.
Effects of procyanidins on the solubility of cholesterol in micelles. Values are presented as the means of three independent tests. Closed circle; CP, cross; monomer, open square; dimer, closed square; trimer, open triangle; tetramer.
Discussion
In this study, ingestion of CP reduced plasma total cholesterol levels in rats fed on a high cholesterol diet (Fig. 1). The accumulation of cholesterol and triglyceride in liver of HCD-fed rats was significantly decreased by addition of 1% CP to the diet (Table 2). In addition, fecal excretion of neutral sterol and triglyceride in the 1% CP group on the HCD was significantly increased at 2 weeks but not significantly increased at 4 weeks (Fig. 2). This difference between 2 and 4 weeks might indicate a certain type of adaptation. According to these data, CP might inhibit intestinal absorption of dietary cholesterol and triglyceride. Then we examined the effect of CP and its components on cholesterol micellar solubility. All procyanidins used in this study (from monomers to tetramers) precipitated micellar cholesterol and this ability was dependent on their molecular weight. Ikeda et al. reported that tea catechins inhibited the intestinal absorption of cholesterol [21]. Of the tea catechins, free catechins such as (−)-epicatechin and (−)-epigallocatechin showed slight activity, while their gallate esters such as (−)-epicatechin gallate and (−)-epigallocatechin gallate were more effective. Effectiveness was correlated with ability to precipitate cholesterol in mixed bile salt micelles. Ikeda et al. suggested that the catechins were co-precipitated with cholesterol, and modified cholesterol was converted to insoluble forms that could not be absorbed from the intestine. In this report, we confirmed that procyanidin monomers (like free catechins [(+)-catechin and (−)-epicatechin]) have low activity, as previously reported. The present data show that the ability of procyanidins to precipitate cholesterol correlated with degree of polymerization. To elucidate the interaction of cholesterol and procyanidins, a more detailed study is necessary.
Buijsse et al. analyzed the relationship between habitual cocoa intake and cardiovascular diseases in 470 elderly men participating in the Zutphen Elderly Study. They reported that the adjusted relative risk of cardiovascular mortality for men in the highest tertile of cocoa intake compared with the lowest tertile was 0.5, and concluded that there was an inverse relationship between cocoa intake and 15-year cardiovascular mortality. Cocoa is a rich source of bioactive compounds such as dietary fiber and magnesium as well as procyanidins that have potential benefit for reducing the risk of coronary artery disease. We have previously reported that the contribution to prevention of cardiovascular disease seems to be higher for procyanidins than for other components. Kurosawa and Kusanagi-hypercholesterolemic rabbits (a spontaneous familial hypercholesterolemic model) fed on a diet containing 1.0% cocoa procyanidins fraction for 6 months showed significant reduction in atherosclerotic lesion formation, and this reduction was similar to that in other rabbits fed on a diet containing an equivalent amount of cocoa procyanidins [23, 24].
Moreover, a recent intervention trial revealed that supplementation with cocoa improves several risk factors for coronary heart disease, such as blood pressure, insulin sensitivity, vascular endothelial function, and the susceptibility of LDL to oxidation. In addition, we reported that cocoa ingestion improves plasma lipid levels in our latest intervention trial. Daily ingestion of two cups of cocoa for 12 weeks significantly decreased LDL in volunteer participants with total cholesterol level more than 200 mg/dl [25]. These data suggested that the improvement in plasma lipid values is partially due to the ability of cocoa or its procyanidin fractions to inhibit intestinal absorption of cholesterol. Among the wide range of actions by CP on risk factors for coronary heart disease, the inhibition of cholesterol or bile acid absorption is one of the most important.
Abbreviations
- CP
cacao procyanidins
- HCD
high cholesterol diet
- LDL
low-density lipoprotein
- AIN
American Institute of Nutrition
References
- 1.Hammerstone J.F., Lazarus S.A., Mitchell A.E., Rucker R., Schmitz H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999;47:490–496. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- 2.Hatano T., Miyatake H., Natsume M., Osakabe N., Takizawa T., Ito H., Yoshida T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry. 2002;59:749–758. doi: 10.1016/s0031-9422(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 3.Sanbongi C., Osakabe N., Natsume M., Takizawa T., Gomi S., Osawa T. Antioxidative polyphenols isolated from Theobroma cacao. J. Agric. Food Chem. 1998;46:454–457. doi: 10.1021/jf970575o. [DOI] [PubMed] [Google Scholar]
- 4.Arts I.C.W., Hollman P.C.H., Feskens E.J.M., Bueno de Mesquita H.B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Arts I.C.W., Jacobs D.R. Jr., Harnack L.J., Gross M., Folsom A.R. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Osakabe N., Baba S., Yasuda A., Iwamoto T., Kamiyama M., Takizawa T., Itakura H., Kondo K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.F., Schramm D.D., Holt R.R., Ensunsa J.L., Fraga C.G., Schmitz H.H., Keen C.L. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000;130:S2115–S2119. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 8.Wan Y., Vinson J.A., Etherton T.D., Proch J., Lazarus S.A., Kris-Etherton P.M. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 9.Osakabe N., Natsume M., Adachi T., Yamagishi M., Hirano R., Takizawa T., Itakura H., Kondo K. Effects of cacao liquor polyphenols on the susceptibility of low-density lipoprotein to oxidation in hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2000;7:164–168. doi: 10.5551/jat1994.7.164. [DOI] [PubMed] [Google Scholar]
- 10.Heiss C., Dejam A., Kleinbongard P., Schewe T., Sies H., Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 11.Keen C.L., Holt R.R., Oteiza P.I., Fraga C.G., Schmitz H.H. Cocoa antioxidants and cardiovascular health. Am. J. Clin. Nutr. 2005;81:S298–S303. doi: 10.1093/ajcn/81.1.298S. [DOI] [PubMed] [Google Scholar]
- 12.Holt R.R., Schramm D.D., Keen C.L., Lazarus S.A., Schmitz H.H. Chocolate consumption and platelet function. JAMA. 2002;287:2212–2213. doi: 10.1001/jama.287.17.2212. [DOI] [PubMed] [Google Scholar]
- 13.Schramm D.D., Wang J.F., Holt R.R., Ensunsa J.L., Gonsalves J.L., Lazarus S.A., Schmitz H.H., German J.B., Keen C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001;73:36–40. doi: 10.1093/ajcn/73.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Ale-Agha N., Stahl W., Sies H. (−)-Epicatechin effects in rat liver epithelial cells: stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Biochem. Pharmacol. 2002;63:2145–2149. doi: 10.1016/s0006-2952(02)01021-3. [DOI] [PubMed] [Google Scholar]
- 15.Loest H.B., Noh S.K., Koo S.I. Green tea extract inhibits the lymphatic absorption of cholesterol and alpha-tocopherol in ovariectomized rats. J. Nutr. 2002;132:1282–1288. doi: 10.1093/jn/132.6.1282. [DOI] [PubMed] [Google Scholar]
- 16.Park Y.B., Do K.M., Bok S.H., Lee M.K., Jeong T.S., Choi M.S. Interactive effect of hesperidin and vitamin E supplements on cholesterol metabolism in high cholesterol-fed rats. Int. J. Vitam. Nutr. Res. 2001;71:36–44. doi: 10.1024/0300-9831.71.1.36. [DOI] [PubMed] [Google Scholar]
- 17.Yamakoshi J., Kataoka S., Koga T., Ariga T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–149. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 18.Auger C., Caporiccio B., Landrault N., Teissedre P.L., Laurent C., Cros G., Besancon P., Rouanet J.M. Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic golden Syrian hamsters (Mesocricetus auratus) J. Nutr. 2002;132:1207–1213. doi: 10.1093/jn/132.6.1207. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi M., Osakabe N., Takizawa T., Osawa T. Cacao liquor polyphenols reduce oxidative stress without maintaining alpha-tocopherol levels in rats fed a vitamin E-deficient diet. Lipids. 2001;36:67–71. doi: 10.1007/s11745-001-0669-9. [DOI] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Sloane-Stanley G.H.. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Ikeda I., Imasato Y., Sasaki E., Nakayama M., Nagao H., Takeo T., Yayabe F., Sugano M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 22.Natsume M., Osakabe N., Yamagishi M., Takizawa T., Nakamura T., Miyatake H., Hatano T., Yoshida T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000;64:2581–2587. doi: 10.1271/bbb.64.2581. [DOI] [PubMed] [Google Scholar]
- 23.Kurosawa T., Itoh F., Nozaki A., Nakano Y., Katsuda S., Osakabe N., Tsubone H., Kondo K., Itakura H. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis. 2005;179:237–246. doi: 10.1016/j.atherosclerosis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kurosawa T., Itoh F., Nozaki A., Nakano Y., Katsuda S., Osakabe N., Tsubone H., Kondo K., Itakura H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2005;12:20–28. doi: 10.5551/jat.12.20. [DOI] [PubMed] [Google Scholar]
- 25.Baba S., Natsume M., Yasuda A., Tamura T., Osakabe N., Kanegae M., Kondo K. Plasma LDL and HDL cholesterol and oxidized LDL concentration are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007;137:1436–1441. doi: 10.1093/jn/137.6.1436. [DOI] [PubMed] [Google Scholar]