Abstract
The objective of the present study was to assess the ascorbic acid (AA) levels in seminal plasma of the fertile and infertile men and to investigate its relationship with sperm count, motility and normal morphology. Semen samples were provided by fertile [smoker (n = 25), nonsmoker (n = 21)] and infertile men [smoker (n = 23), nonsmoker (n = 32)]. A simplified method of reverse phase high performance liquid chromatography (RP-HPLC) procedure using UV detection was applied for the determination of seminal AA. Fertile subjects, smoker or not, demonstrated significantly higher seminal AA levels than any infertile group (p<0.01). Nonsmokers had high, but no significant, mean AA levels in their seminal plasma compared with smokers. Seminal AA in fertile and infertile (smokers or nonsmokers) males correlated significantly with the percentage of spermatozoa with normal morphology (p<0.01). Seminal AA decreased significantly in infertile men. Decrease of seminal plasma AA is a risk factor for low normal morphology of spermatozoa and idiopathic male infertility. Measurement of seminal AA in the seminal plasma of males with a history of subfertility or idiopathic infertility is necessary and can be helpful in fertility assessment.
Keywords: ascorbic acid, sperm quality, seminal plasma, male infertility, RP-HPLC
Introduction
Oxidative stress induced by reactive oxygen species (ROS) has recently been proposed as one of the major causes for human male infertility [1, 2]. It occurs as a consequence of imbalance between the production of ROS and the available antioxidants defense against them [3]. Excessive ROS can be produced by immature sperm and leukocyte cells [4]. It can impair normal sperm function by peroxidation of unsaturated fatty acids in membrane of spermatozoa and by DNA fragmentation [4, 5].
In order to counteract the toxic effects of ROS, human seminal plasma with low molecular weight antioxidants able to act as a free radical scavenger and hence it protects spermatozoa against free radicals [2, 6–9]. This defense mechanism compensates the loss of sperm cytoplasmic enzymes when the cytoplasm is extruded during maturation [10, 11]. Among low molecular weight antioxidants, the outstanding role of ascorbic acid has received particular attention. The higher concentrations of ascorbic acid in the epididymal fluid and seminal plasma compared with blood plasma have been reported previously for several species [12, 13]. High concentrations of ascorbic acid in seminal plasma may protect sperm from ROS and maintain the genetic integrity of sperm cells by preventing oxidative damage to DNA [14–16]. Generally, ascorbic acid is thought to act as an excellent reducing agent since it is also able to reduce catalytically active transition metal ions and free radicals [17]. In this process, ascorbic acid oxidizes and become a radical itself (semidehydroascorbate radical, ascorbate free radical, AFR) [18]. Unlike other free radicals, AFR is relatively unreactive and it can decay through disproportionation to ascorbic acid and dehydroascorbic acid (DHAA), and consequently it can interrupt the propagation of free radical chain reactions [18]. Hence, seminal plasma is extremely sensitive to a decrease in body levels of ascorbic acid [19]. There are conflicting reports on the effect of seminal ascorbic acid (AA) on the parameters of sperm quality. In the present study, it was hypothesized that major changes in the level of seminal plasma AA is related to low quality of sperm and poor fertilizing capacity. This study focused primarily on AA levels in the seminal plasma of fertile and infertile subjects (smokers and nonsmokers). The association of AA with sperm quality in the seminal plasma of all groups was evaluated.
Numerous principles of assays such as spectrophotometric determination and enzymatic method with ascorbic acid oxidase have been described for ascorbic acid detection in biological fluids and tissues [20, 21]. Rapid, reliable and sensitive AA determinations have been performed by high performance chromatography. High-performance liquid chromatography is the most common method for identification and quantification of antioxidant vitamins in biological fluids [18]. Recently several HPLC methods such as ion exchange and ion paired reverse phase HPLC, mostly coupled with electrochemical detection (ECD), have been presented for the determination of vitamin C in serum or seminal plasma [21–25]. In the present research we applied a simple RP-HPLC method, coupled with UV detection, for seminal AA determination by using a simple pretreatment procedure.
Materials and Methods
Semen populations and collections
One hundred and-one semen samples were collected from fertile nonsmokers (n = 21), fertile smokers (n = 25), infertile nonsmokers (n = 32) and infertile smokers (n = 23) referred to the Fatemeh Zahra in-vitro fertilization (IVF) center in Babol, Iran. Semen samples were obtained by masturbation into a sterile container after sexual abstinence for 2–3 days. Before semen analysis, a questionnaire was distributed to obtain information on smoking habits; alcohol use; use or abuse of other substances and drugs; and a history of orchitis, testicular trauma, sexually transmitted disease, varicocele, surgery for inguinal hernia, and cryptorchism. Fertile and infertile patients who smoked cigarettes regularly or who were nonsmokers (never previously smoked) were included in the study.
After collection, semen specimens were allowed to liquefy at room temperature for 30 min and used for analysis. On microscopic examination, sperm count, percentage of motile sperm, and sperm with normal morphology were objectively evaluated. Sperm count and percentage of motile sperm were evaluated according to standards set by the World Health Organization (WHO) [26]. Sperm morphology was evaluated according to Kruger’s criteria [27].
Measurement of ascorbic acid in seminal plasma
Chemical and equipment
Ascorbic acid stock solutions were prepared from L (+) ascorbic acid (Merck, Darmstadt, Germany). The HPLC grade methanol and acetic acid glacial were purchased from Fluka (Buchs, Switzerland). Deionized water obtained with a Milli-Q purification system (Millipore, Bedford, MA), and filtered through 0.45 µm Millipore solvent filter, was used for all reagent and standard solutions. A stock solution of Vitamin C was prepared at 5 mg/lit in methanol. The stock solutions were further diluted with methanol to give a series of working standards. Pure methanol was stored at −20°C and used to initiate protein precipitation in seminal plasma.
Samples preparation for HPLC
Semen sample was centrifuged at 4000 g for 5 min. A 100 µl aliquot of supernatant was admixed with 900 µl of cold methanol (100%). All samples were vortexed for 20 seconds and cold-centrifuged at 10000 g for 3 min. Each supernatant was kept at −20°C until a 20 µl aliquot was injected onto the HPLC system.
HPLC system
The HPLC system consisted of a model 1525 pump, Rheodyne 7725i injector equipped with 20 µL sample loop, Spherisorb C18 column (150 × 4.6 mm, 5 µm particle size) and model 2487 UV detector all from Waters (Waters Assoc., Milford, MA). For data acquisition, Waters Breeze software was used. The experiments were performed in isocratic mode in different mobile phase composition. The mobile phase consisted of 2.5% acetic acid glacial and 80% methanol in ddH2O, and then it was filtered through a 0.45 µm filter under vacuum and degassed before use. The flow rate was maintained at 1 ml/min and spectrophotometric detection at 254 nm was employed. The isocratic chromatographic system was conditioned by passing the eluent through the column until a stable base line was observed. Then, repeatable retention times were obtained for three subsequent injections.
Statistical analysis
An independent t test was considered to compare the scores of each of the measures and some of the parameters data between the two groups. The ANOVA model was utilized for statistical analyses of elements concentration between all groups. The Pearson correlation and linear regression model was used for utilize and examine the relation between seminal AA and sperm parameters quality. A p value<0.05 was considered statistically significant. Data were analyzed using SPSS, version 11.5 (FAQs).
Results
The mean values of examined sperm parameters in the fertile and infertile men (smokers and nonsmokers) are shown in Table 1. Sperm count, motility and normal morphology in fertile group (smokers or nonsmokers) were significantly higher than those in infertile group. A trend toward a higher quality of sperm was seen for nonsmokers compared with smokers (Table 1).
Table 1.
Comparison of sperm parameters quality and ascorbic acid in the fertile and infertile smoker-nonsmoker men
| Variable | Nonsmoker men (n = 53) |
Smoker men (n = 48) |
||
|---|---|---|---|---|
| Fertile (n = 21) | Infertile (n = 32) | Fertile (n = 25) | Infertile (n = 23) | |
| Age (years) | 31.38 ± 4.36 | 29.55 ± 4.46 | 29.7 ± 5.14 | 31.37 ± 7.4 |
| Volume (ml) | 4.14 ± 1.36 | 3.85 ± 1.53 | 4.35 ± 1.47 | 3 ± 1.53 |
| Sperm count (×106 ml) | 80 ± 29.63 | 36.90 ± 29.91* | 71 ± 28.81 | 31.81 ± 21*** |
| Total sperm (×106) | 330.71 ± 145.60 | 137.11 ± 124.33* | 310.5 ± 150.63 | 60.63 ± 47.04*** |
| Motility (%) | 73.1 ± 16.3 | 50.06 ± 29.69† | 72 ± 16.73 | 43.75 ± 32.99*** |
| Normal morphology (%) (by Kruger criteria) | 14.93 ± 3.63 | 5.96 ± 4.36* | 12.9 ± 4.78 | 3.75 ± 2.11*** |
| Ascorbic acid (µmol/l) | 448.71 ± 98.13 | 412.81 ± 114.51** | 440.04 ± 103.31 | 383.13 ± 94.89†† |
Values are mean ± SD. *p<0.001 by using 1-way ANOVA followed by post hoc Newman-Keuls test when values of infertile nonsmokers are compared with fertile nonsmokers. **p<0.05 by using 1-way ANOVA followed by post hoc Newman-Keuls test when values of infertile nonsmokers are compared with fertile nonsmokers. †p<0.01 by using 1-way ANOVA followed by post hoc Newman-Keuls test when values of infertile nonsmokers are compared with fertile nonsmokers. ***p<0.001 by using 1-way ANOVA followed by post hoc Newman-Keuls test when values of infertile smokers are compared with fertile smokers. ††p<0.01 by using 1-way ANOVA followed by post hoc Newman-Keuls test when values of infertile smokers are compared with fertile smokers.
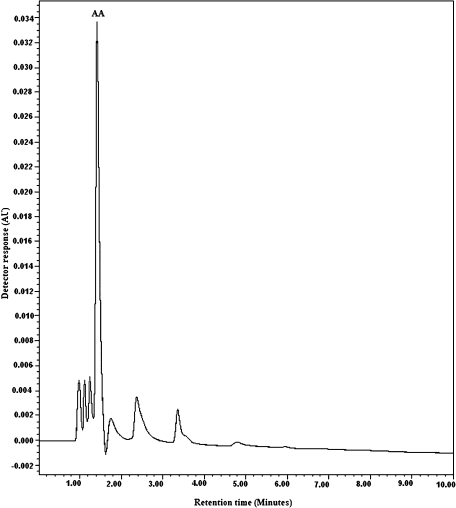
The concentrations of seminal ascorbic acid in both groups were measured by RP-HPLC method. Peak identity was proven both by the correspondence of standard solution and sample retention times (<5 min), and the coelution of added and endogenous AA. A typical chromatogram of seminal AA is shown in Fig. 1. Under the conditions employed, all peaks of interest were well resolved from interfering peaks, and eluted in <5 min. Mean concentrations of ascorbic acid in the seminal plasma of all samples can be seen in Table 1. Total seminal plasma ascorbic acid content ranged from 206.7 to 678.2 µmol/l in all individuals. Fertile groups (smokers or nonsmokers) demonstrated significantly higher AA levels in their seminal plasma than any infertile groups (p<0.01). A trend was observed for a lower mean AA level in seminal plasma of smokers compared with nonsmokers. Fertile nonsmokers had significantly high levels of AA in their seminal than infertile nonsmokers (p<0.05), moreover, fertile smokers had significantly high levels of AA in their seminal plasma compared with infertile smokers (p<0.01).
Fig. 1.
Representative chromatograms from ascorbic acid (AA) in seminal plasma. Samples were prepared as described in materials and methods. The mobile phase consisted of 2.5% acetic acid glacial and 80% methanol in ddH2O; flow rate: 1 ml/min and spectrophotometric detection at 254 nm; column: a Spherisorb C18 column (150 × 4.6 mm, 5 µm particle size).
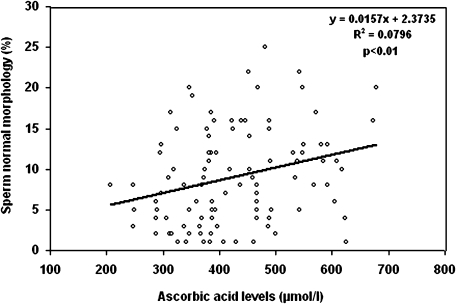
Fig. 2 shows the correlation between ascorbic acid and the percentage of spermatozoa with normal morphology. The ascorbic acid in seminal plasma was positively correlated to the spermatozoa with normal morphology (r2 = 0.79, p<0.01).
Fig. 2.
Correlation between the seminal plasma AA content with sperm with normal morphology. Seminal plasma AA was positively correlated with sperm with normal morphology (r2 = 0.0796, p<0.01). The Pearson correlation test was used to analyze and examine the relationship between AA with sperm parameters.
Discussion
Ascorbic acid as the principle antioxidant in seminal plasma of fertile men, can contribute up to 65% of its total chain breaking antioxidant capacity [3]. This system scavenges harmful ROS before they react with other importance molecules and terminates the subsequent chain reaction [28]. There have been conflicting reports on the effect of seminal AA on sperm quality [29–31]. Some studies indicated that there is no significant difference between AA content in the fertile and infertile men [32], but others found a significant difference between them [12, 33–35], which was in agreement with our study. Some authors have reported a positive relationship between seminal AA and quality of sperm in the seminal plasma of fertile and infertile men [35, 36], whereas others reported no significant difference [37–41]. Thiele et al. [12] observed that infertile patients had significantly lower ascorbic acid in their seminal plasma than fertile men, and ascorbic acid was positively correlated with the percentage of sperm with normal morphology. In our study ascorbic acid levels in seminal plasma of all subjects ranged from 206.7 to 678.2 µmol/l. Our results were agree with the findings of other studies [16, 33, 42]. In this investigation, fertile men had significantly high levels of ascorbic acid in their seminal plasma than infertile groups, and ascorbic acid was positively correlated with the percentage of sperm with normal morphology. In other report, Mostafa et al. [32] analyzed differences in ascorbic acid levels in the seminal plasma of smokers and nonsmokers. They found a significant decrease in the mean seminal plasma ascorbic acid levels in smokers versus nonsmokers. In addition, seminal plasma ascorbic acid levels were positively correlated with the sperm concentration, motility and sperm with normal morphology [32]. In our study, a trend was observed for a lower mean AA level in seminal plasma of smokers compared with nonsmokers. Our results suggested that smokers are susceptible to AA deficiency in their seminal fluid. On the other hand, decreased of seminal plasma ascorbic acid can not be a main effect of cigarette smoking on sperm parameters quality in smokers, which merits further study.
The mechanism by which ascorbic acid in the seminal plasma of infertile patients (smokers or nonsmokers), is depleted has not been fully elucidated. Evidence suggests that one consequence of ascorbic acid deficiency can be an increase in oxidative damage induced by ROS [43]. Recent studies have indicated that high levels of ROS are detected in the semen of 25 to 45% of infertile men [5, 43]. A small amount of ROS is necessary for sperm to acquire fertilizing capabilities [2], but it appears that high levels of seminal ROS may decrease the effective concentration of seminal ascorbic acid. Increased ROS in the seminal plasma of infertile men may decrease the effective concentration of ascorbic acid, increasing the harmful effects of ROS to sperm cells that are associated with abnormal sperm parameters [18, 44]. Several studies supported this hypothesis, noting that supplementation of ascorbic acid cause significant reduction in ROS concentration [45], sperm membrane lipid peroxidation [46] and sperm DNA oxidation [47]. In addition, ascorbic acid treatment had a positive effect on sperm quality and supplementation of biologic ascorbic acid was an effective method for the treatment of infertile males with poor sperm quality [14, 45–47]. Men who smoke inhale a host of toxic substances such as free radicals that can be absorbed, so a causal relationship is suspected. Recently studies have shown that cigarette smoking lead to increased seminal ROS by several mechanisms: (i) cigarette smoking itself contains high levels of ROS, (ii) smoking metabolites may induce an inflammatory reaction in the male genital tract with a subsequent release of chemical mediation of inflammation that can recruit and active leukocytes. Activated leukocytes can generation high levels of ROS in semen and (iii) toxic metabolites of cigarette smoke may impair spermatogenesis, resulting in the production of abnormal spermatozoa, which is an important source of ROS and oxidative stress [48]. High levels of ROS induced by smoking may overwhelm the antioxidant strategies (especially ascorbic acid effective concentration), resulting in oxidative stress which associated with low quality of sperm in smokers [48]. Dawson et al. [49] showed also the beneficial effect of ascorbic acid oral administration on sperm concentration, morphology and viability in smokers. Therefore, infertile men, smoker or nonsmoker, are particularly susceptible to seminal ascorbic acid deficiency. Hence, the ascorbic acid supplementation may improve the sperm parameters quality, however, antioxidant therapy in men can be improved sperm quality is particularly dose dependent [50].
In conclusion, our findings suggested that idiopathic infertile men have significantly low levels of ascorbic acid in their seminal plasma than fertile men. Association of seminal ascorbic acid with normal morphology of sperm may indicate that the decrease in seminal ascorbic acid content can be a risk factor for sperm abnormality and idiopathic male infertility. In the present study, smokers were susceptible to seminal ascorbic acid deficiency, compared with nonsmokers. Therefore, the analysis of ascorbic acid in seminal plasma of idiopathic infertile patients, smokers or nonsmokers, may afford valuable evidence in exploring the mechanism of male infertility.
References
- 1.Sikka S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996;1:e78–e86. doi: 10.2741/a146. [DOI] [PubMed] [Google Scholar]
- 2.Aiken R.J., Buckingham D.W., Harkiss D. Use of Xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J. Reprod. Fertil. 1993;97:441–450. doi: 10.1530/jrf.0.0970441. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A. Role of antioxidants in treatment of male infertility. Reprod. Biomed. Online. 2004;8:616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R.K., Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 5.de Lamirande E., Leduc B.E., Iwasaki A., Hassouna M., Gagnon C. Increased reactive oxygen species formation in semen of patients with spinal cord injury. Fertil. Steril. 1995;64:637–642. [PubMed] [Google Scholar]
- 6.Agarwal A., Prabakaran S.A. Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J. Exp. Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 7.Saleh A., Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J. Androl. 2002;23:737–752. [PubMed] [Google Scholar]
- 8.Aiken R.J., Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994;16:259–267. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong J.S., Rajasekaran M., Hellstrom W.J.G., Sikka S.C. Antioxidant potential of human serum albumin: role in the recovery of high quality human spermatozoa for assisted reproductive technology. J. Androl. 1998;19:412–419. [PubMed] [Google Scholar]
- 10.Sikka S.C. Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J. Androl. 2004;25:5–18. doi: 10.1002/j.1939-4640.2004.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 11.Aitken R.J. A free radical theory of male infertility. Reprod. Fertil. Dev. 1994;6:19–23. doi: 10.1071/rd9940019. [DOI] [PubMed] [Google Scholar]
- 12.Thiele J.J., Freisleben H.J., Fuchs J., Ochsendorf F.R. Ascorbic acid and urate in human seminal plasma: detection and interrelationship with chemiluminescence's in washed semen. Hum. Reprod. 1995;10:110–115. doi: 10.1093/humrep/10.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Smith R., Vantman D., Ponce J., Escobar J., Lissi E. Total antioxidant capacity of human seminal plasma. Hum. Reprod. 1996;11:1655–1660. doi: 10.1093/oxfordjournals.humrep.a019465. [DOI] [PubMed] [Google Scholar]
- 14.Dawson E.B., Harris W.A., Rankin W.E., Charpentier L.A., McGanity W.J. Effect of ascorbic acid on male fertility. Ann. NY Acad. Sci. 1987;498:312–323. doi: 10.1111/j.1749-6632.1987.tb23770.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A., Gagnon C. Formation of reactive oxygen species in spermatozoa in infertile patients. Fertil. Steril. 1992;57:404–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- 16.Fraga C.G., Motchnik P.A., Shigenaga M.K., Helbock H.J., Jacob R.A., Ames B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buettner G.R., Jurkiewicz B.A. Catalytic metals, ascorbate and free radicals: combination to avoid. Radiat. Res. 1991;145:532–541. [PubMed] [Google Scholar]
- 18.Patriarca M., Menditto A., Morisi G. Determination of ascorbic acid in blood or serum and in seminal plasma using a simplified sample preparation and high performance liquid chromatography coupled with UV detection. J. Liq. Chromatogr. 1991;14:297–312. [Google Scholar]
- 19.Chinoy N.J., Buchnee R.P., Mehta R.R., Seethalakshmi L., Sharma J.D., Chinoy MR. Effects of vitamin C deficiency on physiology of male reproductive organs of guinea pigs. Int. J. Fertil. 1986;31:232–239. [PubMed] [Google Scholar]
- 20.Pachia L.A., Reynolds D.L., Kissinger P.T. Analytical methods for determining ascorbic acid in biological samples, food products and pharmaceuticals. J. Assoc. Anal. Chem. 1985;68:1–12. [PubMed] [Google Scholar]
- 21.Kyaw A. A simple colorimetric method for ascorbic acid determination in blood plasma. Clin. Chim. Acta. 1978;86:153–157. doi: 10.1016/0009-8981(78)90128-6. [DOI] [PubMed] [Google Scholar]
- 22.Omaye S.T., Schaus E.E., Kutnink M.A., Hawkes W.C. Measurement of vitamin C in blood components by High-Performance Liquid Chromatography. Ann. NY Acad. Sci. 1987;498:389–401. doi: 10.1111/j.1749-6632.1987.tb23776.x. [DOI] [PubMed] [Google Scholar]
- 23.Hernanz A. High-performance liquid chromatographic determination of ascorbic acid in serum using paired-ion chromatography and UV spectrophotometric detection. J. Clin. Chem. Clin. Biochem. 1988;26:459–461. [PubMed] [Google Scholar]
- 24.Liau L.S., Lee B.L., New A.L., Ong C.N. Determination of plasma ascorbic acid by high-performance liquid chromatography with ultraviolet and electrochemical detection. J. Chromatogr. 1993;612:63–70. doi: 10.1016/0378-4347(93)80368-e. [DOI] [PubMed] [Google Scholar]
- 25.Chang W.Y., Chang J.K., Szeto Y.T., Tomlinson B., Benzie I.F. Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clin. Biochem. 2001;34:623–627. doi: 10.1016/s0009-9120(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Laboratory manual for the examination of human semen and semen-cervical mucus interaction, 4th ed. UK7C Ambridge University Press; Cambridge, New York: 1999. pp. 1–50. [Google Scholar]
- 27.Kruger T.F., Menkveld R., Stander F.S.H., Lombard C.J., Van der Merwe J.P., Van Zyl J.A. Sperm morphologic features as a prognostic factor in in-vitro fertilization. Fertil. Steril. 1986;46:1118–1123. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 28.Fujii J., Iuchi Y., Matsuki S., Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. J. Androl. 2003;5:231–242. [PubMed] [Google Scholar]
- 29.Dawson E.B., Harris W.A., Powell L.C. Relationship between ascorbic acid and male fertility. World Rev. Nutr. Diet. 1990;62:1–26. doi: 10.1159/000417532. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 31.Luck M.R., Jeyaseelan I., Scholes R.A. Ascorbic acid and fertility. Biol. Reprod. 1995;52:262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- 32.Mostafa T., Tawadrous G., Roaia M.M., Amer M.K., Kader R.A., Aziz A. Effect of cigarette smoking on seminal plasma ascorbic acid in infertile and fertile males. Andrologia. 2006;38:221–224. doi: 10.1111/j.1439-0272.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 33.Saleh R.A., Agarwal A., Nada E.A., Tonsy M.H., Sharma R.K., Meyer A., Nelson D.R. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil. Steril. 2003;79:1597–1605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 34.Lewis S.E.M., Boyle P.M., McKinney K.A., Youngi S., Thompson W. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil. Steril. 1995;64:868–870. doi: 10.1016/s0015-0282(16)57870-4. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y.C., Sun H.M., Shang X.J., Zhu P.Y., Huang Y.F. Total antioxidant capacity of seminal plasma in fertile and infertile men. Zhonghua. Nan. Ke. Xue. 2005;11:915–917. [PubMed] [Google Scholar]
- 36.Shi Y.C., Shang X.J., Wang X.L., Huang Y.F. Correlation of total antioxidant capacity in seminal plasma with sperm motility of infertile men. Zhonghua Nan Ke Xue. 2006;12:703–705. [PubMed] [Google Scholar]
- 37.Potts R.J., Jefferies T.M., Notarianni L.J. Antioxidant capacity of the epididymis. Hum. Reprod. 1999;14:2513–2516. doi: 10.1093/humrep/14.10.2513. [DOI] [PubMed] [Google Scholar]
- 38.Rolf C., Cooper T.G., Yeung C.H., Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebocontrolled, double-blind study. Hum. Reprod. 1999;14:1028–1033. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- 39.Abel B.J., Carswell G., Elton R., Hargreave T.B., Kyle K., Orr S., Rogers A., Baxby K., Yates A. Randomised trial of clomiphene citrate treatment and vitamin C for male infertility. Br. J. Urol. 1982;54:780–784. doi: 10.1111/j.1464-410x.1982.tb13647.x. [DOI] [PubMed] [Google Scholar]
- 40.Hargreave T.B., Kyle K.F., Baxby K., Rogers A.C.N., Scott R., Tolley D.A., Abel B.J., Orr P.S., Elton R.A. Randomised trial of mestrolone versus vitamin C for male infertility. Scottish Infertility Group. Br. J. Urol. 1984;56:740–744. doi: 10.1111/j.1464-410x.1984.tb06160.x. [DOI] [PubMed] [Google Scholar]
- 41.Donnelly E., McClure N., Lewis S.E.M. Antioxidant supplementation in vitro does not improve human sperm motility. Fertil. Steril. 1992;72:484–495. doi: 10.1016/s0015-0282(99)00267-8. [DOI] [PubMed] [Google Scholar]
- 42.Sharma R.K., Pasqualotto F.F., Nelson D.R., Thomas A.J., Agarwal A. The reactive oxygen species—total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999;14:2801–2807. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal A., Ikemoto I., Loughlin K.R. Relationship of sperm parameters to levels of reactive oxygen species in semen specimens. J. Urol. 1994;152:107–110. doi: 10.1016/s0022-5347(17)32829-x. [DOI] [PubMed] [Google Scholar]
- 44.Aitken R.J., Baker M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Donnelly E.T., McClure N., Lewis S.E. Glutathione and hypotaurine in vitro: effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis. 2000;15:61–68. doi: 10.1093/mutage/15.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Kodama H., Yamaguchi R., Fukuda J., Kasi H., Tanaka K. Increased oxidative deoxyribonucleic acid damage in spermatozoa of infertile male patients. Fertil. Steril. 1997;68:519–524. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly E., McClure N., Lewis S.E.M. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide induced DNA damage in human spermatozoa. Mutagenesis. 1999;14:505–512. doi: 10.1093/mutage/14.5.505. [DOI] [PubMed] [Google Scholar]
- 48.Saleh R.A., Agarwal A., Sharma R.K., Nelson D.R., Thomas A.J. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men. Fertil. Steril. 2002;78:491–499. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 49.Dawson E.B., Harris W.A., Teter M.C., Powell L.C. Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil. Steril. 1992;58:1034–1039. [PubMed] [Google Scholar]
- 50.Verma A., Kanwar K.C. Human sperm motility and lipid peroxidation in different ascorbic acid concentrations: an in vitro analysis. Andrologia. 1998;30:325–329. doi: 10.1111/j.1439-0272.1998.tb01178.x. [DOI] [PubMed] [Google Scholar]