Abstract
Epidemiologic investigations indicate a close relationship between colorectal cancer and fat intake. However, to date the effects of lipid peroxidation-derived products that are formed from fat (especially free or esterified unsaturated fatty acids) on the initiation or progression of colorectal cancer have not been investigated extensively. Therefore, in the present study, we examined the effects of fatty acids, fatty acid hydroperoxides and aldehydes on the growth of human colorectal cancer cell line HT-29. At concentrations of 1 and 10 µM, linoleic, arachidonic and eicosapentaenoic acids, and 13-hydroperoxyoctadecadienoic and 15-hydroperoxyeicosapentaenoic acids had no significant effects on the growth of HT-29 cells. 4-Hydroxynonenal and 4-hydroxyhexenal had no significant effects on the growth of HT-29 cells up to 10 µM, whereas 4-oxononenal potently inhibited HT-29 cell growth (1–10 µM, 16–85% inhibition). Further experiments concerning DNA fragmentation, expression levels of Bax and Bcl-2 mRNA, expression levels of pro-caspase-3 and caspase-3 proteins, and activity of caspase-3 suggested that 4-oxononenal may increase the sensitivity of HT-29 cells to apoptosis through a decreased expression level of Bcl-2 and then increased formation of caspase-3 from pro-caspase-3.
Keywords: lipid peroxidation, 4-oxononenal, apoptosis, colorectal cancer, proliferation
Introduction
Colon cancer is a serious health problem in most developed countries and is the main cause of cancer mortality throughout the world [1]. Epidemiological studies have shown a significant difference in colon cancer incidence among different ethnic groups. The incidence of colon cancer is much higher in the United States and European countries compared with Asian countries, such as Japan and China, which is believed to be partially due to dietary habits [1, 2]. One of the major differences in diet between these populations is that Americans and Europeans consume larger amounts of fats than Japanese and Chinese. Animal fats, especially saturated fats, have been regarded as the most important nutritional influence on the development of colon cancer [3].
Lipid peroxidation is a free-radical-initiated chain oxidation of polyunsaturated fatty acids (PUFAs). PUFAs are converted into hydroperoxides and aldehydes under oxidative stress. To date, there has been no overall systemic investigation concerning the influences of lipid hydroperoxides and aldehydes on the growth of colorectal cancer.
The present study demonstrated that among lipid hydroperoxides and aldehydes, 4-oxononenal (4-ONE) strongly inhibited the growth of human colorectal cancer cell line HT-29. Furthermore, the present study indicated that 4-ONE induced apoptosis of HT-29 cells through the formation of activated caspase-3 from pro-caspase-3 by the decreased expression of Bcl-2.
Materials and Methods
Materials
Linoleic (LA), arachidonic (AA) and eicosapentaenoic (EPA) acids were obtained from Sigma Chemical Co. (St. Louis, MO). 4-Hydroxynonenal (4-HNE), 4-hydroxyhexenal (4-HHE) and 4-ONE were purchased from Cayman Chemical Co. (Ann Arbor, MI). 13-Hydroperoxyoctadecadienoic (13-HPODE) and 15-hydroperoxyeicosapentaenoic (15-HPEPE) acids were obtained from Cascade Biochem Ltd. (Berkshire, UK). All other reagents were of analytical grade.
Cell culture
Human colorectal cancer cell line HT-29 cells (European Collection of Cell Cultures, Wiltshire, UK) were cultured in McCoy’s 5A medium (Gibco BRL, Life Technologies, Carlsbad, CA) with 10% fetal calf serum (Nichirei Biosciences Inc., Tokyo, Japan) at 37°C in 5% CO2 and 95% humidity. HT-29 cells were subcultured at appropriate intervals to maintain subconfluent growth conditions. All drugs were prepared in Me2SO and added to the medium at up to 0.25%. Preliminary experiments demonstrated no significant effects of this Me2SO concentration on the cell growth and apoptosis of HT-29.
Cell growth assay
Cell growth was quantified using an MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay]. HT-29 cells were seeded in 6-well plates at 50,000 cells/2 mL/10 cm2 well. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 48 h. The medium was removed and the cells incubated with 1.1 mL of MTT solution (0.1 mL of 5 mg/mL MTT in 1 mL of medium) for 4 h. MTT is converted to a blue formazan product by mitochondrial succinate dehydrogenase. The product was eluted from cells by the addition of an equal volume of 20% SDS/0.01 N HCl, and absorbance at 595 nm was determined using a plate reader (model 550, Bio-Rad, Richmond, CA). Cell viability was calculated according to the equation: cell viability (%) = (the absorbance of experiment group/the absorbance of control groups) × 100.
Apoptosis detection
HT-29 cells were seeded in flexiPERM (Greiner bio-one, Monroe, NC) put on slides at 25,000 cells/1 mL/5 cm2 well. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 24 h. Apoptotic cells in the culture were analyzed by an in situ TUNEL assay using an ApopTag Plus Fluorescein in situ Apoptosis Detection Kit from Chemicon International Inc. (Temecula, CA). Apoptosis was also confirmed by DNA ladder formation analysis. In this analysis, cells were seeded in dishes at 500,000 cells/10 mL/75 cm2. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 24 h. At the end of the incubation, the cells were collected by centrifugation and washed with ice-cold PBS. Genomic DNA was extracted using a DNA Extractor WB kit from Wako Pure Chemical Industries, Limited (Tokyo, Japan). After being electrophoresed in 2% agarose gels, DNA was visualized by ethidium bromide staining and visualized using a luminoimage analyzer (model LAS-3000, Fuji-film, Tokyo, Japan).
Western blot analysis
HT-29 cells were seeded in dishes at 500,000 cells/10 mL/75 cm2. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 12 h. At the end of the incubation, the cells were collected by centrifugation, washed with ice-cold PBS, and then lysed. Cell lysates were analyzed using a SDS-7.5% polyacrylamide gel. Proteins were transferred to nitrocellulose membranes by electroblotting and the membranes were incubated overnight in TBS-T (0.14 M NaCl, 20 mM Tris and 0.1% Tween 20, pH 7.4) containing primary antibodies (anti-pro-caspase 3 and anti-caspase-3 from Cayman Chemical Co.) and 3% nonfat dry milk. Proteins were detected using an ECL detection method (Amersham-Pharmacia Corp., Buckingham, UK).
Quantitative real-time RT-PCR analysis
HT-29 cells were seeded in dishes at 500,000 cells/10 mL/75 cm2. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 12 h. At the end of the incubation, the cells were collected by centrifugation, washed with ice-cold PBS, and total RNA was extracted using an RNeasy midi kit (Qiagen, Germantown, MD). Total RNA (2.5 µg) was reverse transcribed into cDNA using a Transcriptor First Strand cDNA synthesis kit (Roche Diagnostics, Indianapolis, IN), and quantitative real-time PCR was carried out as described previously [4, 5] using a LightCycler-FastStart DNA master SYBR Green I kit (Roche Diagnostics) and LightCycler apparatus (Roche Diagnostics). The expression levels were normalized by that of β-actin (Search LC, Heidelberg, Germany). The primers were as follows: Bcl-2, F: 5'-TGC ACC TGA CGC CCT TCA C-3', R: 5'-AGA CAG CCA GGA GAA ATC AAA CAG-3'; Bax, F: 5'-ACC AAG AAG CTG AGC GAG TGT C-3', R: 5'-ACA AAG ATG GTC ACG GTC TGC C-3'. The presence of the expected PCR products after quantitative real-time RT-PCR reactions (293 bp for Bcl-2, 332 bp for Bax, and 329 bp for β-actin) were confirmed by an agarose gel electrophoresis (data not shown).
Caspase-3 assay
Changes in caspase-3 activity were assayed using a Caspase-3 Colorimetric Assay kit (Alexis Biochemicals, Lausen, Switzerland). HT-29 cells were seeded in dishes at 500,000 cells/10 mL/75 cm2. One day after seeding, the medium was changed, and the cells were incubated with the test compounds for 12 h. The medium was removed, and the cells were lysed. After centrifugation, the supernatant fractions obtained were incubated with DEVD-p-nitroanilide (200 µM) for 2 h at 37°C, and the absorbance at 400 nm was measured.
Statistics
Results are means ± SE. Statistical significance was determined by Student’s t test.
Results and Discussion
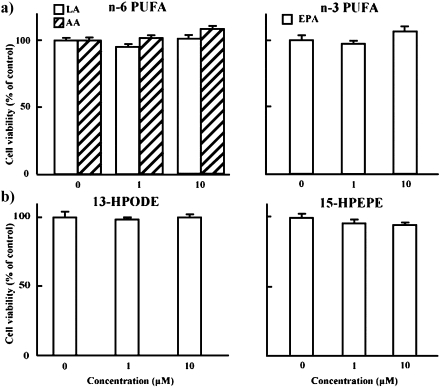
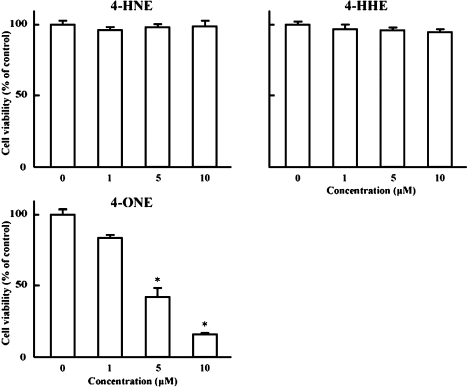
Fig. 1 shows the results of cell viability after 48 h treatment with n-6 (LA and AA) and n-3 (EPA) PUFAs, and hydroperoxy adducts of LA (13-HPODE) and EPA (15-HPEPE). These fatty acids and hydroperoxides had no significant effect on the growth of HT-29 cells at concentrations of 1 and 10 µM. On the other hand, Fig. 2 illustrates the efficacies of unsaturated aldehydes generated from 13-HPODE (4-HNE and 4-ONE) and from 15-HPEPE (4-HHE) [6] on the growth of HT-29 cells. No significant alteration was observed with 4-HNE and 4-HHE at concentrations ranging from 1 to 10 µM, whereas 4-ONE potently reduced HT-29 cell growth at concentrations of 5 and 10 µM (59 and 85% inhibition).
Fig. 1.
Effects of linoleic (LA), arachidonic (AA) and eicosapentaenoic (EPA) acids, and 13-hydroperoxyoctadecadienoic (13-HPODE) and 15-hydroperoxyeicosapentaenoic (15-HPEPE) acids on the growth of HT-29 cells. HT-29 cells were incubated with or without 1 or 10 µM LA, AA, EPA, 13-HPODE and 15-HPEPE for 48 h at 37°C in a humidified atmosphere of 5% CO2. Cell growth was assayed by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]. Each bar indicates the mean of 3 experiments; vertical lines show SE. PUFA, polyunsaturated fatty acid.
Fig. 2.
Effects of 4-hydroxynonenal (4-HNE), 4-hydroxyhexenal (4-HHE) and 4-oxononenal (4-ONE) on the growth of HT-29 cells. HT-29 cells were incubated with or without 1, 5 or 10 µM 4-HNE, 4-HHE and 4-ONE for 48 h at 37°C in a humidified atmosphere of 5% CO2. Cell growth was assayed by an MTT. Each bar indicates the mean of 4–5 experiments; vertical lines show SE. *p<0.01 vs control.
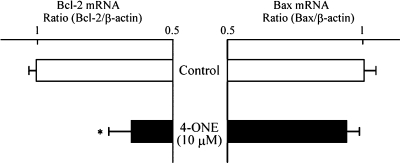
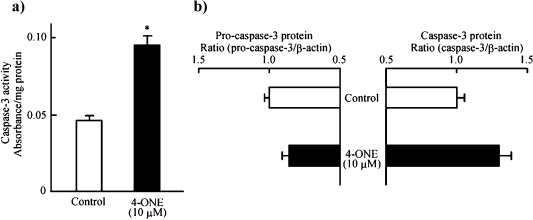
To clarify the mechanism by which 4-ONE inhibited HT-29 cell growth, its effects on the DNA fragmentation (Fig. 3), the expression levels of Bcl-2 and Bax mRNA (Fig. 4), the activity of caspase-3 and protein expression levels of pro-caspase-3 and caspase-3 (Fig. 5) were observed. Using the TUNEL method, DNA fragmentation was found as the fluorescence of small DNA particles in nuclei, when 4-ONE (10 µM) was added to HT-29 cells (Fig. 3a). The fragmentation of DNA in nuclei by incubation with 4-ONE (10 µM) was also visualized by agarose-gel electrophoresis of nuclear extracts (Fig. 3b); 4-ONE treatment resulted in small-sized DNA debris electrophoresed from the origin. Thus, 4-ONE showed DNA fragmentation in HT-29 cells in the same manner as TNF-α (positive control [7, 8]). As shown in Fig. 4, 4-ONE (10 µM) did not have any effect on Bax mRNA expression level, whereas it dramatically reduced the expression level of Bcl-2. Furthermore, 4-ONE (10 µM) enhanced the activity of caspase-3 in HT-29 cells (Fig. 5a). This might be partially due to a tentative increase in activated caspase-3 concomitant with a tentative decrease in pro-caspase-3 (Fig. 5b).
Fig. 3.
Detection of 4-ONE-induced DNA degradation in HT-29 cells using fluorescence microscopy (a) and agarose gel electrophoresis (b). HT-29 cells were incubated with or without 10 µM 4-ONE and 20 ng/mL of TNF-α for 24 h at 37°C in a humidified atmosphere of 5% CO2. Data are representative of at least three independent experiments.
Fig. 4.
Effects of 4-ONE on the mRNA expression level of Bcl-2 and Bax in HT-29 cells. HT-29 cells were incubated with or without 10 µM 4-ONE for 12 h at 37°C in a humidified atmosphere of 5% CO2. Each bar indicates the mean of 4–5 experiments; vertical lines show SE. *p<0.05 vs control.
Fig. 5.
Effects of 4-ONE on the activity of caspase-3 (a), and on the protein expression levels of pro-caspase-3 and caspase-3 (b) in HT-29 cells. HT-29 cells were incubated with or without 10 µM 4-ONE for 12 h at 37°C in a humidified atmosphere of 5% CO2. Each bar indicates the mean of 3–4 experiments; vertical lines show SE. *p<0.01 vs control.
It has been reported that overexpression of Bcl-2 in transgenic models leads to the protection of many cell types against apoptosis, suggesting that Bcl-2 exerts an anti-apoptotic function [9, 10]. Anti-apoptotic activity of Bcl-2 was found to be antagonized by a homologous Bax protein, which was able to form heterodimers with Bcl-2. Proapoptotic Bax forms the channels for cytochrome c release that may initiate the activation of caspase-3 identified as the major caspase that contributes to the hallmark of apoptosis, while anti-apoptotic Bcl-2 prevents the opening of mitochondrial transition pores by binding Bax [11, 12]. Thus, the present study indicated that 4-ONE induces cell apoptosis by affecting the ratio of Bax to Bcl-2, activating cytochrome c release, and inducing proteolytic cleavage of pro-caspase-3 to activated caspase-3.
Also, is there any specificity to a particular cell, tumor or histological type in the growth inhibitory and cytotoxic effects of 4-ONE? When 4-ONE (10 µM) was added to a human colorectal cancer cell CACO-2, human hepatocellular carcinoma cell HepG2, mouse preadipocyte cell 3T3-L1 and rat primary hepatocyte cell, the growth inhibition was detected in all these cells (67–93% inhibition). So, it seems likely that 4-ONE can be an inhibitor of cell proliferation or cell growth, independent of cell types.
Lipid peroxidation is a paradox of aerobic life, affecting human health and quality of life [13]. Biological systems are lipid-rich matrices susceptible to autoxidation unless protected by either endogenous enzymatic or non-enzymatic mechanisms. On the other hand, various lipid peroxidative products are generated physiologically and some of them are known as haemostatic regulators in cells [13]. Lipid peroxidation has been suggested to be involved in the control of cell division [14, 15]. Dreher and Junod [16] also showed that low levels of oxygen free radicals stimulate cell proliferation, whereas high levels induce cytotoxicity and cell death in later stages of carcinogenesis. Pillai et al. [17] and Srihari et al. [18] have reported that, using 1,2-dimethylhydrazine (DMH)-induced rat colon carcinogenesis, levels of lipid peroxidation byproducts, thiobarbituric acid-reactive substances (TBARS) and conjugated dienes (CD), in the rat liver are higher, whereas those levels in the colon and caecum are significantly lowered, as compared with control rats. Kamaleeswari and Nalini [19] have also shown that, in addition to TBARS and CD, lipid peroxide levels in the colon cancer tissue are lower in DMH-treated rats, as compared with control rats. Furthermore, in vivo studies on human colon adenocarcinoma have shown decreased TBARS and 4-HNE contents in the tumor tissue related to malignancy progression [20, 21]. The chemical reactions that lead to 4-ONE production have been the subject of considerable investigation. A likely pathway involves the breakdown of n-6 derived polyunsaturated fatty acid hydropeoxides (e.g., 13-HPODE) to 4-hydroperoxynonenal (4-HPNE), which can dehydrate to 4-ONE or be reduced to 4-HNE [6]. Both 4-HPNE and 4-ONE have been isolated as products of lipid peroxidation, and 4-ONE has been demonstrated to be a powerful electrophile, capable of reacting with protein and DNA at rates significantly greater than 4-HNE [22, 23]. The physiological concentration of 4-HNE in rat tissues can reach up to 3 µM [6], and therefore it might be possible that 4-ONE also appears in the tissues at micromolar concentration range under normal physiological states. Cancer cells acquire particular characteristics that benefit their proliferation [24]. Namely, the present study might suggest that decreased 4-ONE, which is speculated to be associated with tumor progression, favors the proliferation of colon cancer.
References
- 1.Potter J.D., Slattery M.L., Bostick R.M., Gapstur S.M. Colon cancer: a review of the epidemiology. Epidemiol. Rev. 1993;15:499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 2.Messina M., Barnes S. The role of soy products in reducing risk of cancer. J. Natl. Cancer Inst. 1991;83:541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- 3.Pickering J.S., Lupton J.R., Chapkin R.S. Dietary fat, fiber, and carcinogen alter fecal diacylglycerol composition and mass. Cancer Res. 1995;55:2293–2298. [PubMed] [Google Scholar]
- 4.Lanzillo J.J., Yu F.-S., Stevens J., Hassoun P.M. Determination of xanthine dehydrogenase mRNA by a reverse transcription-coupled competitive quantitative polymerase chain reaction assay: regulation in rat endothelial cells by hypoxia and hyperoxia. Arch. Biochem. Biophys. 1996;335:377–380. doi: 10.1006/abbi.1996.0519. [DOI] [PubMed] [Google Scholar]
- 5.Yeo S.-J., Yoon J.-G., Yi A.-K. Myeloid differentiation factor 88-dependent post-transcriptional regulation of cyclooxygenase-2 expression by CpG DNA. J. Biol. Chem. 2003;278:40590–40600. doi: 10.1074/jbc.M306280200. [DOI] [PubMed] [Google Scholar]
- 6.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H., Shiraki K., Ohmori S., Sakai T., Deguchi M., Yamanaka T., Okano H., Nakano T. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int. J. Mol. Med. 2002;9:521–525. [PubMed] [Google Scholar]
- 8.Vaculova A., Hofmanova J., Soucek K., Kovarikova M., Kozubik A. Tumor necrosis factor-alpha induces apoptosis associated with poly (ADP-ribose) polymerase cleavage in HT-29 colon cancer cells. Anticancer Res. 2002;22:1635–1639. [PubMed] [Google Scholar]
- 9.Reed J.C. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourzand C., Rossier G., Reelfs O., Borner C., Tyrrell R.M. The overexpression of Bcl-2 inhibits UVA-mediated immediate apoptosis in rat 6 fibroblasts: evidence for the involvement of Bcl-2 as an antioxidant. Cancer Res. 1997;57:1405–1411. [PubMed] [Google Scholar]
- 11.Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J.J., Mazzei G., Maundrell K., Gambale F., Sadoul R., Martinou J.C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 12.Zamzami N., Marzo I., Susin S.A., Brenner C., Larochette N., Marchetti P., Reed J.K., Kroemer G. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of bcl-2 by enforcing mitochondrial permeability transition. Oncogene. 1998;16:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- 13.Davies K.J. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 1995;6:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 14.Dianzani M.U. Lipid peroxidation and cancer. Crit. Rev. Oncol. Hematol. 1993;15:125–147. doi: 10.1016/1040-8428(93)90052-6. [DOI] [PubMed] [Google Scholar]
- 15.Diplock A.T., Rice-Evans C.A., Burdon R.H. Is there a significant role for lipid peroxidation in the causation of malignancy and for antioxidants in cancer prevention? Cancer Res. 1994;54:1952–1956. [PubMed] [Google Scholar]
- 16.Dreher D., Junod A.F. Role of oxygen free radicals in cancer development. Eur. J. Cancer. 1996;32:30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 17.Pillai M.G., Thampi B.S.H., Menon V.P., Leelamma S. Influence of dietary fibre from coconut kernel (Cocus nucifera) on the 1,2-dimethylhydrazine-induced lipid peroxidation in rats. J. Nutr. Biochem. 1999;10:555–560. doi: 10.1016/s0955-2863(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 18.Srihari T., Sengottuvelan M., Nalini N. Dose-dependent effect of oregano (Origanum vulgare L.) on lipid peroxidation and antioxidant status in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J. Pharm. Pharmacol. 2008;60:787–794. doi: 10.1211/jpp.60.6.0015. [DOI] [PubMed] [Google Scholar]
- 19.Kamaleeswari M., Nalini N. Dose-response efficacy of caraway (Carum carvi L.) on tissue lipid peroxidation and antioxidant profile in rat colon carcinogenesis. J. Pharm. Pharmacol. 2006;58:1121–1130. doi: 10.1211/jpp.58.8.0014. [DOI] [PubMed] [Google Scholar]
- 20.Biasi F., Tessitore L., Zanetti D., Cutrin J.C., Zingaro B., Chiarpotto E., Zarkovic N., Serviddio G., Poli G. Associated changes of lipid peroxidation and transforming growth factor beta 1 levels in human colon cancer during tumor progression. Gut. 2002;50:361–367. doi: 10.1136/gut.50.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanetti D., Poli G., Vizio B., Zingaro B., Chiarpotto E., Biasi F. 4-Hydroxynonenal and transforming growth factor-beta1 expression in colon cancer. Mol. Aspects Med. 2003;24:273–280. doi: 10.1016/s0098-2997(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 22.Doorn J.A., Petersen D.R. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W.H., Liu J., Xu G., Yuan Q., Sayre L.M. Model studies on protein side chain medication by 4-oxo-2-nonenal. Chem. Res. Toxicol. 2003;16:512–523. doi: 10.1021/tx020105a. [DOI] [PubMed] [Google Scholar]
- 24.Schmelz E.M., Sullards M.C., Dillehay D.L., Merrill A.H. Colonic cell proliferation and agerrant crypt foci formation are inhibited by dairy glycosphingolipids in 1,2-dimethylhydrazine-treated CF1 mice. J. Nutr. 2000;130:522–527. doi: 10.1093/jn/130.3.522. [DOI] [PubMed] [Google Scholar]