Abstract
Whether the speed of body mass (BM) reduction influences the body composition is uncertain. To investigate the effects of rapid vs slow body mass reduction on body composition, rats were divided into three groups; fed ad libitum for 16-day (Control, C); received restricted food intake during 16-day to decrease BM slowly (Slow, S); or fed ad libitum for 13-days and fasted for the last 3 days to rapidly reach a BM comparable to that of S (Rapid, R). Drinking water was restricted for R on day 16 to rapidly decrease their BM. All rats trained during the study. Final BM and adipose tissues mass were similar for R and S, and both were lesser than C. The skeletal muscle mass did not decrease in R and S. The liver mass was lower in R and S than C, and the decrease tended to be greater in R than S. Both the stomach and small intestine masses were significantly lower in R than C, but did not differ between S and C. In conclusion, differences of the speed of BM reduction affect the splanchnic tissues, and the decrease in splanchnic tissue mass was greater with rapid than slow BM reduction.
Keywords: speed of weight loss, tissue composition, splanchnic tissues
Introduction
Rapid body mass reduction is commonly employed by wrestlers in Japan [1]. Because body fat is very low in elite wrestlers [2, 3], especially in the lighter weight divisions [4], it is difficult to reduce body mass by decreasing body fat. Thus, a decrease in total body mass can be achieved only through decreasing lean body mass. Therefore, wrestlers have to choose rapid body mass reduction methods that include dehydration and diet restriction for a short period of time.
However, rapid body mass reduction is not recommended [5] because of the loss of fat-free mass [6], which has adverse effects such as the decline of muscle power and strength [7] and increased susceptibility to heat stroke due to dehydration [8]. While slow body mass reduction is recommended [4, 9], some studies have reported that slow reduction also decreases fat-free mass in wrestlers [10, 11].
Information on the effect of rapid vs slow body mass reduction on body composition in wrestlers is limited. Kukidome et al. [6] showed that rapid body mass reduction caused a greater loss of fat-free mass than body fat, when compared to slow body mass reduction. However, the composition and mass of skeletal muscles and splanchnic tissues were not determined. Other studies on body mass reduction speed focused on body fluid status [12] or exercise performance [13] in wrestlers and whole body protein kinetics [14] or glutathione synthesis [15] in non-athletes but did not investigate tissue composition. Currently, many wrestlers reduce body mass based on their own experience, and it is not well understood whether the speed of body mass reduction influences body composition.
In animal, no study has directly compared the effects of rapid vs. slow body mass reduction on body composition as well as in humans. We previously compared the rapid with slow body mass reduction on body composition in rats and found that the mass and protein content of the skeletal muscles and adipose tissues did not differ between the groups whereas the splanchnic tissues were lower in rapid body mass reduction than slow reduction (unpublished data). However, we could not determine what tissues decreased because our preliminary study was lacking in the data before body mass reduction as control.
Other reports showed that the skeletal muscle mass and protein was conserved while the adipose tissues and splanchnic tissues significantly decreased during the short periods fasting in rats [16, 17]. Therefore, we presumed that skeletal muscle mass and protein may not decrease in both rapid and slow body mass reduction, whereas the decrease of splanchnic tissues may be greater in rapid reduction in rats. In addition, we hypothesized that the greater loss of fat free mass in rapid body mass reduction compared to slow reduction in humans [6] may be related to the greater loss of splanchnic tissues in rapid body mass reduction.
The purpose of the present study was to investigate whether the speed of body mass reduction influences body composition, including that of skeletal muscles, adipose tissues, and splanchnic tissues in rats.
Materials and Methods
Animals and study design
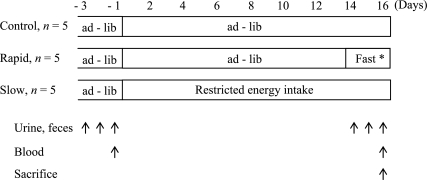
The design of the study is summarized in Fig. 1. Male Sprague-Dawley rats (5 weeks old, n = 15) were obtained from Japan Clea, Inc. (Tokyo, Japan) and kept individually in metabolic cages. The animal room was maintained at 23°C with a 12-h light-dark cycle (lights on from 2100 to 0900 h). The rats were fed ad libitum a standard rat chow (CE-2, Japan Clea, Inc.) and allowed free access to water for 6 weeks before body mass reduction. All rats were subjected to a climbing exercise. The exercise was accomplished utilizing a wire mesh cage (length 38 × width 28 × height 53 cm) which was placed on a 53°C electric hot plate. This temperature is the minimum temperature to provoke the rats to climb. When the rats were positioned at the bottom of the climbing apparatus during exercise, they were motivated to climb the apparatus by the heating plate. Initially, the rats were familiarized with the wire mesh cage by practicing climbing the apparatus from the bottom to the top cage for 7 days, and then the climbing exercise regimen started. The exercise regimen consisted of 6 times a week (10 min × 3 sets/day) during 6 weeks to mimic the situation in human athletes who engage in routine physical training. Rats were allowed to rest for 10 min between training sessions. All study procedures were conducted in conformity with the Guiding Principles in the Care and Use of Animals.
Fig. 1.
Outline of experimental protocol. *Drinking water withdrawn for the last 21 h of the fast. All rats trained with a climbing cage 6 times weekly (10 min × 3 sets/day). Ad - lib indicates ad libitum feeding.
After the 6-week training period, the rats (11-week-old) were divided into three groups; one group continued to receive the standard chow diet ad libitum for the entire 16-day study period (Control, C; n = 5); a second group received restricted food intake during the 16 days to decrease body mass slowly (Slow, S; n = 5); the third group was fed ad libitum for 13 days and fasted thereafter for the last 3 days (Days 14–16) of the study period to rapidly reach a body mass comparable to that of the S group (Rapid, R; n = 5). Drinking water was not allowed for group R for the last 21 h before sacrifice, to mimic the method of rapid body mass reduction [6]. Other group ingested drinking water freely until sacrifice. This is because the water restriction is not used in wrestler who decreases body mass slowly. During the 16-day period, all rats continued the climbing exercise training. The degree of energy restriction was selected as the amount which decreases body mass gradually in rats of this age observed in our preliminary studies and the periods (2–3 weeks) are commonly used in human athletes. The fasting period was selected to produce a clear loss of body energy without causing emaciation [16, 18] and was roughly conformed to the magnitude of the weight loss in human athlete [6].
Body mass, food intake, water consumption, and weight of urine and feces were recorded daily at 0900 h. On the day before the commencement of weight reduction (Day-1), blood was taken from the tail vein at 0830 h and was immediately centrifuged at 3000 rpm for 15 min at 4°C. Urine and feces from all rats were also collected on Day -1 and the last 3 days of the study. On Day 16, blood was taken from the tail vein at 0830 h, and then all rats were euthanized under ether anesthesia. Skeletal muscles (gastrocnemius, soleus, plantaris, extensor digitorum longus, and tibialis anterior), abdominal adipose tissues (perirenal and epididymal), and splanchnic tissues (liver, stomach, and small intestine) were excised, and the masses of these tissues were determined. The masses of the stomach and small intestine were measured after removing the contents of these organs with saline.
Measurement of tissue composition
All excised tissue and collected feces samples were lyophilized. The sample water was determined as the difference of the mass before and after lyophilization. The nitrogen content in the samples was measured by the Kjeldahl method and converted to protein content by multiplying by 6.25. The total lipid content of the samples was measured by the Folch method [19]. The glycogen content was measured according to the method reported by Lo et al. [20].
Nitrogen balance
The nitrogen content in urine and feces was measured with the Kjeldahl method, and the nitrogen balance was calculated as the difference between ingested nitrogen and nitrogen excreted in urine and feces.
Urinary 3-methylhistidine
The concentration of 3-methylhistidine in a pooled urine sample of the last 3 days was determined by high-performance liquid chromatography with post-column reaction using o-phthalaldehyde (as fluorescence reagent) as reported elsewhere [21].
Blood analysis
Plasma glucose concentration was measured with the mutarotase-glucose oxidase method (Glucose CII-test Wako, Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Statistical analysis
All values are expressed as mean and SD. Data were assessed by one-way ANOVA and Fisher’s PLSD test. Statistical significance was assigned if p<0.05. All analyses were performed with a statistical package (Stat View J-5.0, SAS Institute Inc., Cary, NC).
Results
Food intake and body mass
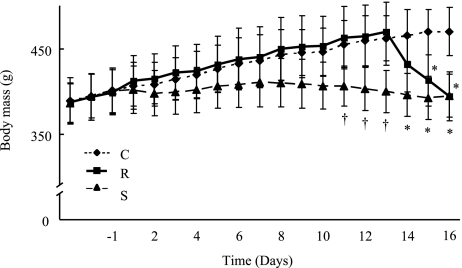
Food intake was restricted gradually in S during the study and it was 41% lower than C at the last day of the study (C 26.3 g/day (SD 3.7), S 15.5 g/day (0.9)). Body masses during the study are shown in Fig. 2. The initial body mass was comparable in the groups (C 400.8 g (26.4), R 398.8 (26.3), S 401.2 (26.0)). The final body mass was 16% lower in both R and S than C (p<0.05), while it was comparable in R and S (C 470.0 g (28.3), R 394.0 (28.6), S 394.8 (25.1)). Although the decrease in the body mass of S was small, rats of this age gained body mass as C. We thus regarded S as a group of slow body mass reduction because its body mass was lower than C.
Fig. 2.
Body mass during the 16-day study. Change of body mass for control (C), rapid body mass reduction (R), and slow body mass reduction (S) groups during the study. Values are means (SD) for 5 rats. †p<0.05 vs C and R; *p<0.05 vs C.
Tissue mass and tissue compositions
After the 16 days of the study, the mass of all excised skeletal muscles did not differ in the 3 groups (Table 1). In addition, the sum of all excised skeletal muscle masses was not significantly different among the groups. As shown in Table 2, the water, protein, total lipid, and glycogen content in the gastrocnemius and tibialis anterior were similar in the 3 groups, regardless of whether it was expressed in absolute or relative value, except for glycogen (mg/g tissue) in the tibialis anterior.
Table 1.
Skeletal muscle mass on day 16 of the study period
| Skeletal muscle, g |
||||||
|---|---|---|---|---|---|---|
| Gastrocnemius | Soleus | Plantaris | Extensor digitorum longus | Tibialis anterior | Sum of skeletal muscles | |
| C | 4.644 (0.217) | 0.298 (0.021) | 0.899 (0.041) | 0.457 (0.038) | 1.708 (0.055) | 8.006 (0.246) |
| R | 4.400 (0.333) | 0.300 (0.028) | 0.849 (0.054) | 0.452 (0.028) | 1.560 (0.192) | 7.561 (0.603) |
| S | 4.211 (0.360) | 0.306 (0.028) | 0.849 (0.106) | 0.429 (0.038) | 1.580 (0.104) | 7.375 (0.600) |
Values are means (SD) for 5 rats. C, control; R, rapid weight reduction; S, slow weight reduction.
Table 2.
Tissue water, protein, total lipid and glycogen content on Day 16 of the study period
| Water |
Protein |
Total lipid |
Glycogen |
|||||
|---|---|---|---|---|---|---|---|---|
| mg/g | g | mg/g | g | mg/g | g | mg/g | g | |
| Gastrocnemius | ||||||||
| C | 750 (4) | 3.482 (0.169) | 213 (2) | 0.990 (0.037) | 19 (2) | 0.088 (0.012) | 0.7 (0.2) | 0.0033 (0.0010) |
| R | 751 (2) | 3.304 (0.247) | 213 (4) | 0.936 (0.084) | 18 (1) | 0.078 (0.003) | 0.7 (0.3) | 0.0030 (0.0011) |
| S | 750 (5) | 3.159 (0.281) | 212 (5) | 0.893 (0.065) | 18 (1) | 0.078 (0.007) | 0.9 (0.4) | 0.0038 (0.0018) |
| Tibialis anterior | ||||||||
| C | 741 (5) | 1.266 (0.042) | 219 (3) | 0.373 (0.011) | 18 (2) | 0.031 (0.003) | 0.3 (0.1)b | 0.0006 (0.0002) |
| R | 743 (4) | 1.160 (0.148) | 216 (5) | 0.337 (0.038) | 17 (2) | 0.026 (0.002) | 0.6 (0.3)ab | 0.0009 (0.0006) |
| S | 743 (5) | 1.173 (0.083) | 215 (3) | 0.340 (0.018) | 18 (2) | 0.029 (0.002) | 0.8 (0.2)a | 0.0012 (0.0004) |
| Perirenal adipose | ||||||||
| C | 120 (27) | 0.951 (0.059)a | 11 (2)b | 0.089 (0.025) | 836 (28)a | 6.895 (1.386)a | 0.2 (0.1)a | 0.0016 (0.0009)a |
| R | 180 (39) | 0.580 (0.187)b | 23 (9)a | 0.071 (0.018) | 766 (45)b | 2.699 (1.391)b | 0.1 (0.0)b | 0.0004 (0.0003)b |
| S | 148 (43) | 0.672 (0.131)b | 14 (2)b | 0.063 (0.016) | 805 (40)ab | 3.892 (1.480)b | 0.1 (0.0)b | 0.0004 (0.0002)b |
| Liver | ||||||||
| C | 694 (11) | 10.706 (0.540)a | 211 (9)b | 3.260 (0.257)a | 48 (6) | 0.745 (0.125)a | 15.1 (7.4)a | 0.2345 (0.1170)a |
| R | 700 (7) | 6.911 (0.791)c | 230 (4)a | 2.272 (0.259)b | 47 (7) | 0.469 (0.101)b | 0.7 (0.2)b | 0.0065 (0.0024)b |
| S | 700 (8) | 7.782 (0.011)b | 221 (7)a | 2.466 (0.224)b | 44 (4) | 0.487 (0.082)b | 7.1 (5.3)b | 0.0791 (0.0599)b |
| Stomach | ||||||||
| C | 763 (12) | 1.390 (0.123)a | 143 (5)b | 0.260 (0.019)a | 66 (13) | 0.121 (0.034)a | 0.6 (0.1) | 0.0010 (0.0002) |
| R | 769 (12) | 1.108 (0.160)b | 153 (5)a | 0.220 (0.025)b | 48 (10) | 0.069 (0.018)b | 0.6 (0.2) | 0.0008 (0.0003) |
| S | 769 (4) | 1.344 (0.106)ab | 157 (3)a | 0.274 (0.020)a | 47 (6) | 0.082 (0.013)ab | 0.5 (0.0) | 0.0010 (0.0003) |
| Small intestine | ||||||||
| C | 771 (11) | 4.705 (0.658)a | 156 (5) | 0.953 (0.122)a | 46 (7) | 0.282 (0.059)a | 0.5 (0.0) | 0.0028 (0.0002)a |
| R | 766 (19) | 3.269 (0.234)b | 163 (8) | 0.696 (0.031)b | 43 (9) | 0.185 (0.040)b | 0.5 (0.0) | 0.0019 (0.0001)c |
| S | 758 (23) | 3.852 (0.964)ab | 156 (15) | 0.776 (0.099)b | 59 (14) | 0.300 (0.095)a | 0.5 (0.0) | 0.0022 (0.0003)b |
Values are means (SD) for 5 rats. Means in a column without common superscripts differ, p<0.05.
In contrast to skeletal muscles, the perirenal adipose tissue mass decreased by 58% and 42% in R and S, respectively (p<0.01, Table 3), due mainly to decreasing total lipid in this tissue (Table 2), but the decrease did not differ significantly between R and S. When the total lipid content of the perirenal adipose tissue was expressed per gram of tissue, it was lower in R than C; however, no statistically significant difference was observed between R and S.
Table 3.
Adipose tissue and splanchnic tissue mass on day 16 of the study period
| Adipose tissues, g |
Splanchnic tissues, g |
||||||
|---|---|---|---|---|---|---|---|
| Perirenal | Epididymal | Sum of adipose tissue | Liver | Stomach | Small intestine | Sum of digestive organs | |
| C | 8.208 (1.417)a | 6.029 (1.520)a | 14.236 (2.682)a | 15.434 (0.928)a | 1.824 (0.173)a | 6.098 (0.832)a | 7.922 (0.819)a |
| R | 3.453 (1.615)b | 4.276 (0.692)b | 7.729 (2.004)b | 9.874 (1.135)b | 1.440 (0.200)b | 4.264 (0.236)b | 5.705 (0.374)b |
| S | 4.796 (1.665)b | 4.324 (0.869)b | 9.120 (2.531)b | 11.126 (0.776)b | 1.748 (0.139)ab | 5.064 (1.144)ab | 7.016 (1.314)ab |
Values are means (SD) for 5 rats. C, control; R, rapid weight reduction; S, slow weight reduction. Means with different superscripts in a column are significantly different according to the Fisher’s PLSD test.
The liver mass decreased by 36% and 28% in R and S, respectively (p<0.01, Table 3) and it tended to be lower in R than S (p = 0.061). The content of water, protein, total lipid, and glycogen (Table 2) in the liver were significantly lower in both R and S compared with C, and the water content was significantly lower in R than in S and the glycogen content was lower by 92% in R than in S. When these components are expressed as relative to the liver mass, the content of water, protein, and total lipid did not differ between R and S, whereas the glycogen content in the liver was 90% lower in R than S (p = 0.075). The stomach (p = 0.017), small intestine (p<0.01), and the sum of these digestive organ (p<0.01) masses were significantly lower in R than C, while these values did not differ between S and C (Table 3). The stomach mass was 18% lower in R than S (p = 0.069). The water, protein, and total lipid content of the stomach and small intestine were also significantly lower in R than C, whereas these measurements showed no difference between S and C, excluding the protein content in the small intestine, which was significantly lower in S than C (Table 2). Glycogen in the small intestine did not differ when expressed per gram of tissue, while it was significantly lower in R than S when expressed per the whole organ.
Nitrogen balance
As shown in Table 4, the nitrogen balance did not differ among the groups on the day before the study (Day -1). The nitrogen balance became negative during the 3-day fast (Days 14–16) in R, and it was significantly lower in R than in both C and S. The amount of nitrogen excreted into urine and feces in R decreased gradually during the 3-day fast. As a result, the nitrogen balance approached a positive value. In S, the nitrogen balance remained slightly positive during the last 3 days of the study in spite of an approximately 40% decrease in nitrogen intake. This was due to a decrease in excretion of urinary nitrogen during this period. The nitrogen balance was significantly lower in S than C.
Table 4.
Nitrogen intake, urinary and fecal nitrogen excretion, and nitrogen balance on the day before (Day –1) and last 3 days (Days 14–16) of the study
| Day -1 | Day 14 | Day 15 | Day 16 | ||
|---|---|---|---|---|---|
| Nitrogen intake, g/day | C | 0.987 (0.082) | 1.116 (0.200)a | 1.125 (0.108)a | 1.069 (0.151)a |
| R | 0.979 (0.066) | 0 (0)c | 0 (0)c | 0 (0)c | |
| S | 1.013 (0.081) | 0.547 (0.039)b | 0.583 (0.045)b | 0.632 (0.037)b | |
| Urinary nitrogen excretion, g/day | C | 0.478 (0.049) | 0.610 (0.089)a | 0.666 (0.065)a | 0.653 (0.109)a |
| R | 0.417 (0.099) | 0.312 (0.025)c | 0.268 (0.028)c | 0.227 (0.040)c | |
| S | 0.525 (0.227) | 0.394 (0.020)b | 0.428 (0.033)b | 0.436 (0.030)b | |
| Fecal nitrogen excretion, g/day | C | 0.125 (0.031) | 0.194 (0.037)a | 0.187 (0.033)a | 0.200 (0.034)a |
| R | 0.170 (0.028) | 0.101 (0.028)b | 0.024 (0.020)c | 0.012 (0.008)c | |
| S | 0.149 (0.027) | 0.092 (0.031)b | 0.114 (0.021)b | 0.105 (0.013)b | |
| Nitrogen balance, g/day 1 | C | 0.383 (0.072) | 0.312 (0.091)a | 0.272 (0.082)a | 0.216 (0.038)a |
| R | 0.392 (0.106) | −0.413 (0.035)c | −0.291 (0.038)c | −0.239 (0.037)c | |
| S | 0.339 (0.168) | 0.061 (0.028)b | 0.041 (0.022)b | 0.090 (0.017)b |
Values are means (SD) for 5 rats. 1Nitrogen balance = (nitrogen intake) – (urinary and fecal nitrogen excretion). Means in a column without common superscripts differ, p<0.05.
Urinary 3-methylhistidine excretion
The urinary excretion of 3-methylhistidine during the last 3 days of the study was 81% and 138% greater in R than C and S, respectively. However, no statistically significant difference was observed among the 3 groups (C 1893.2 nmol/3 days (1868.2), R 3420.9 (2745.0), S 1439.8 (709.3)).
Plasma glucose concentration
The plasma glucose concentration did not differ among the groups on Day -1 (C 135.3 mg/dl (6.5), R 123.2 (10.9), S 139.8 (13.4)). On Day 16, it was significantly lower in R than C and S and was significantly lower in S than C (C 130.8 mg/dl (7.0), R 94.7 (2.8), S 110.7 (6.5)).
Water balance
Water balance calculated by subtracting urine volume from water intake did not differ among the groups on Day -1 (Table 5). It was negative or slightly positive during the first 2 days of fasting (Day 14 and 15) in R, as a result of decreased drinking water consumption. On the final day of fasting (Day 16), water balance turned negative again in R. Water balance was positive throughout the study in S.
Table 5.
Water consumption, urine volume, and water balance on the day before (Day –1) and last 3 days (Days 14–16) of the study
| Day -1 | Day 14 | Day 15 | Day 16 | ||
|---|---|---|---|---|---|
| Water consumption, g/day | C | 40.1 (6.8)ab | 49.4 (13.8)a | 49.9 (13.3)a | 54.3 (16.6)a |
| R | 37.3 (5.5)b | 31.3 (8.7)b | 30.0 (11.2)b | 7.9 (4.3)b | |
| S | 49.7 (9.3)a | 48.2 (5.5)a | 44.4 (10.4)ab | 47.1 (7.1)a | |
| Urine volume, g/day | C | 22.2 (4.7)ab | 27.8 (9.1) | 29.8 (11.4) | 33.4 (14.4)a |
| R | 19.4 (4.3)b | 33.1 (7.8) | 27.8 (10.1) | 10.8 (3.0)b | |
| S | 29.7 (7.7)a | 29.5 (2.7) | 33.1 (8.1) | 29.8 (5.6)a | |
| Water balance, g/day 1 | C | 17.9 (4.5) | 21.6 (5.8)a | 20.1 (4.9)a | 20.8 (4.2)a |
| R | 17.8 (4.2) | −1.9 (4.4)b | 2.1 (1.8)c | −2.8 (1.6)b | |
| S | 20.1 (2.2) | 18.6 (5.3)a | 11.3 (3.5)b | 17.3 (2.2)a |
Values are means (SD) for 5 rats. 1Water balance = (water consumption) – (urine volume).
Means in a column without common superscripts differ, p<0.05.
Hematocrit
Hematocrit did not differ among the groups on Day -1 (C 47.4% (2.4), R 48.0 (1.5), S 47.2 (1.8)), whereas it was significantly higher in R than both C and S on Day 16 (C 45.6% (2.2), R 51.2 (2.4), S 46.4 (4.1)).
Discussion
The primary finding of the present study is that differences between rapid and slow body mass reduction were observed in splanchnic tissues but not in skeletal muscle or adipose tissue. The decrease in splanchnic tissue mass was greater in rapid than slow body mass reduction. No differences in mass reduction were observed in skeletal muscle and adipose tissue. In addition, the decrease in skeletal muscle by both rapid and slow reduction was minimal.
As shown in Tables 1 and 2, the mass and protein content of skeletal muscle did not decrease significantly in either rapid or slow reduction. The results observed in R in the present study are in agreement with reports that skeletal muscle mass remained unchanged during 3 days of fasting in 12-week-old rats [22] and that skeletal muscle protein was maintained during 5 days of fasting in 16-week-old rats [16].
It is considered that the loss of skeletal muscle protein was associated with body fat stores [17, 23, 24]. Goodman et al. demonstrated that there is a strong connection between circulating lipid fuels (free fatty acid and ketone bodies) and protein sparing during starvation and that the diminution in these fuels may trigger protein loss in rats [17, 18, 24]. Although the present study did not determine plasma free fatty acids and ketone bodies, the total lipid content in the perirenal adipose tissue decreased by 61% in R (Table 2). Assuming that the total lipid content in the whole body in rat is 10% of body mass [25], the rat (470 g body mass) we used would contain 47 g total lipid in the whole body. If the total lipid in the whole body decreases by same ratio as total lipid in the perirenal adipose tissues, the loss of total lipid by the fasting is 29 g and it would represent 261 kcal. Since the basal metabolic rate of the rats (470 g body mass) for 3 days is approximately 165 kcal/day [26], it is enough to meet energy needs of the 3 day fasted rats. Thus, it appears plausible that lipid fuels contributed to the conservation of skeletal muscle protein during fasting in R. Total lipid in the adipose tissue was also decreased in S (Table 2), and the decrease did not differ significantly from R. Therefore, lipid fuels derived from adipose tissue might supply energy so that skeletal muscle protein could be spared in S, as well.
The mechanism responsible for maintenance of skeletal muscle protein during fasting in R in the current study was not clear. The rats in the present study performed climbing exercise 3 times a week. Therefore, it is possible that exercise training could prevent skeletal muscle loss during body mass reduction. However, in our pilot study, which employed a comparable energy restriction protocol to that of the current study but no exercise, no significant decrease in skeletal muscle mass was observed. We thus conclude that exercise training would not be associated with the minimal change in skeletal muscle mass in the present study.
In contrast to the findings in skeletal muscle, the decrease in mass of both the liver and the digestive tract tended to be greater in R than S (Table 3). The reason why mass reduction was greater in splanchnic tissues than skeletal muscle in R appears to be related to the higher rate of protein turnover in splanchnic tissues [27, 28]. Furthermore, protein breakdown was greater in the gastrointestinal tissues than skeletal muscle during 3 days of fasting in young rats [29]. Therefore, protein degradation in tissues with rapid protein turnover, such as the digestive organs, would be enhanced to a greater extent in R than S because no exogenous protein/amino acids were supplied during fasting in R. In S, however, protein supplied during food restriction would lessen protein degradation in these tissues. The implication is that rapid body mass reduction is more stressful on the intestinal organs in comparison to the slow body mass reduction. In addition, it was speculated that the greater loss of splanchnic tissues is contributed to the greater loss of the fat free mass with rapid body mass reduction compared with slow body mass reduction [6].
Urinary 3-methylhistidine excretion was not significantly different among the groups. Comparing the means of R and S with the Student t test showed that the excretion tended to be greater in R than S (p = 0.078). Urinary excretion of 3-methylhistidine is usually regarded as a marker of degradation of myofibrillar protein in skeletal muscle [30]. However, it has been reported that 15 to 41% of 3-methylhistidine excreted represents the degradation of protein in smooth muscle such as the digestive tract and skin [28, 31]. Emery et al. [29] have demonstrated that the 3-methylhistidine excretion is derived from the degradation of smooth muscle rather than skeletal muscle during the early period of fasting in rats. In the present study, urinary 3-methylhistidine excretion tended to be higher in R than S. Since skeletal muscle protein did not differ between R and S, increased urinary 3-methylhistidine excretion in R appears to originate from protein degradation in the digestive tract.
In order to reduce body mass rapidly, water intake restriction before competition is commonly used by body mass–conscious athletes including wrestlers [1, 32]. It is well known that these procedures cause dehydration [33]. Thus, drinking water was restricted for the last 21 h of fasting in R. Consequently, water balance turned negative in R, and it was significantly lower in R than both C and S (Table 5), and the hematocrit value was significantly higher in R than both C and S, indicating that the rats in R were dehydrated. The water content of skeletal muscles did not significantly differ among the groups (Table 2); however, the content was somewhat less in R and S than C. Skeletal muscle consists of about 40% of body mass. Thus, the small difference observed among the excised muscles in the present study might be large enough to explain the negative water balance as a whole. The water content in the liver, stomach, and small intestine was significantly greater in C than the other two groups, suggesting that these organs also contributed to the negative water balance.
In conclusion, both adipose tissue and the digestive organs decreased in mass after body mass reduction, and the decrease in the digestive organs was greater in rapid reduction than slow reduction, while no difference was observed in the decrease in adipose tissue. Skeletal muscle did not decrease in mass during either rapid or slow reduction in the present study.
References
- 1.Kukidome T., Aizawa K., Nakajima K., Masujima A. The actual state of weight loss among the contestants for the all Japan wrestling championships. Nippon Rinsyou Supôtu Igaku Kaishi (in Japanese) 2006;14:325–332. [Google Scholar]
- 2.Roemmich J.N., Sinning W.E. Weight loss and wrestling training: effects on nutrition, growth, maturation, body composition, and strength. J. Appl. Physiol. 1997;82:1751–1759. doi: 10.1152/jappl.1997.82.6.1751. [DOI] [PubMed] [Google Scholar]
- 3.Utter A.C., O’Bryant H.S., Haff G.G., Trone G.A. Physiological profile of an elite freestyle wrestler preparing for competition: a case study. J. Strength Cond. Res. 2002;16:308–315. [PubMed] [Google Scholar]
- 4.Oppliger R.A., Utter A.C., Scott J.R., Dick R.W., Klossner D. NCAA rule change improves weight loss among national championship wrestlers. Med. Sci. Sports Exerc. 2006;38:963–970. doi: 10.1249/01.mss.0000218143.69719.b4. [DOI] [PubMed] [Google Scholar]
- 5.Oppliger R.A., Case H.S., Horswill C.A., Landry G.L., Shetler A.C. American College of Sports Medicine position stand: weight loss in wrestlers. Med. Sci. Sports Exerc. 1996;28:ix–xii. [PubMed] [Google Scholar]
- 6.Kukidome T., Sato M., Suzuki M. The effect of methods of weight reduction on body composition and subjective physical condition in college wrestlers. Health Sci. (in Japanese) 2001;17:26–31. [Google Scholar]
- 7.Webster S., Rutt R., Weltman A. Physiological effects of a weight loss regimen practiced by college wrestlers. Med. Sci. Sports Exerc. 1990;22:229–234. [PubMed] [Google Scholar]
- 8.From the Centers for Disease Control and Prevention. Hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers—North Carolina, Wisconsin, and Michigan, November–December 1997. JAMA. 1998;279:824–825. [PubMed] [Google Scholar]
- 9.Oppliger R.A., Steen S.N., Scott J.R. Weight loss practices of college wrestlers. Int. J. Sport Nutr. Exerc. Metab. 2003;13:29–46. doi: 10.1123/ijsnem.13.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Horswill C.A., Park S.H., Roemmich J.N. Change in the protein nutritional status of adolescent wrestlers. Med. Sci. Sports Exerc. 1990;22:599–604. doi: 10.1249/00005768-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mourier A., Bigard A.X., de Kerviler E., Roger B., Legrand H., Guezennec C.Y. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int. J. Sports Med. 1997;18:47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- 12.Yankanich J., Kenney W.L., Fleck S.J., Kraemer W.J. Precompetition weight loss and changes in vascular fluid volume in NCAA Division I college wrestlers. J. Strength Cond. Res. 1998;12:138–145. [Google Scholar]
- 13.Fogelholm G.M., Koskinen R., Laakso J., Rankinen T., Ruokonen I. Gradual and rapid weight loss: effects on nutrition and performance in male athletes. Med. Sci. Sports Exerc. 1993;25:371–377. [PubMed] [Google Scholar]
- 14.Afolabi P.R., Jahoor F., Jackson A.A., Stubbs J., Johnstone A.M., Faber P., Lobley G., Gibney E., Elia M. The effect of total starvation and very low energy diet in lean men on kinetics of whole body protein and five hepatic secretory proteins. Am. J. Physiol. 2007;293:E1580–E1589. doi: 10.1152/ajpendo.00169.2007. [DOI] [PubMed] [Google Scholar]
- 15.Faber P., Johnstone A.M., Gibney E.R., Elia M., Stubbs R.J., Duthie G.G., Calder A.G., Lobley G.E. The effect of rate of weight loss on erythrocyte glutathione concentration and synthesis in healthy obese men. Clin. Sci. (Lond) 2002;102:569–577. doi: 10.1042/cs1020569. [DOI] [PubMed] [Google Scholar]
- 16.Goodman M.N., Ruderman N.B. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA, and protein. Am. J. Physiol. 1980;239:E269–E276. doi: 10.1152/ajpendo.1980.239.4.E269. [DOI] [PubMed] [Google Scholar]
- 17.Goodman M.N., Lowell B., Belur E., Ruderman N.B. Sites of protein conservation and loss during starvation: influence of adiposity. Am. J. Physiol. 1984;246:E383–E390. doi: 10.1152/ajpendo.1984.246.5.E383. [DOI] [PubMed] [Google Scholar]
- 18.Goodman M.N., Larsen P.R., Kaplan M.M., Aoki T.T., Young V.R., Ruderman N.B. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am. J. Physiol. 1980;239:E277–E286. doi: 10.1152/ajpendo.1980.239.4.E277. [DOI] [PubMed] [Google Scholar]
- 19.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Lo S., Russell J.C., Taylor A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970;28:234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 21.Fermo I., De Vecchi E., Diomede L., Paroni R. Serum amino acid analysis with pre-column derivatization: comparison of the o-phthaldialdehyde and N,N-diethyl-2,4-dinitro-5-fluoroaniline methods. J. Chromatogr. 1990;534:23–35. doi: 10.1016/s0378-4347(00)82145-3. [DOI] [PubMed] [Google Scholar]
- 22.Johnson G., Roussel D., Dumas J.F., Douay O., Malthiery Y., Simard G., Ritz P. Influence of intensity of food restriction on skeletal muscle mitochondrial energy metabolism in rats. Am. J. Physiol. 2006;291:E460–E467. doi: 10.1152/ajpendo.00258.2005. [DOI] [PubMed] [Google Scholar]
- 23.Cherel Y., Robin J.P., Heitz A., Calgari C., Le Maho Y. Relationships between lipid availability and protein utilization during prolonged fasting. J. Comp. Physiol. B. 1992;162:305–313. doi: 10.1007/BF00260757. [DOI] [PubMed] [Google Scholar]
- 24.Goodman M.N., McElaney M.A., Ruderman N.B. Adaptation to prolonged starvation in the rat: curtailment of skeletal muscle proteolysis. Am. J. Physiol. 1981;241:E321–E327. doi: 10.1152/ajpendo.1981.241.4.E321. [DOI] [PubMed] [Google Scholar]
- 25.Robin J.P., Decrock F., Herzberg G., Mioskowski E., Le Maho Y., Bach A., Groscolas R. Restoration of body energy reserves during refeeding in rats is dependent on both the intensity of energy restriction and the metabolic status at the onset of starvation. J. Nutr. 2008;138:861–866. doi: 10.1093/jn/138.5.861. [DOI] [PubMed] [Google Scholar]
- 26.Terpstra A.H. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J. Nutr. 2001;31:2067–2068. doi: 10.1093/jn/131.7.2067. [DOI] [PubMed] [Google Scholar]
- 27.Millward D.J., Bates P.C. 3-Methylhistidine turnover in the whole body, and the contribution of skeletal muscle and intestine to urinary 3-methylhistidine excretion in the adult rat. Biochem. J. 1983;214:607–615. doi: 10.1042/bj2140607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wassner S.J., Li J.B. N tau-methylhistidine release: contributions of rat skeletal muscle, GI tract, and skin. Am. J. Physiol. 1982;243:E293–E297. doi: 10.1152/ajpendo.1982.243.4.E293. [DOI] [PubMed] [Google Scholar]
- 29.Emery P.W., Cotellessa L., Holness M., Egan C., Rennie M.J. Different patterns of protein turnover in skeletal and gastrointestinal smooth muscle and the production of N tau-methylhistidine during fasting in the rat. Biosci. Rep. 1986;6:143–153. doi: 10.1007/BF01115000. [DOI] [PubMed] [Google Scholar]
- 30.Candow D.G., Chilibeck P.D., Facci M., Abeysekara S., Zello G.A. Protein supplementation before and after resistance training in older men. Eur. J. Appl. Physiol. 2006;97:548–556. doi: 10.1007/s00421-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 31.Brenner U., Herbertz L., Thul P., Walter M., Meibert M., Muller J.M., Reinauer H. The contribution of small gut to the 3-methylhistidine metabolism in the adult rat. Metabolism. 1987;36:416–418. doi: 10.1016/0026-0495(87)90036-9. [DOI] [PubMed] [Google Scholar]
- 32.Alderman B.L., Landers D.M., Carlson J., Scott J.R. Factors related to rapid weight loss practices among international-style wrestlers. Med. Sci. Sports Exerc. 2004;36:249–252. doi: 10.1249/01.MSS.0000113668.03443.66. [DOI] [PubMed] [Google Scholar]
- 33.Bartok C., Schoeller D.A., Randall Clark. R., Sullivan J.C., Landry G.L. The effect of dehydration on wrestling minimum weight assessment. Med. Sci. Sports Exerc. 2004;36:160–167. doi: 10.1249/01.MSS.0000106855.47276.CD. [DOI] [PubMed] [Google Scholar]