Abstract
We have developed a simple ESR spin trapping based method for hydroxyl (OH) radical scavenging-capacity determination, using iron-free OH radical source. Instead of the widely used Fenton reaction, a short (typically 5 seconds) in situ UV-photolysis of a dilute hydrogen peroxide aqueous solution was employed to generate reproducible amounts of OH radicals. ESR spin trapping was applied to quantify OH radicals; the decrease in the OH radical level due to the specimen’s scavenging activity was converted into the OH radical scavenging capacity (rate). The validity of the method was confirmed in pure antioxidants, and the agreement with the previous data was satisfactory. In the second half of this work, the new method was applied to the sera of chronic renal failure (CRF) patients. We show for the first time that after hemodialysis, OH radical scavenging capacity of the CRF serum was restored to the level of healthy control. This method is simple and rapid, and the low concentration hydrogen peroxide is the only chemical added to the system, that could eliminate the complexity of iron-involved Fenton reactions or the use of the pulse-radiolysis system.
Keywords: hydroxyl radical, antioxidant capacity, ESR spin trapping, hydrogen peroxide, photochemistry
Introduction
Because hydroxyl (OH) radicals are known to be ultimate injurious species in biological systems [1, 2], the ability to remove OH radicals is considered to be an important factor for homeostasis. Thus, serum OH radical scavenging activity could be an index for patho-physiological conditions. In OH radical scavenging assay, the selection of OH radical source has been limited and most studies employed the Fenton reaction, i.e.; ferrous ion plus hydrogen peroxide. A majority of OH radical quantification methods are indirect and utilizes OH radical’s ability to damage chromophores or fluorophores, resulting in the loss of color or fluorescence [3, 4]. In contrast, ESR spin trapping method directly measures OH radical level [5–7]. The decrease in OH radical level by the addition of the specimen with antioxidant activity can be readily converted into OH radical scavenging capacity. Nevertheless, the spin trapping data for serum OH radical scavenging activity have been scarce probably because of the lack of appropriate OH radical source.
Patho-physiological OH radical production is believed to be through the Fenton reaction-type process; therefore we speculate that the addition of exogenous iron for the purpose of OH radical generation would make the system more complicated. Tanigawa reported that in ESR spin trapping, when the Fenton reaction was employed as OH radical source, various factors such as ferric ion/hydrogen peroxide concentration, buffer, and chelating agent could affect the outcome [8]. Complex reaction mechanism in the Fenton reaction was suspected as a reason for these variations [9]; furthermore, the interaction between spin trapping agent and iron ion seems make the reaction system more complicated [10]. In summary, drawbacks of the use of the Fenton reaction as the OH radical source are: 1) the reaction is unstoppable and initiated as soon as ferrous ion and hydrogen peroxide are mixed; 2) OH radical yield depends on the sequence of the addition of reactants and the reaction time [8]; and 3) OH radical level could be decreased either by antioxidant’s scavenging activity or inhibition of the Fenton reaction. Other drawbacks specific for spin trapping include: 1) ferric ion catalyzes the formation of OH radical adducts via non-free radical reactions [11]; and 2) the interference of the iron with the reaction between OH radical and antioxidant [10]. The use of iron-free OH radical generating system could eliminate most of these drawbacks.
We have developed a simple method of OH radical scavenging-capacity measurement by using photolytic OH radical generating system. It has been known for many years that UV photolysis of dilute hydrogen peroxide aqueous solution efficiently produces OH radicals. Harbour et al. reported that hydroperoxyl (HO2) and OH radicals could be detected through the spin trapping method with the spin trap PBN (phenyl N-tert-butyl nitrone) or DMPO (5,5-dimethyl-1-pyrroline N-oxide) [12]. When hydrogen peroxide concentration was lower than approximately 300 mM (1%), UV photolysis produced OH radical adduct alone. In addition, the concentration of OH radical adduct was proportional to the illumination time as long as it was kept relatively short to avoid photolytic decomposition of the spin adduct [13]. In the present study, OH radical was produced with in situ photolysis in the absence or presence of antioxidant or serum. The amount of OH radical was quantified using an improved spin trap, CYPMPO (5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide) [14]. Recent spin trapping studies employed either DMPO [15] or DEPMPO (5-diehoxyphosphoryl-5-methyl-1-pyrroline N-oxide) [16] as a spin trap, but unlike DMPO and DEPMPO, CYPMPO is non-hygroscopic solid at room temperature (mp 126°C) and the spin trap solution has a long shelf-life. Moreover, the stability of the spin adducts of OH and superoxide is comparable to those of DEPMPO. Thus, the decrease of the ESR signal of OH radical adduct by the addition of antioxidant was converted into OH radical scavenging capacity after simple kinetic calculation.
We confirmed the validity of this method in several pure bio-chemicals with antioxidant activities. Through the use of iron-free OH radical source, new results were obtained in the sera from chronic renal failure (CRF) patient before and after hemodialysis. Serum could contain various factors that form metal complex with iron; therefore, we expected that the iron-free method could provide more reliable serum OH radical scavenging capacity data.
Experimental Procedures
Materials and equipment
Hydrogen peroxide, dimethyl sulfoxide, mannitol, sodium benzoate, and sodium salicylate were purchased from Kanto Chemical Co. (Tokyo, Japan). Acetyl salicylic acid, glutathione, N-acetylcysteine (NAC), diethylenetriamine pentaacetic acid (DETAPAC), ferrous sulfate, and sodium phosphate were obtained from Nacalai Tesque (Kyoto, Japan); PBN and CYPMPO were from Radical Research Inc. (Tokyo, Japan); and DMPO from Labotec Co. (Tokyo, Japan).
All human subject procedures were conducted with individual consent form and strictly following the protocol that had been approved by Asao Clinic Human Subject Use Committee. Blood was obtained from chronic kidney disease (CKD) patients (n = 10) under going stable maintenance hemodialysis (HD) and healthy volunteers (n = 10). Ten HD patients (5 females and 5 males, mean age 67.1 ± 9.4 years, time on dialysis 71.2 ± 45.8 months, receiving three weekly HD using polysulphone dialysis membrane) were enrolled. The underlying disease was chronic glomerulonephritis in 5, diabetic nephropathy in 2, polycystic kidney disease in 2 and nehrosclerosis in 1. Ten normal controls (5 females and 5 males, mean age 40.6 ± 14.9 years) were enrolled. Serum was separated and stored in the −20°C freezer until use. HD patients’ blood was collected before and after hemodialysis.
A JEOL JES-TE300 X-band ESR spectrometer (Tokyo, Japan) equipped with 100 kHz field modulation and a WIN-RAD operation software (Radical Research Inc.) was employed for ESR measurements. Typical spectrometer settings were: field modulation width 0.1 mT; microwave power 8 mW; field scan width and rate ±7.5 mT/2 min; time constant 0.1 s. UV light source was a medium pressure mercury/xenon arc (RUVF-203SR UV illuminator, Radical Research Inc.).
Photolytic production of OH radical
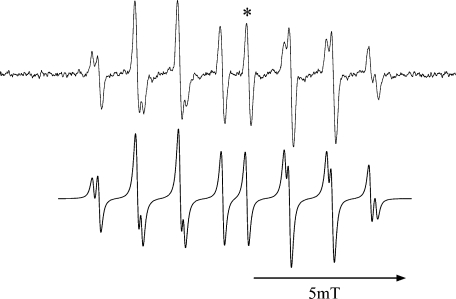
UV light from an RUVF-203SR UV illuminator that is installed with 200 W medium pressure mercury/xenon arc lamp was illuminated in situ to aqueous hydrogen peroxide solution. This UV illuminator is equipped with a quartz-fiber optical guide and its far-end was mechanically fitted to the hole in front of the ESR cavity, therefore, the distance between the light source and the sample can be kept fixed. The illuminator is also equipped with a mechanical shutter, and the illumination period can be controlled within ±0.01 sec. A typical ESR spectrum obtained in this photolysis is shown in Fig. 1 along with computer-simulated spectrum. This spectrum was assigned to OH radical adduct of CYPMPO. The optimization of the photolytic conditions is described in the results section.
Fig. 1.
ESR spectrum of OH radical adduct of CYPMPO (top). OH radical was produced with 5 s UV illumination to 10 mM H2O2 phosphate buffer (50 mM) solution. ESR spectrum was recorded immediately after the illumination was ceased. ESR spectrometer settings were: field modulation width 0.1 mT; microwave power 8 mW; field scan width/rate 15 mT/2 min; time constant 0.1 s. The intensity of the line marked with * was adopted as a measure for the adduct concentration. The bottom chart is a computer simulated ESR spectrum using hyperfine coupling constants; Isomer 1: AN = 1.37 mT, AH = 1.37 mT, AP = 4.88 mT; Isomer 2: AN = 1.25 mT, AH = 1.23 mT, AP = 4.70 mT.
Measurement of antioxidant’s OH radical scavenging capacity
OH radical scavenging capacity (rate) measurement was conducted as follows: 1) in situ illuminate UV light to the solution containing 10 mM hydrogen peroxide and 10 mM CYPMPO in 100 mM sodium phosphate buffer (pH 7.4), and record ESR signal intensity of OH radical adduct (I0); 2) record the ESR signal intensity (I) after UV illumiation from the same solution in the presence of a known amount of the selected antioxidant (AOx); and 3) according to the equation shown in the next section, plot I0/I-1 against [AOx]0/[CYPMPO]0 and calculate k2/k1 (k1: CYPMPO’s OH radical trapping rate constant, k2: AOx’s OH radical scavenging rate constant).
Measurement of serum OH radical scavenging capacity
Serum was dissolved at the rate of 10, 20, and 30% in 100 mM sodium phosphate buffer (pH 7.4), containing 10 mM hydrogen peroxide, 10 mM CYPMPO, and 1 mM DETAPAC. In ambient conditions, UV light was illuminated in situ and the ESR signal intensity I was recorded. The signal intensity I0 in the absence of serum was also recorded, and I0/I-1 was calculated. Serum OH radical scavenging capacity was calculated by dividing I0/I-1 with [serum %]/100([CYPMPO] in mM), e.g., in the case of 30% serum with 10 mM CYPMPO, I0/I-1 will be divided by 30/100 × 10 = 0.03, and the scavenging capacity was obtained in the unit of mM-CYPMPO (mM-CYPMPO equivalent). This number can be converted into mM-DMPO equivalent unit by dividing with 1.34, the ratio of scavenging capacity of CYPMPO and DMPO (Table 1). For the sake of comparison, the Fenton reaction was used to generate OH radicals. Hydrogen peroxide (1 mM), ferrous sulfate (0.1 mM), DETAPAC (1 mM), and CYPMPO (10 mM) were sequentially dissolved in sodium phosphate buffer (20 mM, pH 7.4); 60 s after mixing ESR signal from OH radical adduct was recorded. The same measurement was performed in the presence of serum, and OH radical scavenging capacity was calculated.
Table 1.
Relative OH radical scavenging capacity as measured with photo-generated OH radicals
| vs CYPMPO | vs DMPO |
absolute kAOX | ||
|---|---|---|---|---|
| this work | publisheda | (M-1 s-1)b | ||
| acetylsalicylic acid | 1.99 ± 0.05 | 1.48 | 1.8–3.6 | 5–10E9 [22] |
| DMPO | 1.34 ± 0.07 | (1) | (1) | 2.8–3.6E9 [23–25] |
| dimethylsulfoxide | 2.26 ± 0.33 | 1.69 | 1.9 [8] | 7E9 [26] |
| ethanol | 0.55 ± 0.02 | 0.41 | 0.19–0.31[8] | 0.7–1.1E9 [27] |
| glutathione | 2.48 ± 0.22 | 1.85 | 2.9 | 8E9 [28] |
| mannitol | 0.62 ± 0.01 | 0.46 | 0.27–0.50 [8] | 1–1.9E9 [29, 30] |
| N-acetylcysteine | 3.27 ± 0.11 | 2.44 | 4.9 | 1.36E10 [31] |
| PBN | 2.32 ± 0.12 | 1.73 | 0.93–3.6 | 2.6–10E9 [24, 25] |
| sodium benzoate | 2.00 ± 0.06 | 1.49 | 1.6 [8] | 5.7E9 [32] |
| sodium salicylate | 2.62 ± 0.02 | 1.96 | 4.3 | 1.2E10 [33] |
a Numbers without the reference were calculated from absolute kAOX = 2.8E9 of DMPO. b For example, 5E5 means 5 × 105.
Data analysis
Kinetic formulation of the competitive reaction between the spin trap and the antioxidant against OH radical has been published elsewhere [17, 18]. Briefly, in the presence of the spin trap (ST) and AOx, OH radical scavenging reactions that should occur are:
| OH + ST → OH-adduct rate constant k1 | (1) |
| OH + AOx → EPR-silent product rate constant k2 | (2) |
When the ESR peak intensity of OH radical adduct in the presence of ST alone and both ST and AOx is I0 and I, respectively, the amount of ESR-silent product must be I0 – I. Thus,
| I0/I–1 = k2[OH][AOx] / k1[OH][ST] | (3) |
Because the concentrations of ST and AOx are much higher than OH radical concentration and barely consumed in this reaction, we can replace [ST] and [AOx] with [ST]0 and [AOx]0, respectively, where [ ]0 denotes the initial concentration of the component. Thus,
| I0/I–I = k2/k1 • [AOx]0 / [ST]0 | (4) |
In conclusion, the plot of I0/I–1 against [AOx]0/[ST]0 will give a zero-crossing line with the slope of k2/k1. When the same spin trap and the free radical-generating condition are used, k1 becomes common for all antioxidants. Thus, k2 can be compared each other using k1 as a reference.
In the case of serum specimens, the sera were volumetrically diluted with the rate of by 10, 20, and 30% with phosphate buffer and the scavenging capacity k2/k1 was calculated by dividing I0/I-1 with [serum-vol%/100]/([ST]0 in mM), and those three values were averaged and expressed with standard deviation (SD). In order to evaluate the significance level of difference, control group vs CRF group and pre-HD vs post HD were subjected to Student’s t test and paired Student’s t test, respectively.
Results
Formation of OH radical adduct and its ESR spectrum
When the phosphate buffer (100 mM, pH 7.4) solution containing 10 mM hydrogen peroxide and 10 mM CYPMPO (spin trap) was UV-illuminated for 5 s, we obtained an ESR spectrum shown in Fig. 1. ESR signal was absent when the solution containing 10 mM CYPMPO alone was UV-illuminated for 5 s (data not shown). Based on the previous study this spectrum was assigned to the OH radical adduct of CYPMPO [14]. A computer-simulated spectrum is shown in the bottom panel of Fig. 1. The relative concentration of this species can be obtained using the peak-to-peak line height of the selected line (e.g., marked by * in Fig. 1).
Optimization of the illumination conditions
The crucial point of the present method is to establish the illumination condition to produce reproducible ESR signal intensity for OH radical adduct. The reproducibility of the level of OH radical adducts was determined by loading a fresh 10 mM hydrogen peroxide/10 mM CYPMPO solution into the same sample cuvette, illuminated for exactly 5 s (±0.01 s), and the ESR signal was recorded. This was repeated for five times and the experimental error of the ESR intensity was ±4%. The decay of the ESR signal after the photolysis was ceased was negligible because previous experiments indicated that the half life of the OH radical adduct of CYPMPO in the same condition was approximately 70 min [14]. The wavelength range in the UV light (from 200 W medium pressure mercury/xenon arc) was 250–450 nm; however, the illumination using band-pass filters (RU-330 (250–390 nm), RU-340 (280–370 nm), RU-350 (320–370 nm), and RU-360 (320–380 nm); Radical Research Inc) indicated that OH radical adduct was the sole product at any wavelength. Although molar absorption coefficient of hydrogen peroxide monotonically decreases from 100 M−1m−1 at 250 nm to 0.001 M−1m−1 at 400 nm [19], the yield of OH radical adduct was slightly dependent on the wavelength, indicating that the light intensity was sufficient to override the absorbance difference.
OH radical adduct of CYPMPO (or any nitrone spin traps) but not CYPMPO itself is moderately UV-sensitive and prolonged illumination diminished its ESR intensity [13]. The dependence of the yield of OH radical adduct against the illumination time was measured in 10 mM H2O2/10 mM CYPMPO- sodium phosphate buffer (100 mM) solution (data not shown). The ESR intensity of OH radical adduct linearly increased as a function of the illumination period up to 30 s, and completely leveled off after 180 s illumination. Thus, we adopted 5 s as the illumination time where the photochemical decomposition of OH radical adducts was negligible. When higher H2O2 concentrations were used one could further shorten the illumination time, however, in such a case, errors in the mechanical-shutter speed may become a concerning factor.
The arbitrary selection of relative AOx/ST concentrations was possible because the kinetic equation (4) contains only the ratio [AOx]/[ST], but not absolute concentrations. Using NAC as AOx, we tested the three cases of [NAC]0/[CYPMPO]0 = 0.5, i.e., [NAC]0/[CYPMPO]0 = 2.5 mM/5 mM, 5 mM/10 mM, and 10 mM/20 mM. We obtained k2/k1 = 3.5, 3.4, and 3.8, respectively, indicating that the selection of the spin trap concentration (10 mM) was justifiable.
In summary, once ESR signal of OH radical adduct with the signal-to-noise ratio higher than 100 is obtained was obtained using a UV-illuminator, the experimental parameters that determines the accuracy of the ESR intensity measurement are the illumination time and the concentrations of spin trap and hydrogen peroxide.
Antioxidant’s OH radical scavenging capacity measurement
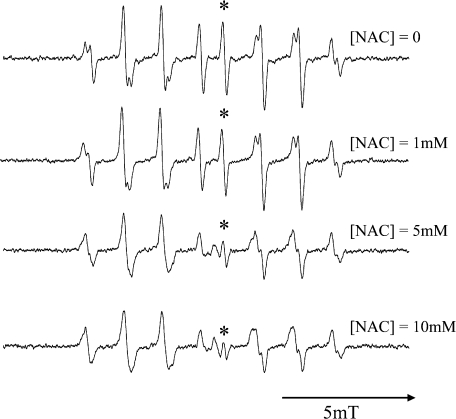
ESR spectrum recorded after UV illumination to 10 mM hydrogen peroxide in 100 mM sodium phosphate buffer was readily assigned to OH radical adduct of CYPMPO (Fig. 1). In the presence of antioxidant, the signal level decreased as a function of the antioxidant concentration. Fig. 2 shows an example when NAC was added as an OH radical scavenging antioxidant. There was a possibility that the OH radical production could be decreased by antioxidant’s UV-absorption. In such a case, the data point for higher antioxidant concentration in the plot like in Fig. 2 should go above the line passing through the origin, because I0 and I would be decreased. But there were no such discrepancies in the plot.
Fig. 2.
Changes in the level of OH radical adduct of CYPMPO as a function of the concentration of the antioxidant N-acetylcysteine (NAC). Five-second in situ UV photolysis was carried out to the sodium phosphate buffer (50 mM) solution of H2O2 (10 mM) and CYPMPO (10 mM). The peak-to-peak intensity of the line marked with * was adopted as I0 or I to calculate relative OH radical scavenging rates.
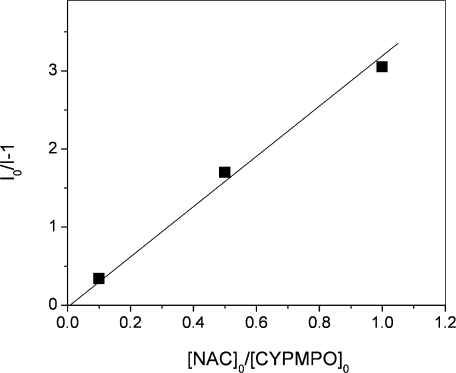
Signal intensities obtained from Fig. 2 were plotted according to Eq. 4 (Fig. 3) and the slope of the line was calculated. Thus, scavenging rate constants (scavenging capacities) relative to CYPMPO for several antioxidants are listed in Table 1. OH radical scavenging capacities of the selected antioxidants were previously measured mainly using pulse radiolysis or ESR spin trapping with the Fenton reaction as OH radical source and DMPO as a spin trap. The present OH radical scavenging capacity was measured with respect to CYPMPO scavenging rate, but in order to facilitate the comparison with the previous data the rates are also expressed in reference to DMPO scavenging capacity (Table 1).
Fig. 3.
A plot of (I0 – I)/I, where I0 and I denote ESR intensity in the absence and presence of NAC, respectively, against [AOx]0/[ST]0, where [AOx]0 and [ST]0 denote the initial concentrations of NAC and CYPMPO, respectively. The slope of the zero-crossing line represents the ratio of scavenging rate constants k2/k1.
Serum OH radical scavenging capacity measurement
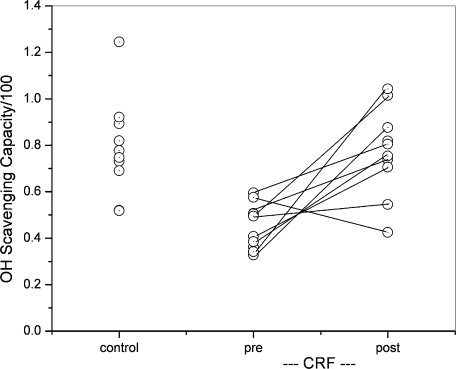
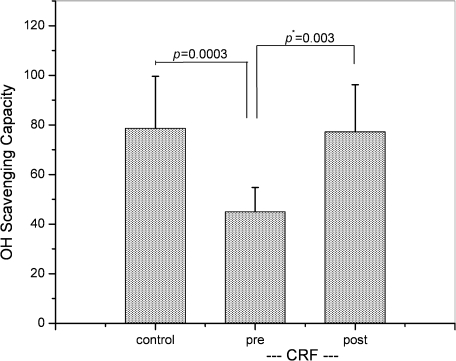
The present method of OH radical scavenging capacity determination was applied to the sera that were collected from 10 HD patients and 10 healthy volunteers. Serum was diluted with phosphate buffer at the rate of 10, 20, and 30%, and the ESR intensity was plotted according to Eq (4). In CRF group, blood was collected before (pre-HD) and after hemodialysis (post-HD). Fig. 4 shows data points for 30 serum specimens in the unit of mM-CYPMPO equivalent. The data point before and after HD from the same subject is connected with a solid line, indicating that scavenging capacity of 9 out of 10 subjects increased after HD. Fig. 5 is a graph for median values and SDs for control, pre-HD, and post-HD, i.e., 79 ± 21, 45 ± 10, and 77 ± 19 mM-CYPMPOeq, respectively. The significance level of difference was estimated using unpaired or paired Student’s t test. p Values for control vs pre-HD (unpaired) and pre-HD vs post-HD (paired) are 0.0003 and 0.003, respectively (Fig. 5), indicating that the differences are statistically significant. Regarding the effect of hemo-concentration after HD on OH-scavenging capacity, the pre/post-dialysis ratios of serum total protein concentrations (TP) and body weight (BW) were calculated to be 1.12 ± 0.04 and 1.04 ± 0.01, respectively. Whereas, the pre/post-dialysis ratio of OH-scavenging capacity was 1.84 ± 1.10, that was significantly higher than that of TP and BW (p<0.001). Table 2 lists the present and past Fenton reaction results in both mM-CYPMPOeq unit and mM-DMPOeq unit.
Fig. 4.
Serum OH radical scavenging capacity of control group (n = 10) and CRF subject (n = 10, Pre and post hemodialysis) in the unit of mM-CYPMPOeq. Serum was volumetrically diluted in buffer solution by 10, 20, and 30%. Each point represents the mean value for the three concentrations. In control, each point represents individual subject. In CRF group, pre-HD and post-HD data points that were obtained from the same individual were connected with a solid line.
Fig. 5.
A graph for mean values of OH radical scavenging capacity for control and pre-HD/post-HD CRF groups with error bars (SD). Post statistical test for the difference with unpaired Student’s t test indicated that the difference between control and pre-HD groups was significant (p = 0.0003). The difference between pre-HD and pos-HD was analyzed with paired Student’s t test and also significant (p* = 0.003).
Table 2.
Serum OH radical scavenging capacity
| Method | Control | Pre-HD | Post-HD | Unit | Source |
|---|---|---|---|---|---|
| UV | 79 ± 21 (n = 10) | 45 ± 10 (n = 10) | 77 ± 19 (n = 10) | mM-CYPMPOeqa | this work |
| 59 | 36 | 55 | mM-DMPOeqb | this work | |
| Fenton | 131 ± 52 (n = 10) | 226 ± 34 (n = 10) | 214 ± 25 (n = 10) | mM-CYPMPOeqa | this work |
| 98 | 120 | 160 | mM-DMPOeq | ref [8] | |
| 110 to 163 | mM-DMPOeqb | this work | |||
| Fenton | 54.0 (plasma) | 59.9 (plasma) | 61.5 (plasma) | mM-DMPOeq | ref [34] |
a Errors are in SD. b These values were calculated by dividing the above numbers with 1.34 (DMPO’s OH radical scavenging capacity in Table 1).
Free radical production from antioxidants.
Some reagents such as glutathione and NAC produced free radicals after scavenging OH radical [20]. Those free radicals were spin trapped by CYPMPO, yielding typical characteristics of S-centered radical adduct. Major component in the bottom spectrum of Fig. 2 is assignable to sulfur-centered NAC radical adduct [20]. Fortunately, when the antioxidant concentration was low, the levels of secondary radical adducts were negligible as compared to OH radical adduct. Dimethyl sulfoxide produced methyl radical adduct after the reaction with OH radical [21], but methyl adduct signal has little overlap with that of OH radical adduct, especially in the central region (data not shown). When PBN and DMPO were tested as antioxidants, naturally OH radical adducts of PBN and DMPO were visible, but the overlap did not interfere with the peak-height measurements (data not shown). Serum specimens did not produce spin adducts other than OH adducts even at 30% concentration.
Discussion
Using iron-free, photochemical OH radical source we were able to measure in vitro OH radical scavenging capacity for several antioxidants as well as sera at room temperature. The accuracy of this method relies on the UV illuminator that produces reproducible amount of OH radicals in repeated experiments. While, in the Fenton reaction, the concentration of OH radical adduct depends on a few laboratory parameters, including the sequence of mixing the reactants and the time interval between the mixing and the ESR measurement. In pure antioxidants, we obtained reasonable agreement to the previous data which were obtained using Fenton reaction/spin trapping or pulse radiolysis (Table 1).
The proposed method is relatively simple and rapid as compared to the Fenton reaction-based method; in addition, the selection of experimental conditions was flexible. We obtained similar results by changing the experimental conditions such as the ratio of hydrogen peroxide/spin trap concentrations and UV intensity/illumination-time within a reasonable range. As opposed to the Fenton system, start and stop of OH radical production was controllable simply by turning on and off the UV-light. Most data from previous Fenton/spin trapping studies were obtained using a single antioxidant concentration, but our results are based on the data taken in three different antioxidant concentrations, thus experimental error could be calculated (Table 1). Absolute second order OH radical scavenging rate constants listed in Table 1 were obtained mainly with pulse radiolysis; this technique is not a method of easy access for a majority of biomedical investigators. Most plots for I0/I-1 vs [AOx]/[ST] like Fig. 3 showed good linear dependence, suggesting that the possibility of reactions other than Eqs. (1) and (2) were unlikely. Dimethylsufoxide, ethanol, NAC, and glutathione reacted with OH radical, and the resultant products were spin trapped by CYPMPO to show ESR signal. In Fig. 2 (bottom panel), in the presence of high concentration of NAC (10 mM) one can see the appearance of spin adducts that was assignable to sulfur-centered radical adduct from NAC. These observations are the direct evidence that the antioxidant and the spin trap were competing to react with OH radical according to Eqs. (1) and (2). Possible drawback of the present method is photochemical decomposition of the specimen. However, the present protocol used very short illumination period in order to avoid such decomposition. Another drawback could be that this method is not applicable to non-transparent or turbid sample solutions.
Use of the Fenton reaction for OH radical source in OH radical scavenging capacity assay may increase the possibility that exogenous iron modifies the biological system of interest. The present method completely eliminated such concern, thus we obtained clear-cut results for human serum OH radical scavenging capacity. In the sera of CKD patients, we clearly showed that OH radical scavenging capacity of pre-HD specimens were significantly lower than control, i.e., 45 unit as compared to 79 unit (Fig. 5, Table 2); whereas, in post-HD sera, it increased to 77 unit, suggesting that hemodialysis was able to restore OH radical scavenging capacity to the control level. The correlation scheme for pre-HD/post-HD pairs in Fig. 4 shows that each individual CRF subject gained the restoration. Paired Student’s t test achieved p value of 0.003, indicating that the restoration was not the result of random averaging. Our Fenton reaction data (Table 1) did show the decrease from control to pre-HD, but there was no change from pre-HD to post-HD. Previously, the similar results were obtained in CKD plasma using the Fenton reaction (Table 2) [34]. It is possible that CKD may cause the increase of certain serum component(s) that inhibits OH radical scavenging activity, and HD can remove such components. The pre/post-dialysis ratios of serum TP and BW were significantly lower than that of OH radical scavenging capacity; i.e., the recovery of this capacity could not be explained by hemo-concentration after dialysis alone; however, it is possible that some dialyzable molecules contribute to this process. Further studies are required to identify such components. Also, there is a possibility that OH radical scavenging assay using the Fenton reaction detects the activity against modified OH radical species.
In conclusion, use of iron-free, photochemical OH radical source in the spin trapping determination of OH radical scavenging capacity may be useful specifically for intact biological fluid.
Acknowledgments
Visiting professorships to YK awarded by International Innovation Center, Kyoto University (2/1/2005–6/30/2005); Institute for Advanced Energy, Kyoto University (4/1/07 to 6/30/07); as well as Japan Society for Promotion of Science (8/1/2005–5/31/2006) are gratefully acknowledged.
Abbreviations
- ESR
electron spin resonance
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- DEPMPO
5-diehoxyphosphoryl-5-methyl-1-pyrroline N-oxide
- CYPMPO
5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide
- PBN
phenyl N-tert-butyl nitrone
- CRF
chronic renal failure
- NAC
N-acetylcysteine
- DETAPAC
diethylenetriamine pentaacetic acid
- CKD
chronic kidney disease
- HD
hemodialysis
- AOx
antioxidant
- TP
total protein concentrations
- BW
body weight
References
- 1.McCord J.M., Wong K. Oxygen free radicals and tissue damage. Excerpta. Medica. 1979;65:343–351. [Google Scholar]
- 2.Halliwell B., Gutteridge J.M.C. Free radicals in biology and medicine. Japan Scientific Societies Press; Tokyo: 1988. [Google Scholar]
- 3.Prior R., Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic. Biol. Med. 1999;27:1173–1181. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 4.Taysi S., Polat F., Gul M., Sari R.A., Bakan E. Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol. Int. 2004;21:200–204. doi: 10.1007/s00296-001-0163-x. [DOI] [PubMed] [Google Scholar]
- 5.Janzen E.G. Spin trapping. Methods Enzymol. 1984;105:188–198. doi: 10.1016/s0076-6879(84)05025-4. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein E., Rosen G.M., Raunkman E.J. Spin trapping. kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Am. Chem. Soc. 1980;102:4994–4999. [Google Scholar]
- 7.Finkelstein E., Rosen G.M., Rauckman E.J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 1980;200:1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- 8.Tanigawa T. Determination of hydroxyl radical scavenging activity by electron spin resonance. J. Kyoto Pref. Univ. Med. 1990;99:133–143. [Google Scholar]
- 9.Goldstein S., Meyerstein D., Czapski G. The Fenton reagents. Free Radic. Biol. Med. 1993;15:435–445. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J., Bank J.F., Scholes C.P. Subsecond time-resolved spin trapping followed by stopped-flow EPR of Fenton reaction products. J. Am. Chem. Soc. 1993;115:4742–4746. [Google Scholar]
- 11.Finkelstein E., Rosen G.M., Rauckman E.J. Production of hydroxyl radical by decomposition of superoxide spin trapped adducts. Mol. Pharmacol. 1982;21:262–265. [PubMed] [Google Scholar]
- 12.Harbour J.R., Chow V., Bolton J.R. An electron spin resonance study of the spin adducts of OH and HO2 radicals with nitrones in the ultraviolet photolysis of aqueous hydrogen peroxide solution. Can. J. Chem. 1974;52:3549–3553. [Google Scholar]
- 13.Kotake Y., Janzen E.G. Decay and fate of the hydroxyl radical adduct of α-phenyl N-tert-butylnitrone in aqueous media. J. Am. Chem. Soc. 1991;113:9503–9506. [Google Scholar]
- 14.Kamibayashi M., Oowada S., Kameda H., Okada T., Inanami O., Ohta S., Ozawa T., Makino K., Kotake Y. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic. Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 15.Janzen E.G., Liu J.I.-P. Radical addition reactions of 5,5-dimethyl-1-pyrroline-1-oxide. ESR spin trapping with a cyclic nitrone. J. Magn. Reson. 1973;9:510–512. [Google Scholar]
- 16.Frejaville C., Karoui H., Tuccio B., Le Moigne F., Culcasi M., Pietri S., Lauricella R., Tordo P. 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: a new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J. Med. Chem. 1995;38:258–265. doi: 10.1021/jm00002a007. [DOI] [PubMed] [Google Scholar]
- 17.Buettner G.R., Mason R.P. Spin-tapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo. Methods Enzymol. 1990;186:127–133. doi: 10.1016/0076-6879(90)86101-z. [DOI] [PubMed] [Google Scholar]
- 18.Huang S.H., Leonard S., Shi X., Goins M.R., Vallyathan V. Antioxiant activity of lazaroid (U-75412E) and its protective effects against crystalline silica-induced cytotoxicity. Free Rad. Biol. Med. 1998;24:529–536. doi: 10.1016/s0891-5849(97)00285-2. [DOI] [PubMed] [Google Scholar]
- 19.Phibbs M.K., Giguére P.A. Hydrogen peroxide and its Analogues. III. Absorption spectrum of hydrogen and deuterium peroxides in the near ultraviolet. Can J. Chem. 1951;29:490–493. doi: 10.1139/v51-058. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H., Joseph J., Zhang H., Karoui H., Kalyanaraman B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001;31:599–606. doi: 10.1016/s0891-5849(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 21.Burkitt M.J., Mason R.P. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: an ESR spin-trapping investigation. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8440–8444. doi: 10.1073/pnas.88.19.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiller K.O., Hodd O.L., Willson R.L. Antiinflammatory drugs: protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem. Biol. Interact. 1983;47:293–305. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- 23.Madden K.P., Taniguchi H. In situ radiolysis time-resolved ESR studies of spin trapping by DMPO: Re-evalution of hydroxyl radical and hydrated electron trapping rates and spin adduct yields. J. Phys. Chem. 1996;100:7511–7516. [Google Scholar]
- 24.Sueishi Y., Yoshioka C., Olea-Azar C., Reinke L.A., Kotake Y. Substituent effect on the rate of the hydroxyl and phenyl radical spin trapping with nitrones. Bull. Chem. Soc. Jpn. 2002;75:2043–2047. [Google Scholar]
- 25.Sridhar R., Beaumont P.C., Powers E.L.J. Fast kinetics of the reaction of hydroxyl radicals with nitrone spin traps. Radioanal. Nucl. Chem. 1986;101:227–237. [Google Scholar]
- 26.Cederbaum A.I., Dricker E., Rubin E., Cohen G. The effect of dimethylsulfoxide and other hydroxyl radical scavengers on the oxidation of ethanol by rat liver microsomes. Biochem. Biophys. Res. Commun. 1984;78:1254–1262. doi: 10.1016/0006-291x(77)91428-0. [DOI] [PubMed] [Google Scholar]
- 27.Anbar M., Neta P. A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms, and hydroxyl radicals with inorganic and organic compounds in aqueous solution. Int. J. Appl. Radiat. Isot. 1967;18:493–523. [Google Scholar]
- 28.Lin W.S., Armstrong D.A. Glutathione mediation of papain inactivation by hydrogen peroxide and hydroxyl radicals. Radiat. Res. 1977;69:434–441. [PubMed] [Google Scholar]
- 29.Goldstein S., Czapski G. Mannitol as an OH. scavenger in aqueous solutions and in biological systems. Int. J. Radiat. Biol. Relat. Biol. 1984;46:725–729. doi: 10.1080/09553008414551961. [DOI] [PubMed] [Google Scholar]
- 30.Rehman A., Whiteman M., Halliwell B. Scavenging hydroxyl radicals but not of peroxynitrite by inhibitors and substrates of nitric oxide synthases. Br. J. Pharmacol. 1997;122:1702–1706. doi: 10.1038/sj.bjp.0701556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 32.Franzini E., Sellak H., Hakim J., Pasquier C. Comparative sugar degradation by (OH)· produced by the iron-driven Fenton reaction and gamma radiolysis. Arch. Biochem. Biophys. 1994;309:261–265. doi: 10.1006/abbi.1994.1111. [DOI] [PubMed] [Google Scholar]
- 33.Amphlett C.B., Adams G.E., Michael B.D. Pulse radiolysis studies of deaerated aqueous salicylate solutions. Advances in Chemistry Series. 1968;81:231–250. [Google Scholar]
- 34.Naito O. Plasma hydroxyl radical scavenging activity in chronic renal failure patients. St. Marianna Med. J. 1995;23:519–530. [Google Scholar]