Abstract
The present study was conducted in order to determine whether oxidative stress during aging involves dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis in association with the emergence of cognitive deficits. When young rats were subjected to oxidative stress in the form of hyperoxia, thiobarbituric acid reactive substances, conjugated diene and lipid hydroperoxides increased markedly in the HPA axis. Vitamin E inhibited such increases in lipid peroxides in each organ. Levels of corticotrophin-releasing hormone in the hypothalamus and plasma levels of adrenocorticotrophic hormone and corticosterone were markedly elevated in young rats exposed to hyperoxia. However, young rats fed vitamin E-supplemented diets showed no abnormal hormone secretion, even after being subjected to hyperoxia. Furthermore, glucocorticosteroid receptors (GR) in pyramidal cells in the Cornus ammonis 1 region of the hippocampus in young rats were markedly decreased by oxidative stress. Similar phenomena were also observed in normal aged rats and young rats fed vitamin E-deficient diet kept in a normal atmosphere. Vitamin E supplementation prevented the decrease in GR in the hippocampus and the increase in corticosterone secretion caused by hyperoxia. These results suggest that oxidative stress induces oxidative damage in the hippocampus and the HPA axis during aging, resulting in a cognitive deficit in rats, and that negative-feedback inhibition on HPA activity was markedly dampened due to an increase in corticosterone levels caused by loss of GR.
Keywords: Oxidative stress, HPA activity, corticosteroid receptor, aging, vitamin E
Introduction
It is known that rats show increased plasma glucocorticoid and adrenocorticotrophic hormone (ACTH) with age under basal and post-stress conditions due to an increase in hypothalamic-pituitary-adrenal (HPA) activity [1–3]. The increase in HPA activity is thought to be associated with a decline in glucocorticoid negative-feedback inhibition over HPA activity [4]. This negative feedback is regulated by corticosteroid receptors in hippocampal pyramidal cells [5].
Aged rats show a loss of corticosteroid receptors in hippocampal cells in association with elevated corticosterone levels in the serum, resulting in the emergence of cognitive deficits [6]. In fact, exogenous treatment with corticosterone reduces HPA suppression in aged rats [7], and adrenalectomized animals show reduced neuron loss in the hippocampus and improved cognitive function [8]. Moreover, Issa et al. showed that HPA dysfunction in aged animals is selectively associated with spatial memory deficits and increased hippocampal neuron loss rather than emergence through the aging process [9]. However, the interrelationship between the emergence of cognitive deficits and the increased corticosterone caused by HPA dysfunction in aged rats remains unclear.
Chronic oxidative stress acting over long periods is thought to produce reactive oxygen species (ROS) in living tissues. The theory proposes that most of the changes with aging are caused by free radical reactions and the formation of lipid peroxides in tissues, thus leading to age-related damage and, eventually, to various aging processes and phenomena [10–11]. Our previous findings revealed that levels of thiobarbitric acid reactive substances (TBARS) and conjugated dienes are markedly increased by oxidative stress through aging in the hippocampus of rats, which modulates cognitive function, and that oxidative stress induces significant deficits in cognitive performance (learning ability and memory retention) accompanied by delayed-type apoptosis of pyramidal cells and accumulation of amyloid β-like substances in the Cornus ammonis 1 (CA1) region of the hippocampus. Moreover, these abnormalities were also observed in aged rats kept in a normal atmosphere [12–14].
Oxidative stress is known to be involved in several neuro-degenerative disorders characterized by progressive cognitive deficits, and to induce dysfunction of the HPA axis in Alzheimer’s disease, resulting in increased corticoid levels in serum [15]. Moreover, increased corticoid levels induce amyloid-β accumulation in a model mouse of Alzheimer’s disease [16]. It is therefore reasonable to infer that chronic oxidative stress for long periods during aging induce oxidative damage in the HPA axis, leading to an increase in serum corticoid due to the loss of corticoid receptors in pyramidal cells in the hippocampus, which modulates cognitive function. The resulting cognitive deficits arise due to toxicity from either abnormally high corticoid levels or oxidative stress.
The present study aimed to determine whether oxidative stress during aging involves dysfunction of the HPA axis in association with the emergence of cognitive deficits. Furthermore, we attempted to evaluate the preventive effects of vitamin E on these phenomena.
Materials and Methods
Animals
All animal experiments were performed with the approval of the Animal Protection and Ethics Committee of the Shibaura Institute of Technology. Young male Wistar rats (age, 3 months; Japan SLC Co., Hamamatsu, Japan), aged male Wistar rats (age, 25 months; obtained from Tokyo Metropolitan Institute of Gerontology) and rats fed vitamin E-supplemented diet (age, 3 months; fed 200 mg of R,R,R-α-tocopherol/kg.body weight/day for 9 weeks from the age of 4 weeks) were used in this study. To assess the effects of oxidative stress on lipid components in the HPA, each rat was subjected to hyperoxia as oxidative stress in a 100% oxygen chamber at room temperature for 48 h, as described previously [14]. Aged rats and rats fed vitamin E-deficient diets (age, 3 months; fed vitamin E-deficient diet for 9 weeks from 4 weeks of age; no tocopherols detected by HPLC; Funabashi Nojyo, Chiba, Japan) were kept in a normal atmosphere for 48 h.
Chemicals
Corticotrophin-releasing hormone (CRH) antibodies were obtained from Gunma University (Institute for Molecular and Cellular Regulation, Gunma, Japan). CRH was purchased from Peptide Institute Inc. (Osaka, Japan); Bovine serum albumin, Hematoxylin, Microperoxidase MP-11, 4-aminophtalhydradine were from Sigma-Aldrich Co. (St. Louis, MO). Elite avidin biotin-peroxidase complex (ABC) kit-PK6101 was purchased from Vector Laboratories Inc. (Burlingame, CA), and o-phenylene diamine-HCl, 3,3'-diamino-benzidinetetrahydro-chloride (DAB) was obtained from Wako Pure Chemical Industries (Osaka, Japan). ACTH (rat) EIA kits were purchased from Phoenix Pharmaceuticals Inc. (Burlingame, CA) and the rat corticosterone [125I] assay system was from Amersham (Buckinghamshire, UK). Finally, mouse glucocorticoid receptor (GR) antibodies were purchased from Affinity Bioreagents Inc. (Golden, CO).
Analyses of lipid peroxides in HPA
The brain was quickly removed and placed on ice, and the hippocampus, hypothalamus and pituitary were dissected. Each portion of the HPA was extracted with a mixture of chloroform/methanol (2:1, v/v). After evaporation of each extract, the residue was dissolved in 200 µl of methanol, and an 80 µl aliquot of the solution was mixed with a chemiluminescent solution (mixture of 0.18 mg isoluminol/mL and 1 mg microperoxidase/ml in 70% methanol, 100:1, v/v). Chemiluminescence of the solution was analyzed using a Luminescencer PSN apparatus (Atto Co., Tokyo, Japan) for LOOH analysis, as described previously [17]. TBARS levels were measured according to the method of Ohkawa et al. [18]. TBARS contents were expressed in terms of nmol in each sample. Conjugated dienes, formed by the peroxidation of unsaturated fatty acids, were analyzed as reported previously [19].
Measurement of HPA axis hormones
In this study, CRH was measured using the homogenate of the hypothalamus, and ACTH and corticosterone were analyzed using the serum. Taking into account circadian physiological rhythms, rats were sacrificed by decapitation at 10 am in each experiment. For analysis of CRH in the hypothalamus, tissue was homogenized in 10 mM Tris-HCl (pH 7.0) using an ultrasonic disruptor XL-2000 (Misonix Inc., New York, NY). This homogenate was centrifuged at 4°C for 15 min at 10,000 × g. Aliquots (50 µl) of supernatant were analyzed by standard ELISA using CRH antibody and biotinylated anti-rabbit horseradish peroxidase secondary antibody (VECTASTAIN, Vector Laboratories Inc., Burlingame, CA). Plasma ACTH was measured using an EIA kit (rat). The serum (50 µl) was placed on the micro plate coated by anti rabbit serum as the second antibody, followed by mixing with rabbit anti ACTH serum and biotinylated peptide in the kit. The mixture in the micro plate was incubated at room temperature for 2 h. After the well of the micro plate washed with an assay buffer in the kit, a streptavidin-horseradish peroxidase solution was added into the well, and incubated at room temperature for 1 h. After an addition of 2 N HCl to stop the reaction, ACTH levels were determined by an absorbance at 450 nm. Levels of corticosterone in the serum were analyzed by the radioimmunoassay method using the rat corticosterone [125I] assay system with a highly specific corticosterone antiserum. Intra- and inter-assay coefficients of variation were 7 and 10%, respectively. In order to displace corticosterone from corticosteroid-binding globulin, the serum was heated for 30 min at 60°C, followed by centrifuging for 10 min at 3000 rpm. The assay was performed in duplicate at room temperature, using rabbit anti-corticosterone serum as the first antibody and anti-rabbit serum coated on polymer particles as the second antibody.
Corticosteroid receptor (GR) analysis
Tissue preparation was carried out by immersion in Mizuhara’s solution (3% paraformaldehyde in 0.1% tannic acid, 2 mM CaCl2 and 1 mM MgCl2; pH 7.2–7.4) [20–21]. Microwave irradiation was used for the rapid penetration of fixative solution. Brains immersed in Mizuhara’s solution were exposed to microwaves in a water bath for 30 s at 25–30°C. After irradiation, samples were left to stand in fixative solution at room temperature overnight. Serial sections were frontally sliced at 50 µm using a microslicer (Vibratome, St. Louis, MO). Changes in GR in the hippocampal area were assessed microscopically by immunochemical staining using rat GR antibodies and Elite ABC kit-PK6101. A positive antigen-antibody reaction was visualized by incubating the slide in 250 µl of 0.3% 3,3'-diaminobenzidine-terahydrochloride (DAB) in 50 mM Tris-HCl (pH 7.6) containing 3% H2O2. Negative staining was performed by the same method, except that the GR antibody was not used. Quantitative analysis of GR was performed in order to evaluate the intensity of stained GR using an Imaging Analyzer LAS-3000 (Fuji Film Co. Tokyo, Japan).
Statistical
Results are presented as means ± SE. All data were assessed by ANOVA analysis and p values of less than 0.05 were considered to be significant.
Results
Changes in lipids in HPA axis caused by oxidative stress
As shown in Table 1, TBARS levels in the HPA in young rats subjected to hyperoxia were significantly higher than those in young control rats. Aged rats and vitamin E-deficient young rats kept under a normal atmosphere also showed increased TBARS levels in the HPA. In contrast, rats fed vitamin E-supplemented diet showed no significant increases in TBARS when compared with young control rats, even when subjected to hyperoxia. Although levels of conjugated dienes in the HPA tended to increase by hyperoxia, aging and vitamin E-deficiency, no significant differences were observed in the hypothalamus. Vitamin E did not decrease the levels in this organ. It was found that other regions showed significant increase in this denatured lipids, and that vitamin E inhibited such increases in conjugated dienes. In contrast, lipid peroxides (LOOH) in the hypothalamus were increased markedly in each rat group. In the pituitary and adrenal, vitamin E supplementation did not show the inhibition of the increases in LOOH caused by hyperoxia.
Table 1.
Effect of oxidative stress on the contents of TBARS, conjugated dienes, and LOOH in hypothalamus, pituitary, and adrenal.
| Parameter | Value for |
||||
|---|---|---|---|---|---|
| Control (Air) | Hyperoxia (100%O2) | Aged (25 month old) kept in air | VE-deficient kept in air | VE-supplement hyperoxia | |
| TBARS (nmol/mg protein) | |||||
| Hypothalamus | 1.0 ± 0.1 | 1.4 ± 0.1* | 1.5 ± 0.2* | 1.5 ± 0.1* | 1.1 ± 0.1# |
| Pituitary | 0.5 ± 0.1 | 0.7 ± 0.1* | 0.8 ± 0.2* | 1.1 ± 0.1** | 0.5 ± 0.1 |
| Adrenal | 1.0 ± 0.1 | 1.3 ± 0.1* | 1.6 ± 0.2* | 1.7 ± 0.1* | 0.9 ± 0.1# |
| Conjugate dienes (pmol/mg protein) | |||||
| Hypothalamus | 13.4 ± 1.7 | 14.8 ± 2.0 | 15.1 ± 2.3 | 15.8 ± 1.0 | 12.2 ± 0.8 |
| Pituitary | 4.8 ± 0.7 | 8.4 ± 1.6** | 9.2 ± 1.1** | 9.7 ± 2.0** | 6.0 ± 0.8# |
| Adrenal | 11.1 ± 1.6 | 16.3 ± 2.0** | 16.7 ± 2.2** | 19.2 ± 1.5* | 9.0 ± 1.3# |
| LOOH (pmol/mg protein) | |||||
| Hypothalamus | 41 ± 6 | 49 ± 8 | 55 ± 10* | 64 ± 4** | 45 ± 5 |
| Pituitary | 50 ± 3 | 62 ± 3* | 56 ± 6 | 87 ± 20* | 53 ± 8 |
| Adrenal | 51 ± 11 | 60 ± 17 | 59 ± 13 | 85 ± 27* | 46 ± 10 |
Values are mean + SE, n = 9, *p<0.05, **p<0.01 vs Control, #p<0.05 vs Hyperoxia, VE means vitamin E
Influence of oxidative stress and aging on hormone secretion in rat HPA axis
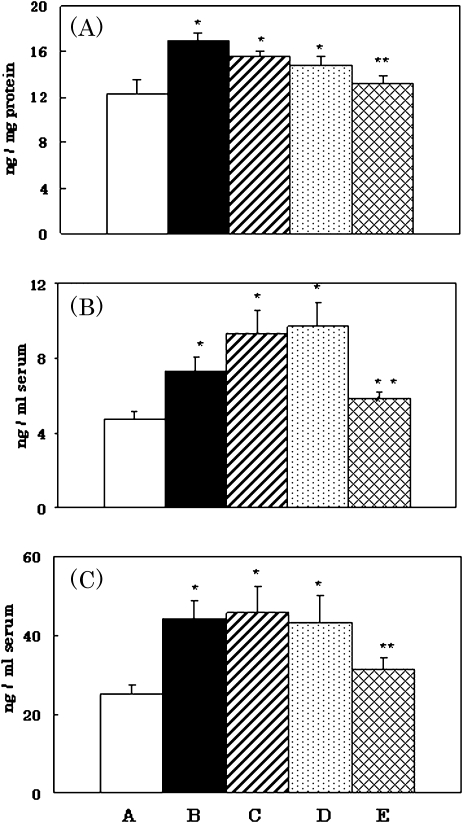
After young rats were subjected to hyperoxia for 48 h, levels of CRH in the hypothalamus increased significantly in comparison with basal levels in normal young rats. Aged rats and vitamin E-deficient young rats kept in a normal atmosphere showed similar increases in CRH secretion. In contrast, young rats fed vitamin E-supplemented diet showed no abnormal hormone secretion, even after exposure to hyperoxia (Fig. 1A).
Fig. 1.
Increases in secretion of CRH (A), ACTH (B) and corticosterone (C) caused by oxidative stress, aging and vitamin E-deficiency, and its prevention by vitamin E. A, Normal young control rats; B, young rats subjected to hyperoxia; C, aged rats kept in normal atmosphere; D, vitamin E-deficient young rats kept in normal atmosphere; and E, vitamin E-supplemented young rats subjected to hyperoxia. *p<0.01 vs normal young controls, **p<0.05 vs young rats subjected to hyperoxia, normal aged rats and vitamin E-deficient rats.
In addition to the abnormal CRH secretion in young rats subjected to oxidative stress, levels of ACTH and corticosterone in serum were markedly elevated. Similar increases in these hormones were observed in aged rats and vitamin E-deficient young rats kept in a normal atmosphere (Fig. 1B and C). Vitamin E treatment in young rats before oxidative stress also prevented increases in these hormones (data not shown). Rats fed vitamin E-supplemented diet showed marked inhibition of increases in these hormones, even after exposure to hyperoxia. The present results suggest that oxidative stress induces an increase in HPA activity, resulting in hypersecretion of CRH, ACTH and corticosterone, and that vitamin E prevents these abnormal hormone secretions.
Changes in GR caused by oxidative stress and aging
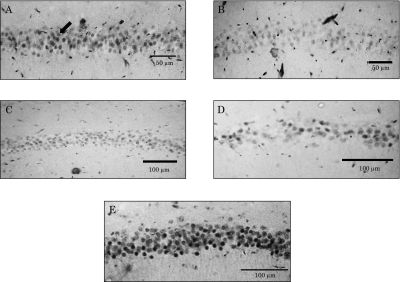
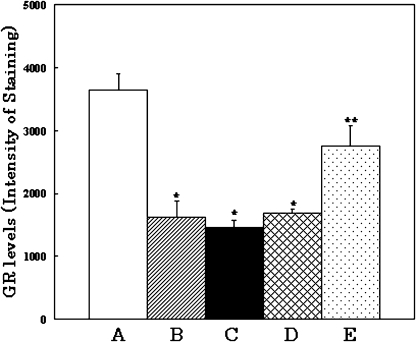
As shown in Figs. 2 and 3, when young rats are subjected to oxidative stress, GR density at the CA1 region of the hippocampus decreased markedly in comparison with that in normal young rats (Fig. 2A and B). Similarly, normal aged rats and vitamin E-deficient young rats kept in a normal atmosphere showed significant decreases in the number of GR (Fig. 2C and D).
Fig. 2.
Glucocorticoid Receptor (GR) loss in hippocampal CA1 region of rats caused by hyperoxia, aging, and vitamin E-deficiency. Samples A to E correspond to the same samples in Fig. 1. Arrow shows immunochemically stained GR in pyramidal cells.
Fig. 3.
GR density (per 0.1 mm2) in hippocampal pyramidal cell field determined by Image Analysis. Samples A to E correspond to the same samples in Fig. 1. *p<0.01 vs A, **p<0.05 vs B, C and D.
In contrast, there were no significant differences in GR density in the hippocampus of vitamin E-supplemented young rats, even after oxidative stress (Fig. 2E). DG density data were analyzed using an Imaging Analyzer for the CA1 region of the hippocampus (Fig. 3).
Discussion
Oxidative stress caused by the imbalance between ROS generation and detoxification by antioxidants in living tissues induces damage in many organs, resulting in physiological dysfunction. Since the nervous system was found to be susceptible to oxidative stress [22], accumulated evidence has suggested a role for oxidative stress in neurodegeneration through brain damage [23–24]. Oxidative stress in the rat brain during aging was manifested by not only increases in lipid peroxides and oxidized protein, but changes in antioxidant enzyme activities, such as superoxide dismutase, catalase and glutathione peroxidase [25]. Furthermore, our previous reports revealed that oxidative damage in the rat hippocampus through hyperoxia underlies delayed-type of pyramidal cell apoptosis in the CA1 region, thereby inducing cognitive deficits, and that the aged rats kept under a normal atmosphere showed similar abnormal phenomena [14]. Consequently, it is evident that neurodegeneration during aging is arisen from oxidative stress.
In order to confirm whether oxidative stress induces the oxidative damage in the HPA, the changes in lipids in the rat HPA caused by hyperoxia were analyzed in this study (Table 1). Levels of TBARS, conjugated dienes and LOOH were increased significantly in the HPA by hyperoxia, aging and vitamin E-deficiency. However, these changes depended on the organ. Especially, levels of conjugated dienes in the hypothalamus did not increase in all animals. Although it is impossible at the present day to explain this organ dependence, it may be presumed that since TBARS is formed from conjugated diene and LOOH, both precursors of TBARS were not accumulated in the hypothalamus because of decomposition of them in the hippocampus, leading to the formation of TBARS. Thus, the HPA in rats is damaged by oxidative stress during aging, resulting in an elevation of the HPA activity. Such an increase in the HPA activity may be arisen from the impairment of negative feedback process which regulates secretion of corticosterone. It has been reported that patients with Alzheimer’s disease are typically subjected to oxidative stress, and show dysfunctions in the HPA axis, resulting in increased corticoid levels in serum [15]. However, the reason of these phenomena has not been well-understood.
As corticosteroid receptors (GR) is known to be inside pyramidal cell, apoptosis caused by oxidative stress may lead to the loss of GR in the hippocampus observed in this study (Figs. 2 and 3). This notion is supported by the theory that decreased glucocorticoid negative feedback inhibition over HPA activity is thought to be associated with a loss of GR in the rat hippocampus [26–27]. It has been recognized that basal levels of HPA activity tend to increase with age in rats, leading to elevated plasma levels of ACTH and corticosterone [9]. However, the cause of this increase in HPA activity with age remains unclear. In this study, it is evident that an increase in the HPA function caused by oxidative stress during aging induces the hyper secretion of CRH, ACTH and corticosterone.
These findings obtained in this study suggest that oxidative stress during aging induces oxidative damage in the hippocampus, thus leading to a prominent GR decrease in the CA1 region and resulting in the hyper secretion of corticosteroid. It is also clear that vitamin E prevents these phenomena through its antioxidant properties.
In conclusion, we confirmed that oxidative stress during aging induces hypersecretion of corticosterone via increased HPA activity caused by hippocampal oxidative damage, and that vitamin E prevents these phenomena via its antioxidant properties. This notion is supported by the fact that patients with Alzheimer’s disease show high levels of corticosterone in plasma, suggesting that they are under oxidative stress [15].
Acknowledgment
This study has been supported, in part, by MEXT HAITEKU (2004), and Grant-in-Aid from Eisai Company Ltd. We would like to gratefully acknowledge Mr. Masao Machida and Miss Sayoko Ohkawara for their excellent technical assistant.
References
- 1.Sencar-Cupovic I., Milkovic S. The development of sex differences in adrenal morphology and responsiveness to stress of rats from birth to end of life. Mech. Age Dev. 1976;5:1–9. doi: 10.1016/0047-6374(76)90002-6. [DOI] [PubMed] [Google Scholar]
- 2.Tang F., Philips J.G. Some age-related changes in pituitary-adrenal function in the male laboratory. J. Gelontol. 1978;33:377–382. [PubMed] [Google Scholar]
- 3.Sapolsky R.M. Do glucocorticoid levels rise with age in the rat? Neurobiol. Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- 4.Sapolsky R.M., Krey L.C., McEwen B.S. The adrenocortical response in the aged rats: impairment of recovery from stress. J. Exp. Gerontol. 1986;18:55–63. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson L., Sapolsky R.M. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 6.Reul J.M., Tonnaer J.A., De Kloet E.R. Neurotropic ACTH analogue promotes plasticity of type I corticosteroid receptors in brain of senescent male rats. Neurobiol. Aging. 1988;9:253–260. doi: 10.1016/s0197-4580(88)80062-9. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky R.M., Krey L.C., McEwen B.S. The adrenocortical axis in the aged rat: impaired sensitivity to both fast and delayed feedback inhibition. Neurobiol. Aging. 1986;7:331–336. doi: 10.1016/0197-4580(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 8.Landfield P., Baskin R.K., Pitler T.A. Brain-aging correlates: retardation by hormonal-pharmacological treatment. Science. 1981;214:581–583. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- 9.Issa A.M., Rowe W., Gauthier S., Meaney M.J. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Free radical theory of aging: the free radical diseases. Age. 1984;7:111–131. [Google Scholar]
- 12.Fukui K., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Annal. NY Acad. Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukui K., Omoi N., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Annal. NY Acad. Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukui K., Takatsu H., Shinkai T., Suzuki T., Abe K., Urano S. Appearance of amyloid β-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J. Alzheimer’s Dis. 2005;8:299–309. doi: 10.3233/jad-2005-8309. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X., Smith M.A., Honda K., Aliev G., Moreira P.I., Nunomura A., Casadesus G., Harris P.L.R., Siedlak S.L., Perry G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green K.N., Billings L.M., Roozendaal B., McGaugh J.L., LaFerla F.M. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2006;30:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omoi N., Arai M., Saito M., Takatsu H., Shibata A., Fukuzawa K., Sato K., Abe K., Fukui K., Urano S. Influence of oxidative stress on fusion of presynaptic plasma membranes of the rats brain with phosphatydyl choline liposomes and protective effect of vitamin E. J. Nutr. Sci. Vitaminol. 2006;52:248–255. doi: 10.3177/jnsv.52.248. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 20.Mizuhira V., Hasegawa H., Notoya M. Microwave fixation and localization of calcium in synaptic vesicles. J. Neurosci. Methods. 1994;55:125–136. doi: 10.1016/0165-0270(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 21.Mizuhira V., Hasegawa H. Microwave fixation method for cytochemistry. For conventional electron microscopy, enzymeimmuno-cytochemistry, autoradiography elemental distribution studies and staining methods. Eur. J. Morphol. 1996;34:385–391. doi: 10.1076/ejom.34.5.385.13055. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B. Oxidants and the central nervous system: some fundamental questions. Acta Neurol. Scand. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield D.A., Lauderback C.M . Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential cause and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 24.Markesbery W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 25.Onodera K., Omoi N., Fukui K., Hayasaka T., Shinkai T., Suzuki S., Abe K., Urano S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Rad. Res. 2003;37:367–372. doi: 10.1080/1071576031000090019. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky R.M., Krey L.C., McEwen B.S. Corticosterone receptors decline in a site specific manner in the aged rat. Brain Res. 1983;289:235–240. doi: 10.1016/0006-8993(83)90024-0. [DOI] [PubMed] [Google Scholar]
- 27.Ritger H., Veldhuis H., De Kloet E.R. Spacial orientation and hippocampal corticosterone receptor systems of old rats: effects of ACTH4-9 analogue ORG2766. Brain Res. 1984;309:393–399. doi: 10.1016/0006-8993(84)90612-7. [DOI] [PubMed] [Google Scholar]