Abstract
Phosphatidylcholine (PC) and its hydrolysates are considered to stimulate intestinal lipid absorption, however, their exact effects on lipoproteins and apolipoprotein (apo) metabolism remain ambiguous. This study aimed to further differentiate the effects of them using fully differentiated enterocyte-like Caco-2 cells. Lipid micelles (oleic acid 0.6, cholesterol 0.05, monooleylglycerol 0.2, taurocholate 2 in mmol/l) with or without choline, PC, and lysoPC (0.2 mmol/l each) were applied apically to Caco-2 cells. 3H-oleic acid and 14C-cholesterol were added to the micelles when necessary. Secreted lipoproteins were analyzed by a HPLC method. LysoPC had the most potent promoting effect on lipid uptake, and lipoprotein and apolipoprotein B-48 secretion among the molecules tested. LysoPC doubled the output of cholesterol and triglyceride as the lipoprotein component, but PC did not. On the other hand, PC only increased the secretion of apoA-IV in the presence of lipid micelles. These findings confirm that the alteration of PC by PLA2 hydrolysis is intrinsically involved in the intestinal lipid absorption process and suggest that PC and its hydrolysis are coordinately associated with not only lipid absorption efficiency but also lipoprotein output and metabolism.
Keywords: apolipoprotein, Choline, lipid absorption, chylomicron, enterocyte
Introduction
Postprandial hyperlipidemia is prevalent during conditions of obesity and insulin-resistance, and has been associated with mediating the accelerated progression of cardiovascular diseases. Interest has been given to reduce or retard lipid absorption by medication and thus further understanding on this process will be of importance for such development. Diet and biliary phospholipids supply phosphatidylcholine (PC) to the intestinal lumen (1–2 g and 11–12 g per day, respectively) and the PC is known to play an important role in intestinal lipid transport [1, 2]. Studies in bile-diverted rats by Tso et al. and others have shown that the addition of PC to a dietary oil-based infusate increases triglyceride (TG) output into the lymph ducts [3]. However, little comparative data is available which PC itself, the hydrolyzed forms, or both facilitate intestinal lipid absorption [4, 5].
Once PC enters into the intestinal lumen, it is readily hydrolyzed to lysoPC by pancreatic phospholipase A2 (PLA2). Fat meal perfusion in humans showed that the concentration of lysoPC in the luminal content increases more than 2.5 mmol/l, while that of PC remained at approximately 0.5 mmol/l [6]. Such increase in the concentration of lysoPC in the lumen is more likely to play an important role in the process than PC, because lysoPC solubilizes lipids more effectively than PC and is well absorbed by the absorptive cells [7, 8]. Furthermore, lysoPC is also involved in the cholesterol absorption. PC inhibits cholesterol absorption [9], while lysoPC greatly stimulates its absorption in Caco-2 cells [10], indicating that the hydrolysis can regulate the intestinal cholesterol absorption.
Choline, a component of PC, has been reported to have a similar stimulatory effect to PC in the intestinal lipid trafficking [4, 11, 12]. Choline-deficient diets cause a decreased fat absorption and a choline supplement restores the decrease [11, 12]. Interestingly, intraperitoneal administration of choline also restores the high rate of fat absorption, indicating that increased emulsification with choline is not involved in the effect, thus suggesting another mechanism [11]. An explanation for the effect with choline is increased phospholipids turnover in the presence of choline [12]. Although evidence suggests that the effect of choline and PC differs from each other, the physiological mechanisms have not been fully studied in comparison with PC and lysoPC.
Differentiated Caco-2 cells on filter membranes have been widely used as a model to examine the intestinal lipid assimilation and transport process, because the cells produce triglyceride (TG)-rich lipoproteins and apolipoproteins (apos), such as B-100, B-48, A-I, and A-IV when lipids are supplied from the apical side (brush border) to the cells. With this in vitro intestinal absorptive cell model, researchers have examined the promoting ability of PC and lysoPC in the lipid absorption [9, 10, 13–16] and revealed effects similar to the observations in animals with a few exceptions. However, the influence of PC and lysoPC on the output of lipids as lipoproteins and apolipoprotein metabolism is unexamined in a comparative manner.
In the present study we attempted to further delineate the effect of choline, PC, and lysoPC on lipid absorption and lipoprotein secretion in Caco-2 cells using a tracer technique and measuring lipoproteins and apolipoproteins. Our results showed that choline, PC, and lysoPC have different effects on the process, suggesting that rapid increases in the concentrations of PC and lysoPC in the intestinal lumen at mealtimes differentially facilitate the dynamic functional changes in the enterocytes to absorb and process dietary lipids.
Materials and Methods
Reagents
Pluronic L81 (BASF Co., Washington, NJ), a hydrophobic surfactant that inhibits lipoprotein secretion, was a kind gift of Patrick Tso (Dept. of Pathology and Laboratory Medicine, University of Cincinnati, OH), 9, 10(n)-3H-oleic acid (specific activity 7.0 Ci/mmol) and [4-14C]-cholesterol (58.0 mCi/mmol) were purchased from GE Healthcare (Piscataway, NJ). Other reagents were obtained from Wako Pure Chemicals (Osaka, Japan) or Sigma-Aldrich (Tokyo, Japan).
Preparation of lipid micelles
Lipid micelles were prepared according to Chateau et al. [17]. In brief, oleic acid (6 µl), 2-monooleylglycerol, and cholesterol (2 µl, respectively) were mixed in a sterile glass tube and dried under a stream of nitrogen and stored at −80°C prior to use. Stock solution of LysoPC or PC was added to the mixture when necessary [2 µl (final concentration, 0.2 mmol/l), respectively]. The resulting dried lipids were dissolved in 83 µl of a sterile solution of 24 mmol/l taurocholate (Nacalai Tesque, Kyoto Japan) in serum-free medium (DMEM, 5 mmol/l glucose and GlutaMAX, Invitrogen, CA) and the tube was soaked in a ultrasound bath for 10 min at room temperature (UT-104, Sharp, Japan). Then one milliliter of serum-free medium was added to the lipid micelles, and the solution was soaked in ultrasound bath again briefly and then mixed well. The final lipid concentrations were oleic acid (0.6 mmol/l), cholesterol (0.05 mmol/l), 2-monooleylglycerol (0.2 mmol/l), and taurocholate (2 mmol/l), a composition that mimics post-digestive duodenal micelles [18]. Lipid micelles were used within 2 h after preparation.
Differentiation of Caco-2 cells on filter membranes
Differentiation of Caco-2 cells on filter membranes was performed according to Chateau et al. [17]. Briefly, Caco-2 cells (between passages 49–55) were plated at a density of 5 × 104 cells per cm2 membrane and grown under a humidified atmosphere containing 10% CO2, at 37°C in Dulbecco’s modified essential medium containing 25 mmol/l glucose and GlutaMAXTM (Product No. 10564-011, Invitrogen) and 20% heat-inactivated fetal calf serum. Cells were grown to confluence for a week and then cultured in asymmetric conditions, with medium containing 5 mmol/l glucose and GlutaMAXTM in the upper compartment and the medium containing 20% FCS in the lower compartment up to the indicated periods. Penicillin/streptomycin (100 IU/ml and 100 µg/ml, respectively) and 1% nonessential amino acids were added to all the media.
Triglyceride-rich lipoprotein secretion by Caco-2 cells
Freshly prepared lipid micelles (1.5 ml) were added to the upper compartment for 24 h. For the tracer experiments, 3H-oleic acid and 14C cholesterol were added to a glass tube to have radioactivities of 2 µCi/ml and 1 µCi/ml, respectively, and dried as well as described above. After incubation for 24 h with the tracer-containing lipid micelles, media were harvested, the cell layers were briefly rinsed twice with ice-cold phosphate-buffered saline (PBS), and the back side of the filters was gently adsorbed onto filter paper to eliminate any basolateral medium. The cell layers were then scraped into 0.5 ml of lysis buffer (1% Triton X-100, 5 mmol/l EDTA in PBS) supplemented with 2% protease inhibitor cocktail, disrupted with a 23G needle and syringe, and immediately frozen at −80° C until analyzed. Cytotoxic effects were assessed by measuring lactate dehydrogenase activity in the apical and basolateral media.
TG-rich lipoproteins secreted into basolateral media were separated from free tracers with a PD-10 desalting column (GE healthcare) with PBS containing 1 mmol/l ETDA and 0.05% NaN3 as eluent. PD-10 is a Sephadex-G25 based desalting column that separates molecules with Mr >5,000 from those with Mr <1,000. Thus, tracers incorporated into lipoproteins appear in the passing-through fractions. Aliquots (50 µl) were suspended in 10 ml of liquid scintillator (Ultima Gold, PerkinElmer Life and Analytical Sciences, Inc., Wellesley, MA), and the radioactivity in each vial was measured with a scintillation counter. The distribution of radioactivity (%) was calculated by comparing to the total counts, that is, the sum of whole counts in the media and cell lysates.
Apolipoprotein B-100, B-48, and A-I assays
ApoB-100 and apoA-I concentrations in the supernatants were determined by an in-house enzyme-linked immunosorbent assay as described previously [19]. In this study we used a LDL fraction obtained from normolipidemic human plasma by ultracentrifugation as assay standard. The protein concentration of the LDL fraction was assayed for protein assay (Bio-Rad) using bovine serum albumin as standard. The purity of the LDL fraction was confirmed by SDS-PAGE with 6% gel. The concentration of apoB-48 was determined with an apoB-48 CLEIA Fujirebio kit and the LUMIPULSE system (Fujirebio, Tokyo, Japan) [20].
Lipoprotein analysis by HPLC
Lipoproteins secreted into the basolateral medium were analyzed by an HPLC column and the following enzymatic detection for TG and cholesterol [21] (Liposearch system, Skylight Biotech., Japan).
Real-time RT-PCR
Total RNA was isolated from the cells with ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer’s instruction. The samples were finally treated with RNase-free DNase I (Invitrogen) for 30 min at 37°C. First-strand cDNA synthesis was performed with a superscriptII reverse transcription kit (Qiagen) after denaturation of total RNA (10 µg, 15 min, 65°C). Reactions were performed in a total volume of 25 µl, containing 2.5 µl of cDNA solution, 12.5 µl of qPCR MasterMix (Applied Biosystems), and 1.25 µl of the TaqMan probe. Reactions were run on an ABI PRISM 7900 Sequence Detector (Applied Biosystems). The cycle threshold (Ct), corresponding to the number of cycles after which the target-DNA concentration increase becomes exponential, was monitored. Results were analyzed using the SDS 2.1 Software (Applied Biosystems). All reactions were done in duplicate.
Western blotting
Lysates of Caco-2 cells were separated on 10% polyacrylamide gel and transferred to PVDF membranes (Amersham Biosciences). Proteins in culture supernatants were precipitated with 20% trichloroacetic acid and then applied for SDS-PAGE. Membranes were blocked with Blocking One (Nacalai). Anti-apoA-IV antibody (1:200, Santa Cruz Biotechnology Inc., CA) and anti-goat-HRP (1:1000, The Binding Site) were successively applied in the same buffer and labeling was visualized by chemiluminescence (ECLTM, Western Blotting Detection Reagents; BD Biosciences) with a high-performance chemiluminescence film (HyperfilmTM ECLTM, BD Biosciences). Visualized proteins were scanned, and each density of the bands was translated into arbitrary unit with Image J (version 1.31; http://rsb.info.nih.gov/ij/).
Statistical analysis
Data are shown as means ± SEM. Each assay was performed in triplicate unless indicated otherwise. For parametric data, means were compared with the Student’s t test. For nonparametric data, the Mann-Whitney U test was used. The statistical analyses were performed with Statview version 5.0 for Win software (SAS Institute).
Results
Lipid absorption and triglyceride-rich lipoprotein secretion
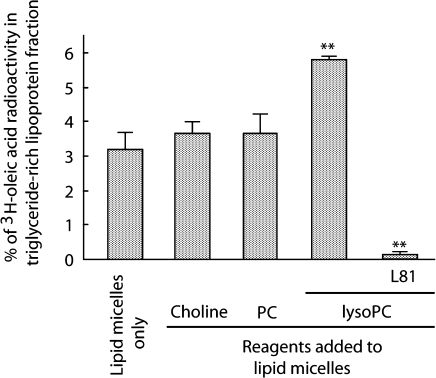
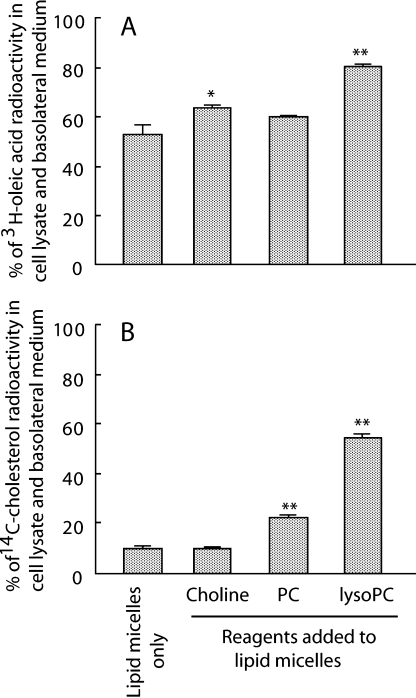
Tracer experiments with radiolabeled oleic acid showed that the addition of lysoPC approximately doubled TG-rich lipoprotein secretion by Caco-2 cells, whereas PC and choline had only a slight and insignificant increase (approximately 15% increases by each) (Fig. 1). We also estimated the effects of the same three reagents on lipid absorption by calculating the percentage of tracer count in the cell lysate and basolateral medium. Choline and lysoPC increased oleic acid absorption by 11% and 27%, respectively (Fig. 2A), while PC and lysoPC increased cholesterol absorption two- and five-fold, respectively (Fig. 2B). LysoPC had the greatest effect in all the above three evaluations. There was little radioactivity for 14C-cholesterol in the basolateral media, probably because the cells preferentially do not utilize exogenous cholesterol, but a cellular source for the lipoprotein assembly. Lactate dehydrogenase release into the medium after adding lipid micelles and test reagents was less than 1%, indicating no apparent cytotoxicity (data not shown), consistent with an earlier report [17].
Fig. 1.
TG-rich lipoprotein secretion by differentiated Caco-2 cell monolayers. Lipid micelles containing radiolabeled tracer of oleic acid and cholesterol were added to Caco-2 cells that had been cultured for 3 weeks on a filter membrane under asymmetric conditions. Oleic acid output as lipoprotein into the basolateral medium was estimated as % of total radioactivity in the passing-through fraction of PD-10 desalting column. Pluronic L81 (L81, 10 µg/ml) was added to lipid micelles as a known inhibitor of chylomicron secretion. ** p<0.01 versus lipid micelles alone. Data are shown as means and SEM of triplicate assays. The concentrations of reagents added to lipid micelles were 0.2 mmol/l, respectively.
Fig. 2.
Oleic acid and cholesterol absorption by Caco-2 cell monolayers. 3H-oleic acid and 14C-cholesterol were added to lipid micelles and incubated for 24 h. Absorbed tracers were calculated as described in the “Materials and Methods” section. A and B, % of absorbed 3H-oleic acid and 14C-cholesterol, respectively. * p<0.05 and ** p<0.01 vs lipid micelles alone. Data are shown as means and SEM of triplicate assays. The concentrations of reagents added to lipid micelles were 0.2 mmol/l, respectively.
Apolipoprotein secretion
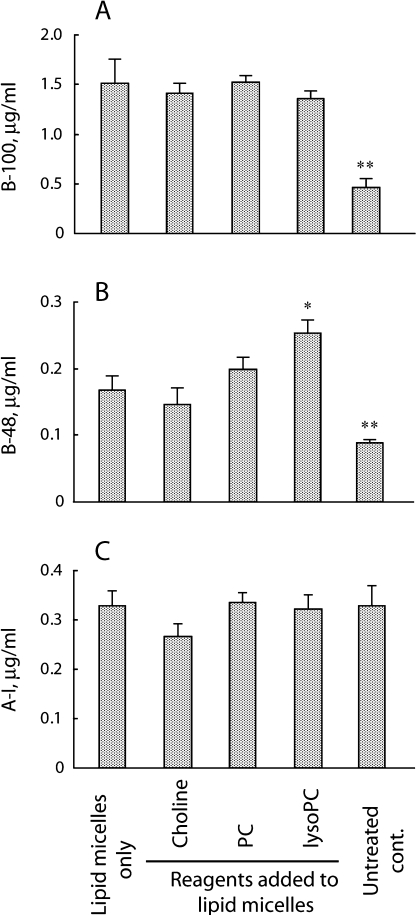
The addition of lipid micelles into the apical medium increased apoB-100 secretion threefold. The secretion level did not differ significantly when choline, PC, or lysoPC was added (Fig. 3A). The addition of lipid micelles also increased apoB-48 secretion twofold, and lysoPC further promoted apoB-48 secretion (1.5-fold versus lipid micelles alone, p = 0.04; Fig. 3B). The addition of PC and lysoPC reversed the suppression fully and partially, respectively. No significant change in apoA-I secretion was observed by adding micelles with or without the test reagents (Fig. 3C).
Fig. 3.
Apolipoprotein secretion into basolateral medium by Caco-2 cell monolayers. After 24 h incubation in the presence or absence of lipid micelles, the basolateral media were assayed for apoB-100 (A), apoB-48 (B), and apoA-I (C). * p<0.05 and ** p<0.01 compared to the data with lipid micelles alone. Data are shown as means and SEM of triplicate assays. The concentrations of reagents added to lipid micelles were 0.2 mmol/l, respectively.
Lysophosphatidylcholine alters secretion and lipid composition of lipoprotein
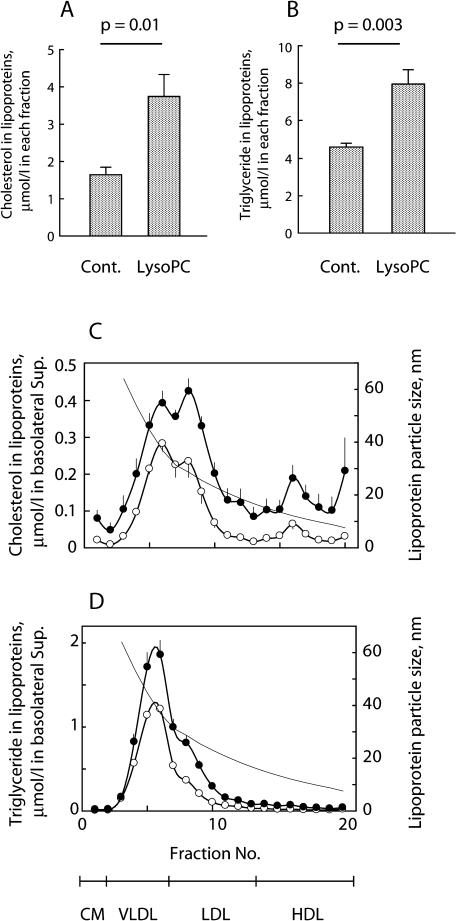
LysoPC was shown to facilitate the apical lipid transport the most. Analysis of the lipoproteins secreted with or without lysoPC with an HPLC method showed that the presence of lysoPC approximately doubled the total TG and cholesterol secretion as lipoprotein components (Fig. 4A and 4B, respectively). The cholesterol content of the lipoproteins was greater in every size of the secreted lipoproteins (Fig. 4C). Moreover, lipoproteins observed in the fractions from 6 to 9 seemed to increase by adding lysoPC (Fig. 4C and D), but since the pattern of the two chromatographs was similar, the presence of lysoPC did not appear to alter the particle sizes of lipoproteins secreted by Caco-2 cells.
Fig. 4.
HPLC analysis of secreted lipoproteins. Lipid micelles were added to Caco-2 cell cultures (n = 5 of each group) with (LysoPC) or without (Cont.) lysoPC (final concentration; 0.2 mmol/l). The lipoproteins secreted were concentrated 10-fold and applied for the HPLC. The total cholesterol and TG concentrations in the lipoproteins are shown in (A) and (B), respectively. The eluate from the column was fractionated into 20 tubes and assayed for cholesterol and TG as shown in C and D. The lines show lipoprotein particle size. Lipoprotein fractionation at the bottom indicates representative lipoprotein subclasses in the particle sizes. Plots and bars show means and SEM, respectively. Open and closed circles, in the absence and presence of lysoPC, respectively. The concentrations of reagents added to lipid micelles were 0.2 mmol/l, respectively.
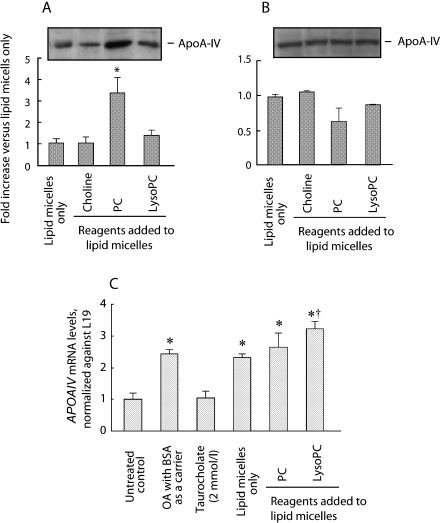
Phosphatidylcholine and taurocholate increase apo-AIV secretion
Apolipoprotein A-IV is now considered to play an important role in the chylomicron assembly and the control of satiety. ApoA-IV production and secretion into the circulation was shown to depend on the fatty acid supply into the lumen. Thus, we examined that the effect of PC and its hydrolyzates on the basolateral secretion of apoA-IV and found that apoA-IV secretion was promoted in the presence of PC, increasing more than threefold compared to those of lipid micelles alone (Fig. 5A). On the other hand, lysoPC showed insignificant difference compared with the non-additive control. There were no significant differences in the cellular apoA-IV levels (Fig. 5B) among the groups tested. Real-Time RT-PCR analysis showed that oleic acid increased apoA-IV mRNA in all the conditions that oleic acid was added (Fig. 5C). The mRNA level in the presence of lysoPC was slightly greater than that of lipid micelles only.
Fig. 5.
Western blotting analysis of basolaterally secreted (A) and cellular (B) apolipoprotein A-IV. * p<0.01 compared to the data from lipid micelles only. Western blot data show representatives of n = 3 experiments. (C) Changes in APOAIV mRNA levels after adding lipid micelles. Messenger RNA levels at 24 h after the cells were treated were determined by RT-PCR with real-time detection as mitochondrial ribosomal protein L19 as a housekeeping gene 24 h after the lipids were added. * p<0.01 vs control. † p<0.05 vs lipid micelles only. Data are shown as means and SEM of triplicate assays. The concentrations of reagents added to lipid micelles were 0.2 mmol/l, respectively.
Discussion
The results of the present study showed that lysophosphatidylcholine (lysoPC) was the most effective in increasing the absorption of oleic acid, cholesterol, the secretions of TG-rich lipoprotein and apoB-48, when lipid micelles were applied simultaneously. LysoPC doubled the output of both cholesterol and triglyceride as lipoprotein components, respectively, while PC increased apoA-IV secretion.
A series of evidence showed that the addition of PC to lipid infusates increases the phospholipid turnover by the enterocytes and TG output into the lymph. Together with our present findings, and the fact that PC is rapidly hydrolyzed by PLA2 in pancreatic juice and in the intestinal absorptive cells [6], PC added to infusates elicits an increased rate of fat absorption after being hydrolyzed to lysoPC. In fact, inhibition of PLA2 attenuates fatty acid absorption in rats [22]. Thus, biliary PC and pancreatic PLA2 are crucial for supplying lysoPC to the intestinal lumen. What was responsible for the considerable difference in the lipid transport efficiency between lysoPC and PC. As the lipids added apically were well solubilized with taurocholate, the efficient lipid emulsification by lysoPC is unlikely. LysoPC is rapidly absorbed by the enterocytes (approximately 70% for 6 h by Caco-2 cell monolayer; [9]), PC is poorly absorbed (less than 2%) unless hydrolyzed [9, 15, 23]. The fact that absorbed fat droplets are surrounded by membranes during their passage through the apical cytoplasm suggests that an extensive membrane system is needed to process the large amount of fat quickly entering the cell [24]. Since lysoPC is incorporated well into lymph phospholipids [7, 8], apically supplied lysoPC seems to provide the sufficient surface coat of lipoproteins, which assists the delivery of dietary lipids by chylomicrons.
The addition of lysoPC doubled or more the cholesterol and TG contents in the lipoproteins secreted. The increase in cholesterol content was independent of the particle size of lipoproteins, suggesting a possibility of the activation of acyl-CoA:cholesterol acyltransferase by accelerated cholesterol uptake by 5-fold in the presence of lysoPC as previously reported [10, 25] (Fig. 1C). Indeed, the absorbed cholesterol is rapidly esterified in Caco-2 cells [9]. PC reduced cholesterol absorption in humans and rats [26, 27]. In the present study, the addition of PC into the apical medium did not reduce the cholesterol absorption, but promoted slightly. Hamada et al. [28] speculated that lysoPC reduced the affinity of cholesterol to the bile salt micelles, promoting the release of cholesterol from them to the cells. Taken together, those data can be explained with the possibility that PC counteracts the effect of lysoPC in the cholesterol absorption process.
Chylomicrons are the physiological carriers of absorbed dietary lipids and apoB-48 plays a crucial role in their assimilation and rapid transport as an exclusive protein of the chylomicron particles [29]. Western blotting method has been utilized to semi-quantify for the basolateral apoB48/B100 secretions [17]. We differentially determined total apoB-100 and apoB-48 concentrations by highly sensitive and specific immunoassays, which have advantages in assay simplicity and reproducibility. LysoPC augmented both TG-rich lipoprotein and apoB-48 secretion. In hepatocytes, lysoPC was also shown to inhibit the degradation of apoB and thus increase the secretion [30]. Furthermore, the secretion of apoB-48 depends on the presence of lipids in the apical side, but does not depend on the surplus intracellular lipids [17]. In the view of the fact that more than 50% of the fatty acid was absorbed and only less than 15% of the apical fatty acid was transported to the basolateral medium in the present study, the fatty acid supply itself does not appear to be a limiting factor for apoB-48-containing TG-rich lipoprotein secretion as suggested [17]. Thus, the increased absorption of oleic acid is not completely attributable to the increased apoB-48 and lipoprotein secretion. We speculate that the supply of lipoprotein surface coat by the absorbed lysoPC is one of the major stimulating factors.
ApoA-IV, a component of chylomicrons, is known to facilitate fat absorption and chylomicron secretion, probably plays an important role in the chylomicron assembly [31]. Moreover, it is a satiety mediator. Increased lipid absorption and TG-rich lipoprotein secretion in the presence of lysoPC was thought to stimulate apoA-IV secretion, but there was no significant change in the secretion of apoA-IV when lysoPC was added. On the other hand, the addition of PC increased the secretion of apoA-IV more than threefold, while the expression level of apoA-IV was dependent on the fatty acid supply as reported [32, 33]. Field et al. [15] showed that taurocholate decreased basolateral secretion of apoB by promoting the intracellular degradation of apoB molecule. This degradation is rescued by adding PC. The less association of PC to Caco-2 cells [15] indicates that PC neither supply fatty acid nor lysoPC in Caco-2 assay system, suggesting a possible regulatory mechanism by PC on lipoprotein output or degradation system.
Choline slightly increased fatty acid absorption, but there was no apparent increase in the lipoprotein output in contrast to the earlier reports in rats. This difference is probably because the investigators examined choline-deficient conditions in rats, whereas the medium we used contains methionine, a precursor of choline; thus, the supply of choline to the cells affected marginally. Other possibilities are that Caco-2 cells may not have proper metabolic activity for phospholipid turnover by the use of choline, or the lack of monoglyceride pathway in Caco-2 cells may reduce the supply of diglyceride to synthesize PC.
In conclusion, the results of the present study confirmed the importance of lysoPC in the lipid absorption process and showed that lysoPC facilitates the efficient intestinal lipid transport by accelerating lipid absorption, lipoprotein assembly, and lipid clearance. On the other hand, PC did not increase lipoprotein output, but increased apoA-IV secretion, suggesting a role of PC in satiety signaling. PC and its hydrolysis appear to be coordinately associated with not only lipid absorption efficiency but also lipoprotein output and metabolism.
Acknowledgments
The authors especially thank Dr. Sylvie Demignot (INSERM, Paris, France) for providing the Caco-2 cells and instruction in regard to the cell culture and lipoprotein secretion experiments and Yuka Nakano for her excellent technical and secretarial assistance for this study.
Abbreviations
- Apo
apolipoprotein
- OA
oleic acid
- PC
phosphatidylcholine
- PLA2
phospholipase A2
- TC
taurocholate
- TG
triglyceride
References
- 1.Tso P., Kendrick H., Balint J.A., Simmonds W.J. Role of biliary phosphatidylcholine in the absorption and transport of dietary triolein in the rat. Gastroenterology. 1981;80:60–65. [PubMed] [Google Scholar]
- 2.O’Doherty P.J., Kakis G., Kuksis A. Role of luminal lecithin in intestinal fat absorption. Lipids. 1973;8:249–255. doi: 10.1007/BF02531899. [DOI] [PubMed] [Google Scholar]
- 3.Voshol P.J., Minich D.M., Havinga R., Elferink R.P., Verkade H.J., Groen A.K., Kuipers F. Postprandial chylomicron formation and fat absorption in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology. 2000;118:173–182. doi: 10.1016/s0016-5085(00)70426-4. [DOI] [PubMed] [Google Scholar]
- 4.Tidwell H.C. Effect of choline, methionine and ethionine on fat absorption. J. Nutr. 1956;58:569–578. doi: 10.1093/jn/58.4.569. [DOI] [PubMed] [Google Scholar]
- 5.Ward J., Haslam R., Schiff L. Effect of choline deficiency and hepatic cirrhosis on absorption of fat in the rat. Proc. Soc. Exp. Biol. Med. 1954;85:401–404. doi: 10.3181/00379727-85-20895. [DOI] [PubMed] [Google Scholar]
- 6.Persson E.M., Nilsson R.G., Hansson G.I., Lofgren L.J., Liback F., Knutson L., Abrahamsson B., Lennernas H. A clinical single-pass perfusion investigation of the dynamic in vivo secretory response to a dietary meal in human proximal small intestine. Pharm. Res. 2006;23:742–751. doi: 10.1007/s11095-006-9607-z. [DOI] [PubMed] [Google Scholar]
- 7.Tso P., Lam J., Simmonds W.J. The importance of the lysophosphatidylcholine and choline moiety of bile phosphatidylcholine in lymphatic transport of fat. Biochim Biophys. Acta. 1978;528:364–372. doi: 10.1016/0005-2760(78)90025-5. [DOI] [PubMed] [Google Scholar]
- 8.Mansbach C.M. 2nd, Arnold A., Cox M.A. Factors influencing triacylglycerol delivery into mesenteric lymph. Am. J. Physiol. 1985;249:G642–G648. doi: 10.1152/ajpgi.1985.249.5.G642. [DOI] [PubMed] [Google Scholar]
- 9.Homan R., Hamelehle K.L. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J. Lipid Res. 1998;39:1197–1209. [PubMed] [Google Scholar]
- 10.Mackay K., Starr J.R., Lawn R.M., Ellsworth J.L. Phosphatidylcholine hydrolysis is required for pancreatic cholesterol esterase- and phospholipase A2-facilitated cholesterol uptake into intestinal Caco-2 cells. J. Biol. Chem. 1997;272:13380–13389. doi: 10.1074/jbc.272.20.13380. [DOI] [PubMed] [Google Scholar]
- 11.Tidwell H.C. Mechanism of fat absorption as evidenced by chylomicrographic studies. J. Biol. Chem. 1950;182:405–414. [Google Scholar]
- 12.Artom C., Cornatzer W.E. Action of choline and fat on the formation of phospholipides in the intestine. J. Biol. Chem. 1946;165:393–394. [PubMed] [Google Scholar]
- 13.Field F.J., Mathur S.N. Intestinal lipoprotein synthesis and secretion. Prog. Lipid Res. 1995;34:185–198. doi: 10.1016/0163-7827(95)00005-k. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M.M., Kedees M.H., Singh K., Athar H., Jamali N.Z. Signposts in the assembly of chylomicrons. Front. Biosci. 2001;6:D320–D331. doi: 10.2741/hussain. [DOI] [PubMed] [Google Scholar]
- 15.Field F.J., Born E., Chen H., Murthy S., Mathur S.N. Regulation of apolipoprotein B secretion by biliary lipids in CaCo-2 cells. J. Lipid Res. 1994;35:749–762. [PubMed] [Google Scholar]
- 16.Mathur S.N., Born E., Murthy S., Field F.J. Phosphatidylcholine increases the secretion of triacylglycerol-rich lipoproteins by CaCo-2 cells. Biochem. J. 1996;314:569–575. doi: 10.1042/bj3140569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chateau D., Pauquai T., Delers F., Rousset M., Chambaz J., Demignot S. Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J. Cell Physiol. 2005;202:767–776. doi: 10.1002/jcp.20173. [DOI] [PubMed] [Google Scholar]
- 18.Hernell O., Staggers J.E., Carey M.C. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041–2056. doi: 10.1021/bi00460a012. [DOI] [PubMed] [Google Scholar]
- 19.Nakano T., Seo M., Komoda T., Kitazato K.T., Uno M., Hamaoki M., Nagata A. Immunoreactive circulating oxidized HDL concentrations do not increase in patients undergoing carotid endarterectomy: a comparative study for oxidized HDL and oxidized LDL concentrations in plasma. Clin. Chim. Acta. 2007;381:179–181. doi: 10.1016/j.cca.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Uchida Y., Kurano Y., Ito S. Establishment of monoclonal antibody against human Apo B-48 and measurement of Apo B-48 in serum by ELISA method. J. Clin. Lab. Anal. 1998;12:289–292. doi: 10.1002/(SICI)1098-2825(1998)12:5<289::AID-JCLA7>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usui S., Hara Y., Hosaki S., Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid. Res. 2002;43:805–814. [PubMed] [Google Scholar]
- 22.Wang S., Noh S.K., Koo S.I. Green tea catechins inhibit pancreatic phospholipase A2 and intestinal absorption of lipids in ovariectomized rats. J. Nutr. Biochem. 2006;17:492–498. doi: 10.1016/j.jnutbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers J.B., O’Brien R.J., Balint J.A. The absorption and subsequent utilization of lecithin by the rat jejunum. Am. J. Dig. Dis. 1975;20:208–211. doi: 10.1007/BF01070723. [DOI] [PubMed] [Google Scholar]
- 24.Ladman A.J., Padykula H.A., Strauss E.W. A morphological study of fat transport in the normal human jejunum. Am. J. Anat. 1963;112:389–419. doi: 10.1002/aja.1001120307. [DOI] [PubMed] [Google Scholar]
- 25.Field F.J., Albright E., Mathur S.N. Regulation of cholesterol esterification by micellar cholesterol in CaCo-2 cells. J. Lipid Res. 1987;28:1057–1066. [PubMed] [Google Scholar]
- 26.O’Mullane J.E., Hawthrone J.N. A comparison of hte effects of feeding linoleic acid-rich lecithin or corn oil on cholesterol absroption and metabolism in the rats. Atherosclerosis. 1982;45:81–90. doi: 10.1016/0021-9150(82)90173-3. [DOI] [PubMed] [Google Scholar]
- 27.Greten H., Raetzer H., Stiehl A., Schettler G. The effect of polyunsaturated phosphatidylcholine on plasma lipids and fecal sterol excertion. Atherosclerosis. 1980;36:81–88. doi: 10.1016/0021-9150(80)90201-4. [DOI] [PubMed] [Google Scholar]
- 28.Hamada T., Ikeda I., Takashima K., Kobayashi M., Kodama Y., Inoue T., Matsuoka R., Imaizumi K. Hydrolysis of micellar phosphatidylcholine accelerates cholesterol absorption in rats and Caco-2 cells. Biosci. Biotechnol. Biochem. 2005;69:1726–1732. doi: 10.1271/bbb.69.1726. [DOI] [PubMed] [Google Scholar]
- 29.Xie Y., Nassir F., Luo J., Buhman K., Davidson N.O. Intestinal lipoprotein assembly in apobec-1-/- mice reveals subtle alterations in triglyceride secretion coupled with a shift to larger lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G735–G746. doi: 10.1152/ajpgi.00202.2003. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Z., Luchoomun J., Bakillah A., Hussain M.M. Lysophosphatidylcholine increases apolipoprotein B secretion by enhancing lipid synthesis and decreasing its intracellular degradation in HepG2 cells. Biochim. Biophys. Acta. 1998;1391:13–24. doi: 10.1016/s0005-2760(97)00200-2. [DOI] [PubMed] [Google Scholar]
- 31.Black D.D., Rohwer-Nutter P.L., Davidson N.O. Intestinal apolipoprotein A-IV gene expression in the piglet. J. Lipid Res. 1990;31:497–505. [PubMed] [Google Scholar]
- 32.Kalogeris T.J., Fukagawa K., Tso P. Synthesis and lymphatic transport of intestinal apolipoprotein A-IV in response to graded doses of triglyceride. J. Lipid Res. 1994;35:1141–1151. [PubMed] [Google Scholar]
- 33.Stan S., Delvin E.E., Seidman E., Rouleau T., Steinmetz A., Bendayan M., Yotov W., Levy E. Modulation of apo A-IV transcript levels and synthesis by n-3, n-6, and n-9 fatty acids in CACO-2 cells. J. Cell Biochem. 1999;75:73–81. [PubMed] [Google Scholar]