Abstract
The present study was undertaken to evaluate the effects of lansoprazole (LPZ) on lipopolysaccharide (LPS)-stimulated toll-like receptor 4 (TLR4) signal transduction systems using the 293hTLR4/MD2-CD14 cells. The cells were incubated and then divided into the following groups: (a) untreated group, (b) non-LPZ treated (1h) group, (c) LPZ-treated (1h) plus non LPS-stimulated (1h) group, (d) LPZ-treated (1h) plus non LPS-stimulated (6h) group, (e) LPZ-treated (1h) plus LPS-stimulated (1h) group, (f) LPZ-treated (1h) plus LPS-stimulated (6h) group, (g) non LPZ-treated (1h) plus LPS-stimulated (1h) group and (h) non LPZ-treated (1h) plus LPS-stimulated (6h) group. Samples from each group were subjected to western blotting for analysis of IkB phosphorylation, intranuclear transfer of NF-kB, phosphorylation of MAP kinase (MAPK), intranuclear transfer of interferon regulatory factor 5 (IRF5), and expression of suppressor of cytokine signaling-1 (SOCS1). In the LPZ-treated groups, neither phosphorylation of MAPK nor intranuclear transfer of IRF5 was suppressed under stimulation with LPS, and enhanced intranuclear transfer of NF-kB and increased expression of SOCS1 were noted by comparison with the group treated with LPS alone. These results suggest that LPZ stimulates the expression of SOCS1 and regulates protein phosphorylation through its activity on TLR4 signal transduction under LPS stimulation.
Keywords: TLR4, lipopolysaccharide, lansoprazole, SOCS1, 293hTLR4/MD2-CD14 cell
Introduction
Toll-like receptors (TLRs) have been identified as a receptor of the innate immunity system which recognizes exogenous microorganisms [1, 2]. Furthermore, analysis of gene-knockout mice demonstrated that TLRs regulates not only innate immunity, but also acquired immunity [3, 4]. It has also been reported that excessive reactions of TLRs are involved in various diseases [5, 6]. Toll-like receptor (TLR) 4 is a receptor capable of recognizing lipopolysaccharide (LPS). TLR4 signal transduction system is regulated the phosphorylation of IkB and NF-kB transfer into the nucleus, the phosphorylation of MAP kinase, interferon regulatory factor (IRF5) transfer into the nucleus. IRF5 transfer into the nucleus is concerned with the production of cytokines and regulates innate or acquired immunity no less than NF-kB [7–9]. Suppressor of cytokine signaling-1 (SOCS1) has been identified as a negative feed back gene involved in control of excessive LPS stimulation [10–13]. SOCS1 regulates the excess production of cytokines under stimulation with LPS. Proton pump inhibitor (PPI), which suppresses acid output, has been reported to additionally possess anti-inflammatory and immune actions [14, 15]. The present study was undertaken to evaluate the effects of PPI on TLR4 signal transduction (protein phosphorylation) in the 293hTLR4/MD2-CD14 cells (transfected with TLR4, MD2 and CD14 genes to yield human fibroblasts as HEK293 cells) under stimulation with LPS.
Materials and Methods
In order to study under the condition of similar living body, we used the 293hTLR4/MD2-CD14 cells in this study. The 293hTLR4/MD2-CD14 cells is a transfected cells with TLR4, MD2 and CD14 genes to yield HEK293 cell. We purchased the 293hTLR4/MD2-CD14 cells from InvivoGen (San Diego, CA). Lansoprazole (LPZ) as PPI was used. The additional concentration of LPZ to the cells was prepared as previously described [16]. The cells were incubated and aliquots of 1.5 × 106 cells were plated on collagen-coated 6-well culture plates (Falcon, Heidelberg, Germany) and cultured in 5% CO2-supplemented room atmosphere. The viability of the 293hTLR4/MD2-CD14 cells was more than 95% as assessed by trypan blue exclusion. And then, the cells divided into the following groups: (a) untreated group (no culture), (b) non LPZ-treated (1h) group, (c) LPZ (10−4 M)-treated (1h) plus non LPS-stimulated (1h) group, (d) LPZ (10−4 M)-treated (1h) plus non LPS-stimulated (6 hours: 6h) group, (e) LPZ (10−4 M)-treated (1h) plus LPS (1 µg/ml)-stimulated (1h) group, (f) LPZ (10−4M)-treated (1h) pus LPS (1 µg/ml)-stimulated (6h) group, (g) non LPZ-treated (1h) plus LPS (1 µg/ml)-stimulated (1h) group and (h) non LPZ-treated (1h) plus LPS (1 µg/ml)-stimulated (6h) group. Extracts of samples from each group were subjected to the following analyses.
Analysis of IkB phosphorylation and NF-kB transfer into nucleus
Phosphorylation of IkB was analyzed by western blotting using phospho-IkB-α (Ser32) antibody and IkB- antibody. NF-kB transfer into the nucleus was analyzed by western blotting of nuclear and cytoplasmic fractions using anti-NF-kBp65 antibody. Phospho-IkB-α antibody, IkB-α antibody and anti-NF-kBp65 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Analysis of phosphorylation of MAP kinase (MAPK: ERK, JNK and p38)
ERK was analyzed by western blotting using phospho-p44/42 MAPK antibody and p44/42 MAPK antibody. JNK was analyzed by western blotting using phospho-SAPK/JNK (Thr183/Tyr185) antibody and SAPK/JNK (56G8) rabbit mAb. P38 was analyzed by western blotting using phospho-p38MAPK (Thr180/Tyr182) antibody and p38MAPK antibody. Phospho-p44/42 MAPK antibody, p44/42 MAPK antibody, phospho-SAPK/JNK antibody, SAPK/JNK rabbit mAb and p38MAPK antibody were purchased from Santa Cruz biotechnology.
Analysis of intranuclear transfer of IRF5
For analysis of IRF5 transfer into the nucleus, the nuclear and cytoplasmic fractions were exposed to the primary antibody (anti-IRF5 antibody) and the HRP-labeled secondary antibody (anti-goat IgG-HRP), followed by western blotting. Anti-IRF5 antibody and anti-gout IgG-HRP were purchased from Santa Cruz Biotechnology.
Analysis of SOCS1 expression
For analysis of SOCS1 expression, samples were exposed to the primary antibody (anti-SOCS1 antibody) and the HRP-labeled secondary antibody (anti-rabbit IgG-HRP), followed by western blotting. Anti-SOCS1 antibody and anti-rabbit IgG-HRP were purchased from Santa Cruz Biotechnology.
Western blot analysis
At the end of the incubation period, the medium was removed and the cells were immediately lysed and transferred by sodium dodecyl sulfate/polyacrylamide gel electrophoresis; proteins were then blotted to nitrocellulose membranes using a semidry transfer apparatus (Pharmacia Biotech, Freiburg, Germany). Blots were blocked for 2 h in 5% (wt/vol) bovine serum albumin containing 20 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, and 0.1% Tween 20 (TBS-T) and then incubated at 4°C overnight with the first antibody. Following washing with TBS-T and incubation with horseradish peroxidase-coupled anti-mouse, anti-goat, or anti-rabbit immunoglobulin G antibody at room temperature for 2 h, respectively, the blots were washed extensively and developed using enhanced chemiluminescent detection (Amersham Pharmacia Biotech, Uppsala, Sweden). Blots were exposed to Kodak X-OMAT AR-5 film (Eastman Kodak Co., Rochester, NY).
Results
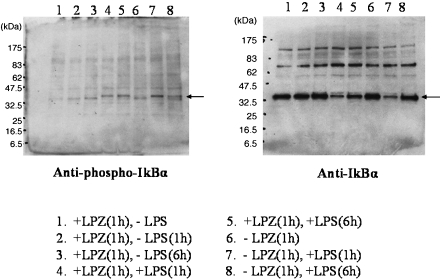
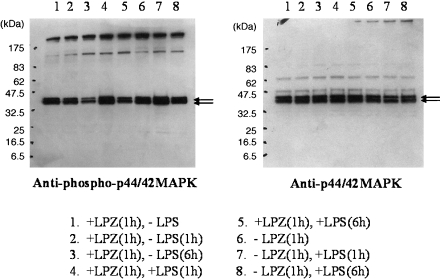
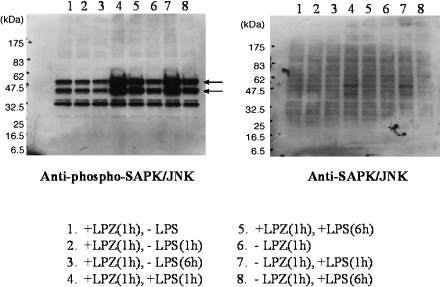
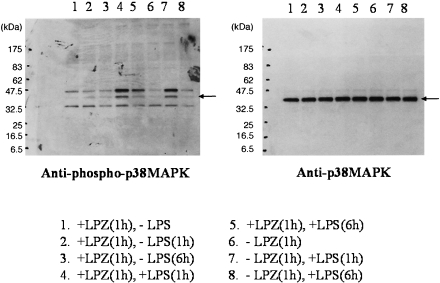
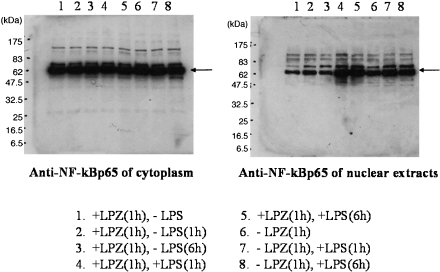
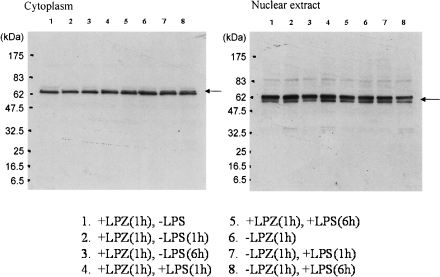
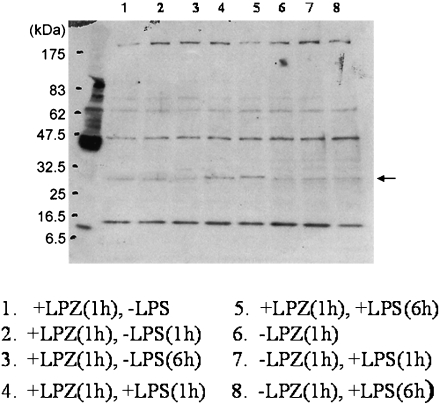
In the LPS-stimulated group, the phosphorylation of IkB, phospho-p44/42 MAPK antibody and p44/42 MAPK antibody, phospho-SAPK/JNK (Thr183/Tyr185) antibody) and phospho-p38MAPK (Thr180/Tyr182) antibody and p38MAPK antibody was suppressed after 6 h of stimulation as compared to that observed after 1h of stimulation (Figs. 1, 2, 3 and 4), and intranuclear transfer of IFR5 and NF-kB was noted (Figs. 5 and 6). In the LPZ-treated group, the phosphorylation of IkB, phospho-p44/42 MAPK antibody and p44/42, phospho-SAPK/JNK (Thr183/Tyr185) antibody) and phospho-p38MAPK (Thr180/Tyr182) antibody and p38MAPK antibody was not suppressed (Figs. 1, 2, 3 and 4), and intranuclear transfer of IFR5 was not suppressed under stimulation with LPS (Fig. 6). The induction of SAPK/JNK (56G8) rabbit mAb was yet presence in all groups (Fig. 3). And then in the LPZ-treated groups, enhanced intranuclear transfer of NF-kB and increased expression of SOCS1 were noted, as compared to the observations in the group stimulated with LPS alone (Fig. 7).
Fig. 1.
The phosphorylation of IkB-α analyzed by western-blotting. An arrow indicates the induction of IkB-α. LPZ and LPS denotes lansoprazole and lipopolysaccharide, respectively.
Fig. 2.
The phosphorylation of ERK (phospho-p44/42 MAPK antibody and p44/42 MAPK antibody) analyzed by western-blotting. Arrows indicate the induction of phospho-p44/42 MAPK antibody and p44/42 MAPK antibody, respectively.
Fig. 3.
The phosphorylation of JNK (phospho-SAPK/JNK (Thr183/Tyr185) antibody and SAPK/JNK (56G8) rabbit mAb) analyzed by western-blotting. Arrows indicate the induction of phospho-SAPK/JNK (Thr183/Tyr185) antibody. The phosphorylation of SAPK/JNK (56G8) rabbit mAb was obscure.
Fig. 4.
The phosphorylation of p38 (phospho-p38MAPK (Thr180/Tyr182) antibody and p38MAPK antibody) analyzed by western-blotting. An arrow indicates the induction of phospho-p38MAPK (Thr180/Tyr182) antibody and p38MAPK antibody, respectively.
Fig. 5.
The result of the transition from cytoplasm to nucleus of NF-kBp65 analyzed by western-blotting.An arrow indicates the induction of NF-kB.
Fig. 6.
The result of the transition from cytoplasm to nucleus of IRF5 analyzed by western-blotting. An arrow indicates the induction of IRF5.
Fig. 7.
The expression of SOCS1 analyzed by western-blotting. An arrow indicates the induction of SOCS1.
Discussion
The present study was undertaken to examine whether or not LPZ might suppress phosphorylation of IkB and MAPK or intranuclear transfer of NF-kB and IRF5 and to evaluate the effects of LPZ on SOCS1 as a negative feedback gene involved in the TLR4 signal transduction system under stimulation with LPS. In the analysis of the effects of LPZ on LPS-stimulated TLR4 signal transduction, LPZ did not suppress either phosphorylation of MAPK (IkB, ERK, JNK or p38) or IRF5 transfer into the nucleus. NK-kB transfer into the nucleus, which might possibly lead to exacerbation of inflammatory reactions, seemed to be stimulated by LPS. In the LPZ-treated group, increase in the expression of SOCS1 was observed. Previous studies using various systems have demonstrated that LPZ exerts anti-inflammatory activity such as the suppression of cytokine formation. Taking into consideration the results of the present study and previous reports, it seems possible that LPZ stimulates the transfer of NF-kB into the nucleus during LPS-stimulated TLR4 signal transduction, which, in turn, leads to the induction of SOCS1 expression and anti-inflammatory activity. Because of limitations related to cell specificity, the results of this study cannot be reasonably extrapolated to other cells. For strict demonstration of the anti-inflammatory activity of LPZ using the test system employed in this study, it is necessary to measure the cytokines formed in both the LPZ-treated groups and non LPZ-treated groups. Furthermore, since cell extracts were used in the present study, it remains unknown whether the results from this study might reflect the events in the cell membrane or the events in the cytoplasm. It has recently been reported that MyD88, an adaptor molecule for TLRs (TLR2, 4), regulates the conduction of stimuli through TLRs, working together with heat shock protein (HSP)-associated molecules, such as HSP70, HSP90, Grp96 and Grp78 [17–21]. Also for the testing system employed in this study, it would be desirable to clarify the involvement of these HSP-associated molecules. In any event, the results of the present study suggest the possibility that LPZ induces SOCS1 and this regulates protein phosphorylation during TLR4 signal transduction.
Conclusion
These results suggest that LPZ stimulates the expression of SOCS1 and regulates protein phosphorylation through its activity on TLR4 signal transduction under LPS stimulation in the 293hTLR4/MD2-CD14 cells. This is a noteworthy new finding. We propose to analysis the particular account of this study using different cell lines or using cell cultures other techniques.
Acknowledgments
This work was supported by research subsidies from Takeda Pharmaceutical Industry Corporation, Japan. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations
- LPZ
Lansoprazole
- LPS
Lipopolysaccharide
- TLRs
Toll-like receptors
- MAPK
MAP kinase
- IRF5
Interferon regulatory factor 5
- SOCS1
Suppressor of cytokine signaling-1
- PPI
Proton pump inhibitor
- HSP
Heat shock protein
References
- 1.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O., Hoshino K., Akira S. TLR 2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 3.Diebold S.S. Activation of dendritic cells by toll-like receptors and C-type lectins. Handb. Exp. Pharmacol. 2009;188:3–30. doi: 10.1007/978-3-540-71029-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Goodridge F.S., Shimada T., Wolf A.J., Hsu Y.M., Becker C.A., Lin X., Underhill D.M. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J. Immunol. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochud P.Y., Chien J.W., Marr K.A., Leisenring W.M., Upton A., Janer M., Rodrigues S.D., Li S., Hansen J.A., Zhao L.P., Adererm A., Boeckn M. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Eng. J. Med. 2008;359:1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richez C., Tasuda K., Watkins A.A., Akira S., Lafyatis R., van Seventer J.M., Rifkin I.R. TLR4 ligands induce IFN-alpha production by mouse conventional dendritic cells and human monocytes after IFN-beta priming. J. Immunol. 2009;182:820–828. doi: 10.4049/jimmunol.182.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paun A., Reiner J.T., Jiang Z., Medin C., Baikhi M.Y., Fitzgerald K.A., Piha P.M. Functional characterization of murine interferon regulatory factor 5 (IRF5) and its role in the innate antiviral response. J. Boil. Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill L.A., Bowie A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 10.Mostecki J., Showalter B.M., Rothman P.B. Early growth response-1 regulate lipopolysaccharide-induced suppressor of cytokine signaling-1 transcription. J. Biol. Chem. 2005;280:2596–2605. doi: 10.1074/jbc.M408938200. [DOI] [PubMed] [Google Scholar]
- 11.Prele C.M., Woodward E.A., Bisley J., Keith-Magee A., Nicholson S.E., Hart P.H. SOCS1 regulates the IFN but not NF kappa B pathway in TLR-stimulated human monocytes and macrophages. J. Immunol. 2008;181:8018–8026. doi: 10.4049/jimmunol.181.11.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansell A., Smith R., Doyle S.L., Gray P., Fenner J.E., Crack P.J., Nicholson S.E., Hilton D.J., O’Neil L.A., Hertzog P.J. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 13.Adib-Conquy M., Adrie C., Fitting C., Gattolliat O., Beyaert R., Cavaillon J.M. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit. Care Med. 2006;34:2377–2385. doi: 10.1097/01.CCM.0000233875.93866.88. [DOI] [PubMed] [Google Scholar]
- 14.Handa O., Yoshida N., Fujita N., Tanaka Y., Ueda M., Takagi T., Kokura S., Naito Y., Okanoue T., Yoshikawa T. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm. Res. 2006;55:476–480. doi: 10.1007/s00011-006-6056-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda M., Yoshida N., Ichikawa H., Takagi T., Okuda T., Naito Y., Okanoue T., Yoshikawa T. Lansoprazole, a proton pump inhibitor, reduces the severity of indomethacin-induced rat enteritis. Int. J. Mol. Med. 2006;17:89–93. [PubMed] [Google Scholar]
- 16.Yoshida N., Yoshikawa T., Tanaka N., Kasai Y., Naito Y., Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors—inhibitory effects on neutrophil-endotherial cell interactions. Aliment. Pharmacol. Ther. 2000;14 Suppl. 1:74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 17.Luo X., Zuo X., Zhang B., Song L., Wei X., Zhou Y., Xiao X. Release of heart shock protein 70 and the effects of extracellular heart shock protein 70 on the production of IL-10 in fibroblast-like synoviocytes. Cell Stress Chaperones. 2008;13:365–373. doi: 10.1007/s12192-008-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asea A. Heat shock proteins and toll-like receptors. Hndb. Exp. Pharmacol. 2008;183:111–127. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- 19.Warger T., Hilf N., Rechtsteiner G., Haselmayer P., Carrick D.M., Jonuleit H., von Landenberg P., Rammensee H.G., Nicchitta C.V., Radak M.P., Schild H. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J. Biol. Chem. 2006;281:22545–22553. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]
- 20.Osterioh A., Breloer M. Heart shock proteins: linking danger and pathogen recognition. Med. Microbiol. Immunol. 2008;197:1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- 21.Brown V., Brown R.A., Ozinsky A., Hesselberth J.R., Fields S. Binding specificity of Toll-like receptor cytoplasmic domains. Eur. J. Immunol. 2006;36:742–753. doi: 10.1002/eji.200535158. [DOI] [PMC free article] [PubMed] [Google Scholar]