Abstract
Although low-dose aspirin is widely used, since it is a cheap and effective means of prevention of cardiovascular events, it can cause hemorrhagic gastrointestinal complications. The aim of this study was to evaluate the efficacy of rebamipide in preventing low-dose aspirin-induced gastric injury. A randomized, double-blind, placebo-controlled, crossover trial was performed in twenty healthy volunteers. Aspirin 81 mg was administered with placebo or rebamipide 300 mg three times daily for 7 consecutive days. The rebamipide group exhibited significant prevention of erythema in the antrum compared with the placebo group (p = 0.0393, respectively). Results for the body and fornix did not differ significantly between the placebo and rebamipide groups. In conclusion, short-term administration of low-dose aspirin induced slight gastric mucosal injury in the antrum, but not in the body or fornix. Rebamipide may be useful for preventing low-dose aspirin-induced gastric mucosal injury, especially which confined to the antrum.
Keywords: low-dose aspirin, healthy subject, rebamipide, prevention
Introduction
Helicobacter pylori (H. pylori) infection [1] and the use of non-steroidal anti-inflammatory drugs (NSAIDs) [2–4] can cause gastrointestinal disease. We have reported that H. pylori-negative, non-NSAID-induced gastrointestinal ulcers are extremely rare [5]. Aging of the population has recently been increasing in Japan, and long-term users of low-dose aspirin are correspondingly increasing in number. Low-dose aspirin is used for primary prevention of cardiovascular events such as myocardial infarction. Randomized controlled trials have demonstrated the preventive effect of administration of low-dose aspirin for patients with prior cardiovascular events [6–7]. Low-dose aspirin is widely used, since it is a cheap and effective means of prevention of cardiovascular events, and now over 5 million Japanese undergo this treatment. However, aspirin use can have hemorrhagic gastrointestinal complications. The recommended dose of aspirin for the prevention of vascular events is in the range of 75–300 mg/day, though when benefits and risks are taken into account the optimal dose is considered to be no more than 100 mg/day [8–10]. Few findings of randomized, placebo-controlled trials are available to clearly determine the risk of upper gastrointestinal ulcer with low-dose aspirin. Several small trials, usually in healthy subjects, have been performed, using a mucosal injury grading system as an outcome measure [11–14]. The clinical utility of such systems is uncertain, and these studies have not provided specific findings on ulcer development.
Recently, a large 12-week endoscopic double-blind study in osteoarthritis patients randomly assigned to placebo (n = 381) or 81 mg enteric-coated aspirin (n = 387) found no significant difference in rate of development of ulcer [15]. However, significant increases in mean number of erosions (mean change: 0.2 vs 0.9) and in the proportion of patients with increase in number of erosions (20% vs 32%) were observed with low-dose aspirin. The increase in rate of clinical events with low-dose aspirin is in general small. It may also be that, though aspirin causes only a very slight increase in rate of development of ulcer, the proportion of patients with complications is much higher due to the anti-platelet effects of aspirin. The increased risk of erosions does indicate that some damage to the upper gastrointestinal tract is occurring. In any case, the real issue in clinical practice is determining the incidence of clinical gastrointestinal events, such as gastrointestinal bleeding, associated with the long-term use of low-dose aspirin.
Rebamipide (2-(4-chlorobenzoylamino)-3-[2-(1H)-quinolinon-4-yl]-propionic acid) (Otsuka Pharmaceutical Co., Tokyo) is a mucosal-protective drug that has many biological effects in the gastric mucosa, such as increasing blood flow, increasing the biosynthesis of prostaglandins, preventing H. pylori-elicited neutrophil-induced mucosal injury, and decreasing oxygen radical levels [16–19]. Rebamipide was also found to be efficacious in reducing gastric injury in healthy subjects taking aspirin 1500 mg once a day [20].
The aim of this study was to evaluate the efficacy of rebamipide in preventing low-dose aspirin-induced gastrointestinal complications in healthy subjects.
Methods
A randomized, double-blind, placebo-controlled, crossover trial was performed in twenty healthy volunteers. The study protocol was approved by the Ethics Committees of Hokkaido University Hospital, and written informed consent was obtained from all subjects.
Inclusion and exclusion criteria
Inclusion criteria were i) lack of gastric conditions such as erosions, erythema, bleeding, and ulcer on endoscopy, and ii) H. pylori negativity on 13C urea breath test. Subjects who habitually smoked or drank alcohol were excluded.
Study design
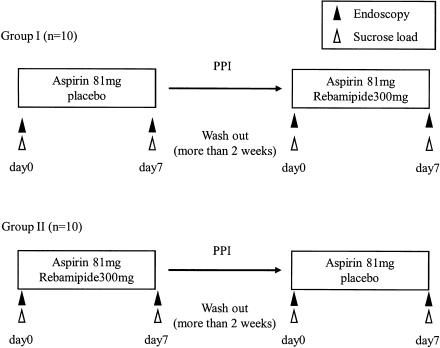
The mean age of the 20 subjects was 24 ± 2 years; fifteen were male and five were female. The 20 healthy subjects were divided two groups, Group I and II. In the first period of this study, aspirin 81 mg with placebo (Group I) or rebamipide 300 mg (Group II) was administered three times daily for 7 consecutive days, while in the second period the two groups were crossed over. The washout period is longer than two weeks between treatments (Fig. 1). The 2-week washout period was determined with reference to the life span of platelets and the half-lives of rebamipide and aspirin.
Fig. 1.
Study design.
Evaluation criteria
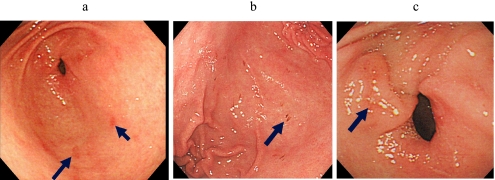
Endoscopic gastric mucosal injury was determined in three gastric areas, the antrum, body, and fornix. The categories of injury were erythema, erosions, and petechiae. Erythema was defined as an area clearly redder than surrounding mucosa, petechia as a bleeding area without mucosal deficit, and erosion as an area with mucosal deficit (Fig. 2). Gastric mucosal injuries detected on endoscopy were evaluated by Lanza score (Table 1).
Fig. 2.
Endoscopic findings of erythema (a), petechiae (b), and erosion (c).
Table 1.
Lanza score.
| 0: | Normal or erythema |
| 1: | mucosal hemorrhage only |
| 2: | one or two erosions ± submucosal hemorrhage or edema |
| 3: | numerous (3–10) erosions ± submucosal hemorrhage or edema |
| 4: | large number of erosions (>10) or an ulcer |
Evaluation of adverse events
The following gastrointestinal symptoms and complications were to be recorded in the symptom diary by all subjects over the entire study period.
Statistical analysis
Endoscopic gastric mucosal injuries were determined in each gastric area, and analyzed by Fisher’s exact test. Findings of p<0.05 were considered significant. All statistical analyses were performed using SAS® version 8.2 (SAS Institute, Cary, NC).
Results
Effects of treatment on endoscopically detectable gastric mucosal injury
The effects of low-dose aspirin on endoscopically detectable gastric mucosal injury in the placebo and rebamipide groups are shown in Tables 2 and 3. Lanza scores in the antrum are shown in Table 2. The rebamipide group exhibited significant prevention of erythema in the antrum, compared with the placebo group (p = 0.0393). The incidence of neither erosions nor petechiae differed significantly between the placebo and rebamipide groups. The results of evaluation of gastric mucosal injury in the body and fornix are shown in Table 3. There were no significant differences between the placebo and rebamipide groups.
Table 2.
Endoscopic evaluation in antrum.
| Lanza score | erythema |
erosion |
petechiae |
||||
|---|---|---|---|---|---|---|---|
| Placebo | Rebamipide | Placebo | Rebamipide | Placebo | Rebamipide | ||
| n = (%) | n = (%) | n = (%) | n = (%) | n = (%) | n = (%) | ||
| antrum | 0 | 11/20 | 17/20 | 9/20 | 13/20 | 15/20 | 17/20 |
| (55.0) | (85.0) | (45.0) | (65.0) | (75.0) | (85.0) | ||
| 1 | 6/20 | 3/20 | 6/20 | 3/20 | 4/20 | 3/20 | |
| (30.0) | (15.0) | (30.0) | (15.0) | (20.0) | (15.0) | ||
| 2 | 3/20 | 0/20 | 2/20 | 1/20 | 1/20 | 0/20 | |
| (15.0) | (0.0) | (10.0) | (5.0) | (5.0) | (0.0) | ||
| 3 | 0/20 | 0/20 | 3/20 | 3/20 | 0/20 | 0/20 | |
| (0.0) | (0.0) | (15.0) | (15.0) | (0.0) | (0.0) | ||
| p-value | 0.0393 | 0.5538 | 0.3105 | ||||
Table 3.
Endoscopic evaluation in body and fornix.
| Lanza score | erythema |
erosion |
petechiae |
||||
|---|---|---|---|---|---|---|---|
| Placebo | Rebamipide | Placebo | Rebamipide | Placebo | Rebamipide | ||
| n = (%) | n = (%) | n = (%) | n = (%) | n = (%) | n = (%) | ||
| body | 0 | 13/20 | 15/20 | 16/20 | 13/20 | 16/20 | 16/20 |
| (65.0) | (75.0) | (80.0) | (65.0) | (80.0) | (80.0) | ||
| 1 | 7/20 | 4/20 | 2/20 | 4/20 | 3/20 | 2/20 | |
| (35.0) | (20.0) | (10.0) | (20.0) | (15.0) | (10.0) | ||
| 2 | 0/20 | 1/20 | 1/20 | 0/20 | 0/20 | 1/20 | |
| (0.0) | (5.0) | (5.0) | (0.0) | (0.0) | (5.0) | ||
| 3 | 0/20 | 0/20 | 1/20 | 3/20 | 1/20 | 1/20 | |
| (0.0) | (0.0) | (5.0) | (15.0) | (5.0) | (5.0) | ||
| p-value | 0.3076 | 0.3308 | 0.6622 | ||||
| fornix | 0 | 17/20 | 16/20 | 18/20 | 19/20 | 18/20 | 20/20 |
| (85.0) | (80.0) | (90.0) | (95.0) | (90.0) | (100.0) | ||
| 1 | 3/20 | 4/20 | 2/20 | 0/20 | 1/20 | 0/20 | |
| (15.0) | (20.0) | (10.0) | (0.0) | (5.0) | (0.0) | ||
| 2 | 0/20 | 0/20 | 0/20 | 1/20 | 1/20 | 0/20 | |
| (0.0) | (0.0) | (0.0) | (5.0) | (5.0) | (0.0) | ||
| 3 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | ||
| p-value | 0.6769 | 0.1233 | 0.2372 | ||||
Adverse events
No gastrointestinal symptoms or complications were recorded in the symptom diary by any subjects during the study period.
Conclusion
Recently, the number of individuals using low-dose aspirin to prevent cardiovascular events has sharply increased in Japan. Increase in the rate of occurrence of lethal bleeding has been predicted with use of this treatment, though that of cardiovascular events should be decreased with it. The frequencies of aspirin-induced gastrointestinal bleeding and ulcer are lower than those of NSAID-induced gastrointestinal bleeding and ulcer, though their severity is very mild. Aspirin produces its anti-thrombotic effects through irreversible acetylation of a serine in cyclooxynase-1 in platelets [21]. This abolishes production of thromboxane A2 for the life of the platelet. Although the half-life of aspirin is short, at 0.4 h, it suppresses platelet aggregation for almost 1 week [22, 23]. This 1-week period is due to the life span of platelets. The effects of aspirin on platelets are a significant problem for chronic aspirin users, compared with NSAID users. Patients with a history of gastric bleeding, who take corticosteroids, or who are elderly readily develop gastrointestinal bleeding [24]. Prevention of aspirin-induced gastric bleeding, especially for high-risk groups, may thus be necessary. Only for proton pump inhibitors is there evidence of prevention of aspirin-induced gastric bleeding [25]. However, administration of long-term acid-suppressive agents carries the risk of infection, such as ventilator-associated pneumonia [26]. Candidate drugs other than acid-suppressing agents are thus needed.
Two reports are available on the use of rebamipide in healthy subjects with drug-induced gastric mucosal injury. The efficacy of rebamipide in reducing NSAID-induced gastric injury has been reported in healthy volunteers on indomethacin treatment [27]. In addition, Dammann et al. reported that rebamipide reduces gastric injury in individuals taking high-dose aspirin (1,500 mg/day) [20]. However, the efficacy of rebamipide in preventing low-dose aspirin-induced gastrointestinal complications has remained unexplored. We therefore tested it as a candidate drug for the prevention of low-dose aspirin-induced gastric injury.
In the present study, rebamipide significantly prevented low-dose aspirin-induced erythema in the antrum compared with placebo (p<0.05) (Table 2). Naito et al. demonstrated that lipid peroxidation mediated by oxygen radicals and activated neutrophil play a crucial role in the pathogenesis of NSAID-induced gastropathy. Rebamipide showed the inhibitory of these parameters [28]. On the other hands, it is well known that reduction of gastric mucosal blood flow (GMBF) induces gastrointestinal disorders [29]. Rebamipide prevented NSAID-induced gastric mucosal injury by maintaining GMBF [30]. Administration of low-dose aspirin frequently induced gastric mucosal injury in the antrum in the control group, though the degree of injury was slight (Table 2). On the other hand, administration of low-dose aspirin did not induce injury in the body and fornix (Table 3). This finding suggests that low-dose aspirin-induced gastric mucosal injury occurs to a slight extent in the antrum but not in the body or fornix. Asaki et al. demonstrated that NSAID-induced gastric ulcers are concentrated in the pyloric region to the antrum, regions which account for three-fourths of all cases of ulcer in the stomach [31]. On the other hand, Daniel et al. have reported that administration of aspirin (900 mg/day) induced more mucosal erosions and submucosal hemorrhages in the antrum than in the fundus (p<0.05) [32]. Gastrokine 1, one of the most abundant transcripts in normal stomach, is down-regulated by H. pylori infection. G. Martin et al. demonstrated that the use of low-dose aspirin led to a significant decrease (3.07 a.u. vs 0.23 a.u., p<0.001) in antrum at day 7, while Gastrokine 1 transcript levels in corpus mucosa were slightly elevated (twofold, p<0.005). This data demonstrated that low-dose aspirin down-regulates Gastrokine 1 expression specifically in antral mucosa, and induces gastrointestinal injuries in antrum [33]. A strategy to prevent low-dose aspirin-induced gastric injuries in the antrum will thus be needed. In addition, many patients undergoing low-dose aspirin treatment are asymptomatic. Sudden hospitalization due to bleeding is a serious problem. In our study, all subjects were asymptomatic (data not shown). Aging of society is progressing in Japan, and the number of low-dose aspirin users will correspondingly be increasing. In addition, periodic endoscopic examination will be needed to detect serious gastrointestinal diseases other than bleeding and ulcer, such as gastric cancer, because of the high prevalence of gastric malignancy in Japan.
Our study has several limitations. It was performed with only short-term administration of low-dose aspirin and was small in size. Although long-term administration of low-dose aspirin is associated with an increased risk of lethal upper gastrointestinal bleeding, the cumulative risk of upper gastrointestinal bleeding is still very low. A large 5-year observational Danish cohort study assessed hospitalization for gastrointestinal bleeding with low-dose aspirin [34]. The incidence of hospitalization for gastrointestinal bleeding was 0.6% per year for individuals taking aspirin alone. It was thus difficult to detect serious cases, such as bleeding, in our small, short-term study. Our study used a mucosal injury grading system (erosion, erythema, and petechia). Evaluation with this grading system is not clinically sufficient. Taking of high-dose aspirin induces submucosal hemorrhages and gastrointestinal ulcers with frequently, however most of low-dose aspirin-induced gastrointestinal injuries are just slight, such as erythema, erosions, and petechiae [31]. These slight injuries may progress to serious injuries, such as ulcer, bleeding, and perforation, under the chronic aspirin use and the additional risk factors that are induced gastropathy, although there is no evidence to resolve about this process. Since findings on patients with long-term use of low-dose aspirin are quite limited, larger long-term clinical research on patients with low-dose aspirin is needed.
In conclusion, administration of short-term low-dose aspirin induced slight gastric mucosal injury in the antrum, but not in the body or fornix. Rebamipide may be useful for the prevention of low-dose aspirin-induced gastric mucosal injury, especially which confined to the antrum.
References
- 1.Marshall B.J. Helicobacter pylori. Am. J. Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 2.Bombardier C., Laine L., Reicin A., Shapiro D., Burgos-Vargas R., Davis B., Day R., Ferraz M.B., Hawkey C.J., Hochberg M.C., Kvien T.K., Schnitzer T.J. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N. Engl. J. Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein F.E., Faich G., Goldstein J.L., Simon L.S., Pincus T., Whelton A., Makuch R., Eisen G., Agrawal N.M., Stenson W.F., Burr A.M., Zhao W.W., Kent J.D., Lefkowith J.B., Verburg K.M., Geis G.S. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis; the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H., Hibi T., Marshall B.J. Helicobacter pylori: present status and future prospects in Japan. J. Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa K., Sugiyama T., Kato M., Ishizuka J., Komatsu Y., Kagaya H., Katagiri M., Nishikawa S., Hokari K., Takeda H., Asaka M. Non-Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur. J. Gastrol. and Hepatol. 2000;12:635–640. doi: 10.1097/00042737-200012060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Antithrombotic Trialists’ Collaboration, author. Collaborative meta-analysis of randomized trials of ant platelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer M.S. Aspirin for primary prevention of coronary events. N. Engl. J. Med. 2002;346:1468–1474. doi: 10.1056/NEJMcp012672. [DOI] [PubMed] [Google Scholar]
- 8.The Dutch TIA Trial Study Group, author. A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N. Engl. J. Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 9.Cryer B., Feldman M. Effect of very low dose daily, long-term aspirin therapy on gastric, duodenal, and, rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17–25. doi: 10.1016/s0016-5085(99)70545-7. [DOI] [PubMed] [Google Scholar]
- 10.Howkey C., Lanes A. Double and certainly about NSAIDs in the year 2000; a multidisciplinary expert statement. On behalf of the Sardinia NSAID meeting participants. Am. J. Med. 2001;110:S79–S100. doi: 10.1016/s0002-9343(00)00651-3. [DOI] [PubMed] [Google Scholar]
- 11.Dammann H.G., Burkhardt F., Wolf N. Enteric coating of aspirin significantly decreases gastroduodenal mucosal legions. Aliment. Pharmacol. Ther. 1999;13:1109–1114. doi: 10.1046/j.1365-2036.1999.00588.x. [DOI] [PubMed] [Google Scholar]
- 12.Hawthorne A.B., Mahida Y.R., Cole A.T., Howkey C.J. Aspirin-induced gastric mucosal damage: prevention by enteric-coating and relation to prostaglandin synthesis. Br. J. Clin. Pharmacol. 1991;32:77–83. doi: 10.1111/j.1365-2125.1991.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole A.T., Hudson N., Hawkey C.J., Hepinstall S. Protection of human gastric mucosa against aspirin-enteric coating or dose reduction? Aliment. Pharmacol. Ther. 1999;13:187–193. doi: 10.1046/j.1365-2036.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 14.Petroski D. A comparison of aspirin granules with plain and buffered aspirin: a report of two studies. Am. J. Gastroenterol. 1986;81:26–28. [PubMed] [Google Scholar]
- 15.Laine L., Maller E.S., Yu C., Quan H., Simon T. Ulcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double-blind trial. Gastroenterology. 2004;127:395–402. doi: 10.1053/j.gastro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K., Okajima K., Uchiba M., Harada N., Johno M., Okabe H., Takatsuki K. Rebamipide attenuates indomethacin-induced gastric mucosal legion formation by inhibiting activation of leukocytes in rats. Dig. Dis. Sci. 1997;42:319–325. doi: 10.1023/a:1018861818023. [DOI] [PubMed] [Google Scholar]
- 17.Kleine A., Kluge S., Peskar B.M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig. Dis. Sci. 1993;38:1441–1449. doi: 10.1007/BF01308601. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M., Miura S., Mori M., Kai A., Suzuki H., Fukumura D., Suematsu M., Tsuchiya M. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–1378. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka S., Ogino K., Hobara T., Yoshimura S., Okazaki Y., Takemoto T., Iida Y. Effects of various mucosal protective drugs on diethyldithiocarbamate-induced antral ulcer in rats. Eur. J. Pharmacol. 1991;197:99–102. doi: 10.1016/0014-2999(91)90370-6. [DOI] [PubMed] [Google Scholar]
- 20.Dammann H.G. Effect of rebamipide on aspirin-induced gastric damage: a case-control study. Eur. J. Gastrol. Hepatol. 1994;6:911–915. [Google Scholar]
- 21.Awtry E.H., Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 22.Benedek I.H., Joshi A.S., Pieniaszek H.J., King S.Y., Kornhauser D.M. Variability in the pharmacokinetics and pharmacodynamics of low dose aspirin in healthy male volunteers. J. Clin. Pharmacol. 1995;35:1181–1186. doi: 10.1002/j.1552-4604.1995.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 23.Patrono C., Ciabattoni G., Pinca E., Pugliese F., Castrucci G., De Salvo A., Satta M.A., Peskar B.A. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb. Res. 1980;17:317–327. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez L.A.G., Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- 25.Lai K.C., Lam S.K., Chu K.M., Wong B.C., Hui W.M., Hu W.H., Lau G.K., Wong W.M., Yuen M.F., Chan A.O., Lai C.L., Wong J. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N. Engl. J. Med. 2002;346:2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 26.Cook D., Guyatt G., Marshall J., Leasa D., Fuller H., Hall R., Peters S., Rutledge F., Griffith L., McLellan A., Wood G., Kirby A. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N. Engl. J. Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 27.Naito Y., Yoshikawa T., Iinuma S., Yagi N., Matsuyama K., Boku Y., Fujii T., Yoshida N., Kondo M., Sasaki E. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig. Dis. Sci. 1998;43:S83–S89. [PubMed] [Google Scholar]
- 28.Naito Y., Iinuma S., Yagi N., Boku Y., Imamoto E., Takagi T., Handa O., Kokura S., Yoshikawa T. Prevention of indometacin-induced gastoric mucosal injury in Helicobacter pylori-Negative healthy volunteers: A comparison study rebamipid vs Famotidine. J. Clin. Biochem. Nutr. 2008;43:34–40. doi: 10.3164/jcbn.2008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace J.L. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract. Res. Clin. Gastroenterol. 2001;15:691–703. doi: 10.1053/bega.2001.0229. [DOI] [PubMed] [Google Scholar]
- 30.Kim H.K., Kim J.I., Kim J.K., Han J.Y., Park S.H., Choi K.Y., Chung I.S. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig. Dis. Sci. 2007;52:1776–1782. doi: 10.1007/s10620-006-9367-y. [DOI] [PubMed] [Google Scholar]
- 31.Asaki S. NSAIDs induced gastroduodenal ulcer in the aged. Nippon Rinsho. 2002;60:1527–1532. [PubMed] [Google Scholar]
- 32.Stiel D., Ellard K.T., Hills L.J., Brooks P.M. Protective effect of enprostil against aspirin-induced gastroduodenal mucosal injury in man. Comparison with cimetidine and sucralfate. Am. J. Med. 1986;81:54–58. doi: 10.1016/s0002-9343(86)80012-2. [DOI] [PubMed] [Google Scholar]
- 33.Martin G., Wex T., Treiber G., Malfeteiner P., Nardone G. Low-dose aspirin reduces the gene expression of gastrokine-1 in the antral mucosa of healthy subjects. Aliment. Pharmacol. Ther. 2008;28:782–788. doi: 10.1111/j.1365-2036.2008.03793.x. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen H.T., Mellemkjaer L., Blot W.J., Nielsen G.L., Steffensen F.H., McLaughlin J.K., Olsen J.H. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am. J. Gastroenterol. 2000;95:2218–2224. doi: 10.1111/j.1572-0241.2000.02248.x. [DOI] [PubMed] [Google Scholar]