Abstract
Intrahepatic and extrahepatic metastases are common findings in hepatocellular carcinoma (HCC). Insulin-like growth factor 2 (IGF2) expression is frequently induced in HCC, and serum IGF2 levels correlate with the presence of extrahepatic metastases. Yet, the role of IGF-induced signaling in the dissemination of HCC remains unclear. We have previously observed elevated IGF2 levels in tumors with metastatic potential in an HCC mouse model. Here, we demonstrate that inhibition of IGF2, or its receptor IGF1R, impairs the migration and invasion activities of murine HCC cells. Furthermore, inhibition of IGF1R also impairs the ability of HCC cells to colonize the lungs after introduction into the circulation through the tail vein but does not impair subcutaneous tumor growth. Collectively, these findings suggest that IGF1R-mediated signaling plays a causative role in tumor dissemination but is not required for tumor growth per se. Although previous studies indicate that IGF ligands can signal through IGF1R/insulin receptor (IR) heterodimers, and IR-A homodimers, we demonstrate that the IR is not required for invasion and metastasis by HCC cells. Finally, we identify matrix metalloproteinase 2 as a mediator of the invasive phenotype downstream of IGF1R-induced signaling. Thus, our studies demonstrate the importance of IGF2-induced signaling in the dissemination of HCC cells.
Introduction
More than 600,000 new cases of hepatocellular carcinoma (HCC) are diagnosed each year, with a survival rate of less than 5% and an average survival of less than 1 year after diagnosis [1]. Various growth factors and their respective receptors have been identified as modulators of cell proliferation and differentiation in HCC. Among these, the insulin-like growth factor (IGF) axis has been implicated as an important mediator of proliferation and response to chemotherapy [2,3].
The IGF system consists of two small, secreted ligands, IGF1 and IGF2; three cell membrane receptors, IGF1 receptor (IGF1R), insulin receptor (IR) and IGF2 receptor (IGF2R); and six high-affinity IGF binding proteins, IGFBP 1 through 6. IGF1R with a 70% homology to IR is a heterotetrameric (α2β2), disulfide-bonded, transmembrane glycoprotein containing extracellular ligand binding and intracellular tyrosine kinase domains [4]. The IGF1Rβ subunit binds cytoplasmic adapter proteins, thereby propagating the signaling cascade.
Binding of IGF1 or IGF2 to IGF1R results in receptor oligomerization, activation of protein tyrosine kinase activity, intermolecular receptor autophosphorylation, and phosphorylation of cellular substrates, such as the IR substrate (IRS) proteins and the Shc adapter protein that, consequently, lead to gene activation, DNA synthesis, and cell proliferation [5]. In addition, IGF-mediated signaling may occur through IGF1R and IR homodimers or through IGF1R/IR hemireceptor complexes [6]. In contrast, IGF2R, which is structurally unrelated to IGF1R, does not exhibit cytoplasmic kinase activity, but it regulates IGF2 turnover and bioavailability through receptor-mediated endocytosis and subsequent degradation [7].
Alterations in the IGF signaling pathway have been described in several human tumors including Wilms tumors, colon, lung, breast, and prostate carcinomas, as well as HCC [8–13]. The expression of IGF2, which is high in the fetal liver and the early neonatal period but strongly reduced in adult humans, mice, and rats, is strongly reactivated in HCC and in animal models of the disease and may contribute to hepatocarcinogenesis through an autocrine mechanism [14–21]. Besides the direct transcriptional induction of IGF2, additional mechanisms, such as reduction of IGFBP levels and inactivation of IGF2R by mutation and loss of heterozygosity, may contribute to elevated IGF2 bioavailability in HCC cells [22–27]. Transgenic expression of IGF2 leads to HCC, further supporting a causative role for IGF2 in this malignancy [28].
Yet, the mechanisms through which IGF2-induced signaling contributes to liver tumorigenesis remain unclear. We previously demonstrated the existence of elevated IGF2 messenger RNA (mRNA) levels in murine HCCs with enhanced metastatic potential relative to those with low metastatic potential [21], consistent with findings correlating serum IGF2 levels and extrahepatic metastases [29]. We therefore sought to determine the role played by IGF2-induced signaling in various tumor-associated phenotypes including metastasis. Here, we show that IGF2-induced signaling through the IGF1R, but not the IR, is required for the ability of HCC cells to migrate, invade, and colonize the lungs. Importantly, the migration and invasion phenotypes seem to require signaling through the downstream adapter IRS2, but not IRS1, and the induction of matrix metalloproteinase 2 (MMP2). These findings confirm the importance of IGF signaling in hepatocarcinogenesis and suggest the significance of different signaling pathways downstream of IGF1R activation.
Materials and Methods
Cell Lines
The MM189 and BL322 HCC cell lines have been previously described [30,31]. RNAi-mediated depletion of IGF2, IRS1, IRS2, IGF1R, and IR was achieved by infecting the MM189 murine HCC cell line with pLKO-based lentiviruses encoding short hairpin RNA (shRNA) targeting the appropriate mRNA (Open Biosystems, Huntsville, AL). Infected cells were selected in 8 µg/ml puromycin (EMD Biosciences, Gibbstown, NJ). Resistant cells were then analyzed by immunoblot analysis to determine gene knockdown efficacy.
Immunoblot Analysis
Immunoblot analysis was conducted as previously described [30]. Conditioned medium was collected from confluent cells grown in serum-free medium for 48 hours and concentrated using centrifugal filter devices according to the manufacturer's protocol (Millipore, Billerica, MA). The following primary antibodies were used: anti-IGF1Rβ (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-IR (1:1000; Santa Cruz), anti-IRS1 (1:1000; Millipore), anti-IRS2 (1:1000; Millipore), IGF2 (1:1000; R&D Systems, Minneapolis, MN), IGF1 (1:1000; R&D Systems), and antiβ-actin (1:5000; Santa Cruz).
Cell Proliferation Assay
Cell proliferation assays were performed as previously described [30]. The proliferation curves were constructed by calculating the mean value of optical density measurement at 490 nm using a 96-well plate reader and repeated at least three times.
Migration and Invasion Assays
Migration and invasion assays were performed as described in our previous studies [30]. The percent invasion was calculated as the number of invading cells divided by the number of migrating cells. The percent invasion was then normalized to get the invasion index, with the value for the control cell population set to 1. All experiments were performed in duplicate and repeated a minimum of three times. Data shown are from representative experiments. For IGF2 rescue, serum-free conditioned medium from confluent cells was filtered using a filter with a 30-kDa size exclusion pore according to the manufacturer's protocol (Millipore). This conditioned medium was used in the transwell insert instead of serum-free medium. For IGF1R inhibition, cells were cultured for 12 hours in the presence of AG1024 (EMD Biosciences) before placement in the chambers.
Lung Colonization Assay
Six-week-old male nude mice were placed in a tail vein injector and restrainer, the heated tail was cleaned with an alcohol swab, and 105 cells in 300 µl of sterile PBS were injected into the tail vein. Pressure was applied to the injection site for several seconds to prevent bleeding. After 25 to 28 days, mice were killed, and their lungs were harvested for paraffin embedding, sectioning, and histologic examination after hematoxylin and eosin staining. The total number of lesions per lung was determined by counting under a light microscope, and the total tumor area was calculated using IPLab software (BD Biosciences, Rockville, MD). Six animals were included in each group, and each experiment was performed at least twice. Data are shown for representative experiments. All experiments were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee and performed in accordance with guidelines approved by the US Public Health Service.
Subcutaneous Tumor Formation
A total of 106 of either knockdown or control cells were suspended in 100 µl of sterile PBS and injected subcutaneously (SC) into left or right flank of the same 6-week-old male nudemice. After 10 to 12 days, mice with SC injection were killed, and the tumors were weighed and processed. Four to six animals were included in each group, and each experiment was performed at least twice. Data are shown for representative experiments.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from cell lines as previously described [21]. Two micrograms of RNA was reverse-transcribed into complementary DNA (cDNA) using Superscript first-stand cDNA synthesis system according to the manufacturer's manual. The cDNA were amplified in a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) using SYBR Green Master PCR mix for Snail, Twist, and MMP3 amplification, or TaqMan Universal Master mix for MMP2, MMP9, and β-actin amplification (Applied Biosystems). All amplifications were performed in quadruplicate. For experiments involving inhibition of signaling pathways, cells were cultured for 36 hours in the presence of AG1024 (EMD Biosciences), PD98059 (Sigma, St. Louis, MO), or LY294002 (Invitrogen, Carlsbad, CA), which are specific inhibitors of IGF1R, MAP kinase kinase, and phosphoinositide 3 (PI3) kinase, respectively, before the isolation of total RNA.
All values were normalized to an endogenous β-actin control. The relative quantification value of each target gene in IGF1R knockdown cells relative to vector control cells is expressed as 2-(Ct - Cc), where Ct and Cc are the mean normalized threshold cycle difference for the knockdown and control cells, respectively. The primer sequences used to amplify individual genes are listed below.
Snail-F: CTGCAACCGTGCTTTTGC
Snail-R: CACATCCGAGTGGGTTTGG
Twist-F: CACGCAGTCGCTGAACGA
Twist-R: GACCTGGTACAGGAAGTCGATGT
MMP3-F: AGGAGCTAGCAGGTTATCCTAAAAGC
MMP3-R: TAGAAATGGCAGCATCGATCTTC
MMP9 (Mm006001164_g1; Applied Biosystems)
MMP2 (Mm00439506_m1; Applied Biosystems)
β-actin (Mm00607939_s1; Applied Biosystems)
Zymography
The identification of MMP2 proteolytic activity was performed using gelatin zymography by electrophoresis of serum-free conditioned medium collected from confluent cells. Serum-free conditioned medium was concentrated using centrifugal filter devices according to the manufacturer's protocol (Millipore). After quantification of protein concentration by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA), a total of 30 µg of concentrated conditioned medium was loaded into 10% SDS-PAGE supplemented with 0.1% gelatin. After electrophoresis, gels were incubated in renaturing buffer (10 mM Tris pH 7.4, 2.5% Triton X-100; Sigma) for 30 minutes at room temperature, then incubated in developing buffer (Invitrogen) for 30 minutes at room temperature, followed by incubation in fresh developing buffer at 37°C for overnight. Gels were stained with Coomassie Brilliant Blue R-250 (Sigma) and washed in a solution containing 10% acetic acid and 30% methanol (Fisher Scientific, Pittsburgh, PA).
Results
IGF2 Induces Migration and Invasion Phenotypes Through IGF1R
Our previously published data indicated that IGF2 mRNA levels correlated with metastatic potential in a mouse model for HCC [21,31], and published work has correlated serum IGF2 levels with extrahepatic metastasis in HCC patients [29]. We have additionally described murine HCC cell lines, derived from tumors induced in our model, with different capabilities in cell culture migration and invasion assays and have shown that increased IGF2 levels correlate with increased invasiveness [30,31]. We therefore sought to determine whether IGF2-induced signaling is required for the migration and invasion activities of HCC cells.
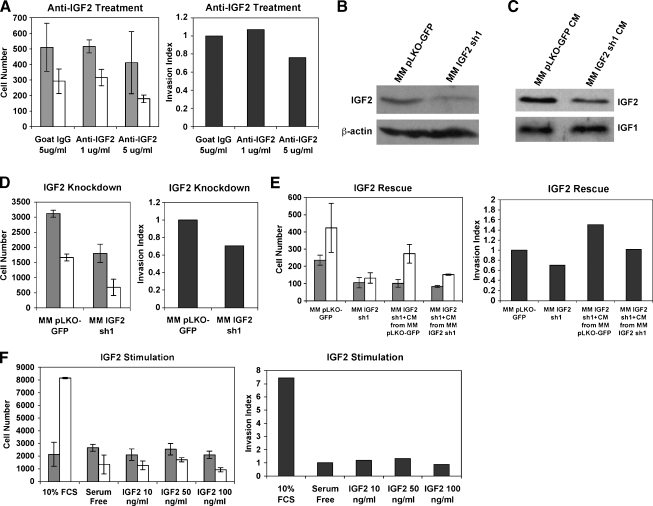
Treatment of MM189 HCC cells possessing high migration and invasion potential, and high expression of IGF2 [31], with a neutralizing antibody (Figure 1A) reduced cell migration and invasion in transwell assays, suggesting that IGF2 is a key component in these phenotypes. To confirm that this effect was due to the specific neutralization of IGF2, we infected MM189 cells with a lentiviral vector encoding IGF2-targeting shRNA. We confirmed knockdown of IGF2 by immunoblot analysis of cell lysates (Figure 1B) and conditioned serum-free medium (Figure 1C). The specificity of the knockdown was confirmed by the maintenance of IGF1 levels in conditioned serum-free medium collected from knockdown cells relative to controls (Figure 1C). ShRNA-mediated knockdown of IGF2 decreased cell migration and invasion (Figure 1D), further supporting a role for IGF2 in these phenotypes. Importantly, incubation of IGF2 knockdown cells with conditioned serum-free medium from control cells more effectively restored their invasion activity than incubation with conditioned medium from knockdown cells (Figure 1E). We next determined whether ectopic administration of IGF2 was sufficient to induce cell migration or invasion. Incubation of MM189 cells in the presence of recombinant IGF2 (but the absence of serum) failed to stimulate cellmigration or invasion (Figure 1F). Together, these findings demonstrate that IGF2 is a critical stimulator of HCC cell migration and invasion but is not able to induce these phenotypes on its own.
Figure 1.
IGF2 is required for HCC cell migration and invasion. (A) Migration (gray bars) and invasion (white bars) activities of MM189 HCC cells after inhibition of IGF2 using neutralizing antibodies. Error shown is SEM. The panel on the right displays the invasion index (see Materials and Methods). Data are from a representative experiment. (B) Immunoblot of whole-cell lysates confirming knockdown of IGF2 in MM189 HCC cells by a targeting shRNA. β-Actin serves as a loading control. (C) Immunoblot of conditioned serum-free medium collected from IGF2 knockdown cells and controls to determine levels of secreted IGF2 and IGF1. (D) Migration (gray bars) and invasion (white bars) activities of MM189 HCC cells after inhibition of IGF2 by shRNA. Error shown is SEM. The panel on the right displays the invasion index. Data are from a representative experiment. (E) Migration and invasion activities of MM189 cells with IGF2 knockdown or controls in the presence of serum-free medium (first two samples) or in the presence of serum-free conditioned medium collected from either control MM189 cells or MM189 cells expressing IGF2 shRNA (last two samples). Error shown is SEM. The panel on the right displays the invasion index. Data are from a representative experiment. (F) Migration (gray bars) and invasion (white bars) activities of MM189 HCC cells stimulated with recombinant IGF2. Error shown is SEM. The panel on the right displays the invasion index. IGF2 concentrations are shown on the x-axis. Data are from a representative experiment.
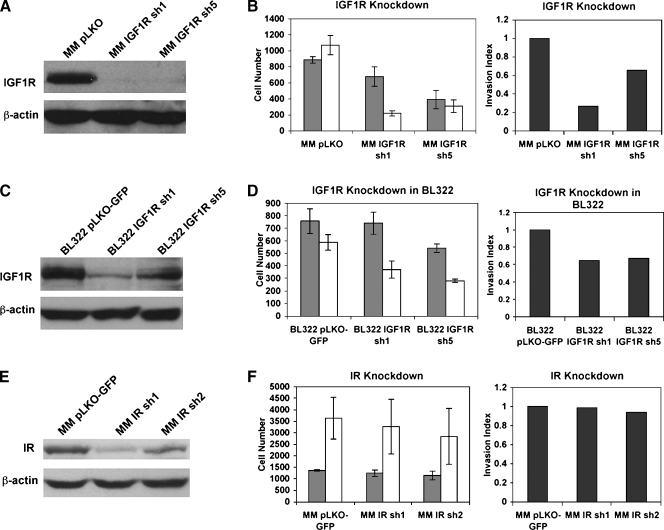
Previous studies have suggested that IGF2 signals through the IGF1R but may also signal through the IR or IGF1R/IR heterodimers [6]. To ascertain whether IGF1R-mediated signaling is required for HCC cell migration and invasion, we introduced lentiviral vectors encoding shRNA targeting IGF1R into MM189 cells and confirmed efficient knockdown of IGF1R expression by two independent targeting shRNA by immunoblot analysis for the IGF1Rβ subunit (Figure 2A). Interestingly, the expression of IGF1R-targeting shRNA inhibited tumor cell migration and invasion (Figure 2B). To confirm that this phenomenon is not restricted to a single cell line, we performed similar experiments in the BL322 HCC cell line [30]. We observed that IGF1R knockdown impaired the migration and invasion activities of these cells (Figure 2, C and D). Thus, these data demonstrate that IGF1R-mediated signaling is required for the migration and invasion activities of HCC cell lines.
Figure 2.
IGF1R-mediated, but not IR-mediated, signaling is required for HCC cell migration and invasion. (A) Immunoblot confirming knockdown of IGF1R in MM189 HCC cells by independent targeting shRNA. β-Actin serves as a loading control. (B) Migration (gray bars) and invasion (white bars) activities of vector controls and MM189 cells with IGF1R knockdown. Error shown is SEM. The panel on the right displays the invasion index. Data are from a representative experiment. (C) Immunoblot confirming knockdown of IGF1R in BL322 HCC cells by independent targeting shRNA. β-Actin serves as a loading control. (D) Migration (gray bars) and invasion (white bars) activities of vector controls and BL322 cells with IGF1R knockdown. Error shown is SEM. The panel on the right displays the invasion index. Data are from a representative experiment. (E) Immunoblot confirming knockdown of IR by targeting shRNA. (F) Migration (gray bars) and invasion (white bars) activities of vector controls and MM189 cells with IR knockdown. The panel on the right displays the invasion index. Data are from a representative experiment.
To determine whether IR-mediated signaling is involved in the migration and invasion phenotypes, we infected MM189 cells with lentiviral vectors encoding IR-targeting shRNA and documented reduced IR expression by immunoblot (Figure 2E). In transwell assays, reduced IR levels did not impair tumor cell migration and invasion (Figure 2F), suggesting that IR-mediated signaling is not required for these phenotypes.
Migration and invasion assays were also performed in the presence of increasing concentrations of the compound AG1024. AG1024 is a competitive inhibitor of IGF1R kinase activity and, at higher concentrations, of IR activity as well [32]. Consistent with the data obtained after shRNA-mediated knockdown of either IGF1R or IR, low concentrations of AG1024, below the half-maximal inhibitory concentration for IR, effectively inhibited the invasion activity of this HCC cell line (Figure W1). Collectively, the previously mentioned data demonstrated that IGF1R but not IR is required for IGF2-mediated HCC cell migration and invasion.
IGF1R-Mediated Signaling Is Required for the Metastatic Phenotype
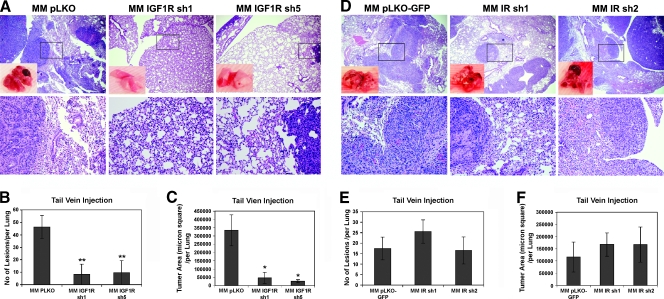
The previously mentioned data demonstrate that IGF2-stimulated IGF1R-mediated signaling is required for HCC cell migration and invasion. To ascertain whether this correlated with an in vivo phenotype, we determined the ability of MM189 cells with IGF1R knockdown, or empty vector controls, to colonize the lungs after introduction into the circulation through the tail vein.We observed that IGF1R knockdown led to a dramatic alteration of the phenotype in this assay. Whereas control cells readily colonized the lungs and formed large masses within this tissue, there was an 80% reduction in the number of lesions and a 90% reduction in the size of lesions in the lung after injection of cells expressing either of two shRNA hairpins targeting IGF1R (Figure 3, A–C). Thus, IGF1R-stimulated signaling is required for lung colonization in vivo.
Figure 3.
IGF1R signaling is required for lung colonization. (A) Representative lung fields of nude mice after the delivery of MM189 cells with IGF1R knockdown or vector controls through tail vein injection. The boxed area in the upper panel is shown at a higher magnification in the lower panel. Quantification of the number of lung lesions (B) and total tumor area (C) in the lungs of mice injected with MM189 cells with IGF1R knockdown or vector controls. Error shown is SEM. *P < .01 by Student's t test. **P < .05 by Student's t test. (D) Representative lung fields of nude mice after the delivery of MM189 cells with IR knockdown or vector controls through tail vein injection. The boxed area in the upper panel is shown at a higher magnification in the lower panel. Quantification of the number of lung lesions (E) and total tumor area (F) in the lungs of mice injected with MM189 cells with IR knockdown or vector controls. Error shown is SEM. P, not significant.
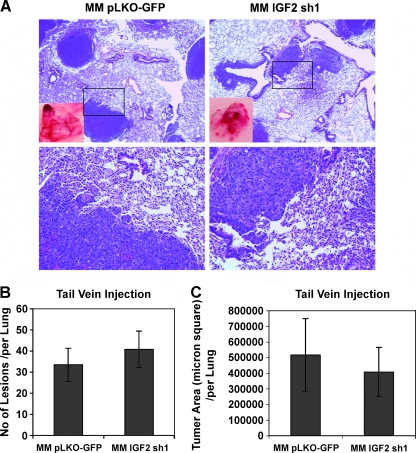
In contrast, and consistent with the findings in the transwell assays, MM189 cells expressing IR-targeting shRNA colonized the lungs as effectively as control cells did (Figure 3D). IR knockdown cells did not display a reduction either in the number of lesions that formed or in the lung area occupied by these lesions (Figure 3, E and F). Interestingly, shRNA-mediated knockdown of IGF2 did not result in a decrease in the number of lesions within the lungs and resulted in a modest but statistically insignificant decrease in tumor area within the lung after tail vein injection (Figure 4, A–C). Together, these data demonstrate that IGF1R-stimulated signaling, but not IR- or IGF1R/IR-stimulated signaling, is important for metastasis-associated phenotypes.
Figure 4.
Inhibition of IGF2 does not block lung colonization. (A) Representative lung fields of nude mice after the delivery of MM189 cells with IGF2 knockdown or vector controls through tail vein injection. The boxed area in the upper panel is shown at higher magnification in the lower panel. Quantification of the number of lung lesions (B) and total tumor area (C) in the lungs of mice injected with MM189 cells with IGF2 knockdown or vector controls. Error shown is SEM. P, not significant.
IGF1R-Mediated Signaling Is Not Required for Subcutaneous Tumor Formation
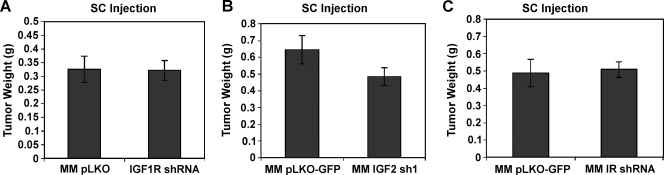
These findings indicate an important role for IGF1R-mediated signaling in HCC cell migration, invasion, and lung colonization. We therefore asked whether IGF1R signaling plays important roles in other phenotypes of HCC cells. We determined whether IGF1R knockdown affected the ability of MM189 HCC cells to form tumors after SC injection into the flanks of nude mice. Interestingly, we did not observe any differences between IGF1R knockdown cells and controls. IGF1R knockdown cells induced tumors with an average weight of 321 ± 101 mg compared with 325 ± 136 mg for controls (Figure 5A). Likewise, RNAi-mediated inhibition of IGF2 did not significantly impair SC tumor formation (Figure 5B), suggesting that IGF2-stimulated IGF1R signaling is not required for tumor formation. Finally, RNAi-mediated inhibition of IR did not inhibit SC tumor formation, suggesting that IR-mediated signaling is not required for this phenotype either (Figure 5C).
Figure 5.
IGF2-induced signaling is not required for SC tumor formation. Effect of RNAi-mediated depletion of IGF1R (A), IGF2 (B), or IR (C) on SC tumor growth of MM189 HCC cells. pLKO- or pLKO-GFP-infected cells are used as vector controls.
Thus, combined with our previously mentioned findings, these results indicate that IGF1R-mediated signaling is dispensable for tumor development but is required for HCC cell migration, invasion, and metastasis.
IGF1R-Stimulated HCC Cell Invasion Involves IRS2
Ligand binding to the IGF1R leads to autophosphorylation of the receptor and binding of adapter IRS proteins [33]. mRNA for IRS 1 to 4 are detectable in the murine liver, and we have demonstrated through reverse transcription-polymerase chain reaction (RT-PCR) that all four are expressed in HCC tissues and cell lines generated in our mouse model (data not shown).
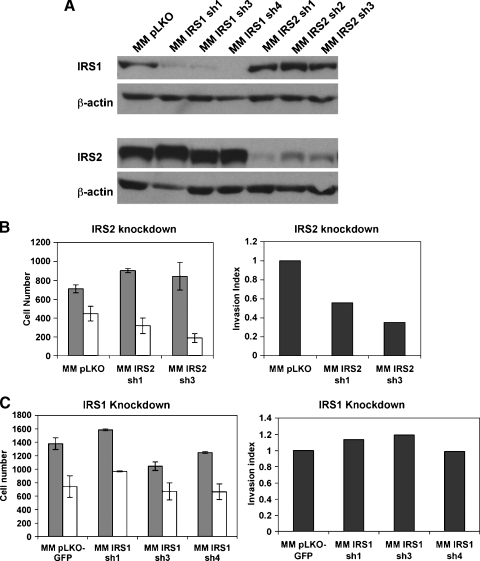
Previous studies have demonstrated opposite roles for IRS1 and IRS2 in the metastasis of mammary tumor cells in a mouse model of the disease [34,35]. We therefore sought to determine whether the signals downstream of IGF1R in HCC cell migration and invasion are mediated by IRS1, IRS2, or both. To explore this question, we generated MM189 cells with shRNA-induced knockdown of IRS1 or IRS2 and measured the migration and invasion activities of these cells. First, we confirmed by immunoblot that knockdown was specific for each of the IRS proteins (Figure 6A). We next measured the migration and invasion activities of the knockdown cells relative to controls. We found that IRS2 knockdown, but not IRS1 knockdown, reduced invasion activity (Figure 6, B and C). Thus, our results indicate that the effect of IGF1R on the invasion activity of HCC cells is primarily mediated by IRS2.
Figure 6.
IGF1R-mediated HCC cell invasion involves IRS2. (A) Immunoblot confirming specific knockdown of either IRS1 or IRS2 using targeting shRNA. β-Actin serves as a loading control. Invasion activity of MM189 HCC cells with knockdown of either IRS2 (B) or IRS1 (C). Migration (gray bars) and invasion (white bars) activities of vector controls and cells with indicated knockdown. Error shown is SEM. The panel on the right displays the invasion index. Data are from representative experiments.
MMP2 Is a Mediator of IGF1R-Mediated HCC Cell Invasion
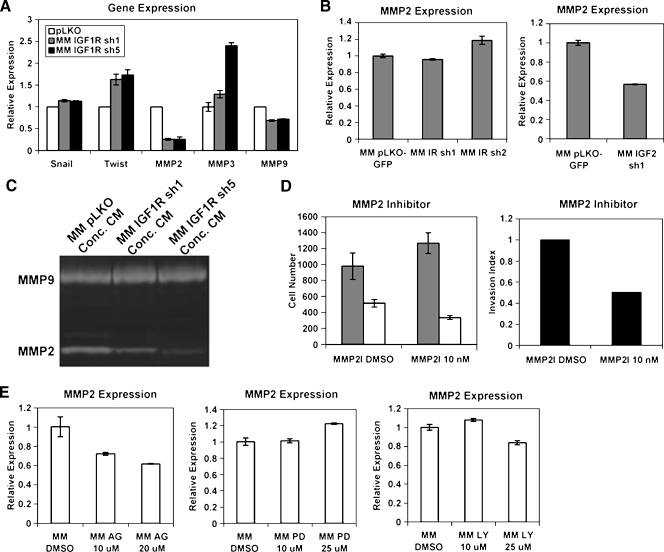
To identify potential mediators of the IGF1R-induced migration and invasion phenotypes in MM189 HCC cells, we assayed the mRNA levels of several candidate genes in IGF1R knockdown cells and controls by quantitative RT-PCR (qRT-PCR). We determined the levels of the transcription factors Snail and Twist and the matrix metalloproteinases MMP2, MMP3, and MMP9 because all of these factors have been previously shown to be involved in tumor cell migration and/or invasion [36,37]. We observed that the levels of Snail are not altered in cells lacking IGF1R, whereas Twist1 mRNA levels are slightly elevated in these cells (Figure 7A). However, MMP2 mRNA levels are greatly reduced in cells with IGF1R knockdown relative to controls (Figure 5A), whereas MMP9 levels are modestly reduced, consistent with regulation of MMP2 by IGF signaling in other cancer cell lines [38–40]. This change does not reflect a general reduction in MMP-encoding mRNA because MMP3 mRNA levels are elevated in IGF1R knockdown cells relative to controls (Figure 7A). Importantly, shRNA-mediated knockdown of IGF2, but not the IR, similarly reduced MMP2 mRNA levels (Figure 7B). Consistent with the RT-PCR data, the activity of MMP2 was reduced in conditioned serum-free medium collected from IGF1R knockdown cells relative to controls (Figure 7C). These data indicate that IGF2-induced IGF1R signaling stimulates the expression of MMP2.
Figure 7.
MMP2 mediates IGF1R-induced cell invasion. (A) qRT-PCR data demonstrating the relative mRNA levels for the indicated genes in IGF1R knockdown cells and vector controls. (B) qRT-PCR measurement of MMP2 mRNA levels in cells with either IGF2 or IR knockdown. (C) Gelatinase zymogaphy demonstrating the activity of secreted MMP2 and MMP9 in IGF1R knockdown cells and vector controls. (D) Migration (gray bars) and invasion activities (white bars) of MM189 cells treated with an MMP2 inhibitor. Error shown is SEM. The panel on the right displays the invasion index. Data are from a representative experiment. (E) Measurement of MMP2 mRNA levels in MM189 HCC cells treated with inhibitors targeting the IGF1R, MAP kinase signaling pathway, or the PI3 kinase signaling pathway.
We next determined whether inhibition of MMP2 reduced the migration and invasion activities of MM189 cells. We found that treatment of MM189 cells with 10 µM of an MMP2 inhibitor reduced cell invasion but not migration (Figure 7D). These data suggest that MMP2 acts downstream of IGF1R to regulate HCC cell invasion.
To determine how IGF signaling may regulate MMP2 levels, we treated MM189 cells with the IGF1R inhibitor AG1024, or with specific inhibitors against PI3-kinase or MAP kinase kinase, and measured MMP2 mRNA levels relative to vehicle-treated controls. We observed that blockade of PI3 kinase signaling modestly reduced MMP2 mRNA levels, whereas MAP kinase signaling blockade did not, suggesting that PI3 kinase signaling mediates, at least in part, IGF2-induced MMP2 expression (Figure 7E).
Discussion
IGF-induced signaling has been implicated in the pathogenesis of several human malignancies [2]. In particular, induction of IGF signaling, by a variety of mechanisms, has been implicated in HCC. Indeed, the induction of IGF2 gene expression is a common event in HCC, and serum levels of IGF2 mRNA correlate well with the presence of extrahepatic metastases in this disease [17,29]. In agreement with these findings, we have previously shown that elevated IGF2 mRNA levels identify tumors with enhanced metastatic potential in a novel HCC mouse model [21].
We therefore sought to determine the role of IGF2-induced signaling in the metastatic capability of HCC cells. Although exogenous IGF2 was insufficient to stimulate HCC cell migration and invasion, we observed that knockdown of IGF2, as well as blockade with anti-IGF2 antibodies, impaired the ability of HCC cells to migrate and invade in transwell assays, indicating that IGF2 is required for these phenotypes and that IGF2 may act in an autocrine manner to induce these effects.
Using an shRNA-mediated inhibition strategy, we found that IGF1R-mediated signaling is required for cell migration and invasion in vitro, indicating that IGF2 signals through this receptor to stimulate these phenotypes. These findings are consistent with a recent report showing that IGF1R inhibition impaired the migration of HCC cells in a wound healing assay [41]. Importantly, we also observed that IGF1R-mediated signaling is required for lung colonization in vivo, providing experimental support for the connection between IGF2 expression and extrahepatic metastasis in HCC patients. Our results are consistent with previous findings indicating roles for IGF1R-mediated signaling in the metastasis of other tumor types [4].
Interestingly, whereas IGF1R knockdown impaired tumor lung colonization, knockdown of IGF2 had only a modest effect, perhaps suggesting that in vivo circulating IGFs produced by the recipient animals may stimulate the IGF1R signaling cascade in the injected HCC cells, circumventing the need for the cells to produce IGF2. Another possibility is that IGF2 is important at an earlier step in metastasis, such as the migration and invasion of tumor cells away from the primary site and into the circulation, a step that is circumvented in this particular assay. This hypothesis is consistent with our in vitro migration and invasion data.
In contrast to the importance of IGF1R-stimulated signaling in lung colonization by HCC cells, we observed that IGF1R-mediated signaling was not required for tumor growth after the SC injection of tumor cells. The different results obtained in the SC tumor assay and the lung colonization assay also suggest the possibility that IGF1R signaling is specifically required for tumor progression but not for tumor development per se. Further experiments are required to test this hypothesis and to provide mechanistic insights into how this might occur. However, these observations are consistent with findings reported in mammary tumorigenesis [42,43].
Our results contrast with those of Nussbaum et al. [41] who found that inhibition of IGF1R signaling using small molecules impaired tumor growth. A potential explanation for this discrepancy is that in addition to impairing IGF1R signaling in tumor cells, treatment of mice with small-molecule antagonists also impairs IGF1R signaling in stromal cells such as fibroblasts and vascular endothelial cells that are critical for tumor growth. Our use of shRNA specifically targets IGF1R signaling within the tumor cells and therefore allows more precise evaluation of the requirement for IGF1R-stimulated signaling cascades in tumor development.
Previous studies have suggested that in addition to IGF1R, IGFs may signal through IGF1R/IR heterodimers and IR-A homodimers [6]. Of significance, our results demonstrate that IGF1R-mediated, but not IR-mediated signaling, is required for many of the phenotypes assayed. These results are consistent with recent findings on the effects of IGF1R, but not IR, on the viability, proliferation, and migration of human HCC cells [41]. These results suggest, that in HCC cells, IGF ligands signal through IGF1R holoreceptors, not IGF1R/IR heterodimers, and that insulin signaling is not important for transformation phenotypes in HCC, despite the fact that the liver is a critical site of insulin action. Further, these results suggest that although the IGF1R and IR share common downstream IRS adapter proteins, the signals stimulated downstream of these receptors are different.
Consistent with this idea, knockdown of IRS2, but not IRS1, partially recapitulated the migration and invasion defect observed in IGF1R depleted cells. The involvement of IRS2 in the invasion phenotype is consistent with its role in tumor metastasis in mouse models of human cancer [35]. However, IRS2 knockdown does not induce as severe a phenotype as IGF1R depletion (nor does concomitant knockdown of IRS1 and IRS2). This suggests that signals propagated downstream of IGF1R independently of the IRS proteins play critical roles in these phenotypes.
Indeed, the identities of the downstream signaling pathways important for the metastasis-related phenotypes induced by IGF1R-stimulated signaling remain unknown. Direct pharmacologic inhibition of these pathways in our HCC cell lines is an unfeasible approach because these inhibitors block the transforming activity of PyMT, the initiating oncoprotein in these cell lines. We have previously demonstrated that treatment of these HCC cell lines with the mTOR antagonist rapamycin impairs invasion [31], and our unpublished results show that blockade of MAP kinase or PI3 kinase signaling potently impairs tumor cell migration and invasion (Y.-W. Chen and B. Lewis, unpublished data). Thus, rescue experiments in which IGF1R mutants defective in stimulating specific downstream signaling pathways are used to reverse the invasion defect caused by IGF1R knockdown will be needed to address this issue.
What also remain unclear are the identities of the terminal effectors that mediate IGF-induced cell migration and invasion. Using a candidate gene approach, we showed that MMP2 is involved in IGF1R-mediated invasion, yet MMP2 inhibition does not produce as severe an impact on cell invasion as IGF1R inhibition, indicating that other critical players remain to be identified. It is likely that a more global approach may identify important players in this process. Indeed, Nussbaum et al. [41] recently reported that IGF signaling induces the expression of several genes involved in motility. An intriguing possibility, yet to be tested experimentally, is that the metastatic phenotype induced by IGF signaling is dependent on the inhibition of FoxO transcription factors, recently shown to act as tumor suppressors in vivo [44].
In summary, we have demonstrated a critical role for IGF-stimulated signaling in metastasis-associated phenotypes in HCC cells. These data provide further support for the pathologic significance of IGF2 gene reactivation in HCCs and suggest that interfering with this signaling pathway may be a critical strategy in treating HCC patients with disseminated disease.
Supplementary Material
Acknowledgments
The authors thank Vicky Appleman, Sharon Magnusson, Annette Nelkenbaum, and Jessica Tatem for technical assistance; Kirsten A. Hubbard and Leslie Shaw for critical review of the manuscript; and members of the Lewis laboratory for thoughtful discussion.
Footnotes
B.C.L. is the recipient of a Career Development Award in the Biomedical Sciences from the BurroughsWellcome Fund and is a Liver Scholar of the American Liver Foundation. This study was supported by National Institutes of Health grant CA121171 (B.C.L.).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 3.Lund P, Schubert D, Niketeghad F, Schirmacher P. Autocrine inhibition of chemotherapy response in human liver tumor cells by insulin-like growth factor-II. Cancer Lett. 2004;206:85–96. doi: 10.1016/j.canlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–S43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- 6.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 7.Oka Y, Rozek LM, Czech MP. Direct demonstration of rapid insulinlike growth factor II receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J Biol Chem. 1985;260:9435–9442. [PubMed] [Google Scholar]
- 8.Reeve AE, Eccles MR, Wilkins RJ, Bell GI, Millow LJ. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985;317:258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- 9.Lambert S, Collette J, Gillis J, Franchimont P, Desaive C, Gol-Winkler R. Tumor IGF-II content in a patient with a colon adenocarcinoma correlates with abnormal expression of the gene. Int J Cancer. 1991;48:826–830. doi: 10.1002/ijc.2910480607. [DOI] [PubMed] [Google Scholar]
- 10.Baghdiguian S, Verrier B, Gerard C, Fantini J. Insulin like growth factor I is an autocrine regulator of human colon cancer cell differentiation and growth. Cancer Lett. 1992;62:23–33. doi: 10.1016/0304-3835(92)90194-z. [DOI] [PubMed] [Google Scholar]
- 11.Favoni RE, de Cupis A, Ravera F, Cantoni C, Pirani P, Ardizzoni A, Noonan D, Biassoni R. Expression and function of the insulin-like growth factor I system in human non-small-cell lung cancer and normal lung cell lines. Int J Cancer. 1994;56:858–866. doi: 10.1002/ijc.2910560618. [DOI] [PubMed] [Google Scholar]
- 12.Toropainen E, Lipponen P, Syrjanen K. Expression of insulin-like growth factor I (IGF-I) in female breast cancer as related to established prognostic factors and long-term prognosis. Eur J Cancer. 1995;31A:1443–1448. doi: 10.1016/0959-8049(94)00466-i. [DOI] [PubMed] [Google Scholar]
- 13.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 14.Kiess W, Yang Y, Kessler U, Hoeflich A. Insulin-like growth factor II (IGF-II) and the IGF-II/mannose-6-phosphate receptor: the myth continues. Horm Res. 1994;41(Suppl 2):66–73. doi: 10.1159/000183963. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Cui H, Sandstedt B, Nordlinder H, Larsson E, Ekstrom TJ. Expression levels of the insulin-like growth factor-II gene (IGF2) in the human liver: developmental relationships of the four promoters. J Endocrinol. 1996;149:117–124. doi: 10.1677/joe.0.1490117. [DOI] [PubMed] [Google Scholar]
- 16.Christofori G, Naik P, Hanahan D. A second signal supplied by insulinlike growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 17.Cariani E, Lasserre C, Seurin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res. 1988;48:6844–6849. [PubMed] [Google Scholar]
- 18.Schirmacher P, Held WA, Yang D, Chisari FV, Rustum Y, Rogler CE. Reactivation of insulin-like growth factor II during hepatocarcinogenesis in transgenic mice suggests a role in malignant growth. Cancer Res. 1992;52:2549–2556. [PubMed] [Google Scholar]
- 19.Yang DY, Rogler CE. Analysis of insulin-like growth factor II (IGF-II) expression in neoplastic nodules and hepatocellular carcinomas of woodchucks utilizing in situ hybridization and immunocytochemistry. Carcinogenesis. 1991;12:1893–1901. doi: 10.1093/carcin/12.10.1893. [DOI] [PubMed] [Google Scholar]
- 20.Liu P, Terradillos O, Renard CA, Feldmann G, Buendia MA, Bernuau D. Hepatocarcinogenesis in woodchuck hepatitis virus/c-myc mice: sustained cell proliferation and biphasic activation of insulin-like growth factor II. Hepatology. 1997;25:874–883. doi: 10.1002/hep.510250415. [DOI] [PubMed] [Google Scholar]
- 21.Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher JA, Varmus HE. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol. 2005;25:1228–1237. doi: 10.1128/MCB.25.4.1228-1237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Nong Z, Ekstrom C, Larsson E, Nordlinder H, Hofmann WJ, Trautwein C, Odenthal M, Dienes HP, Ekstrom TJ, et al. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997;57:2048–2054. [PubMed] [Google Scholar]
- 23.Sohda T, Yun K, Iwata K, Soejima H, Okumura M. Increased expression of insulin-like growth factor 2 in hepatocellular carcinoma is primarily regulated at the transcriptional level. Lab Invest. 1996;75:307–311. [PubMed] [Google Scholar]
- 24.Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T, Taniyama M, Nakamura S, Uemura M, Takuma Y, et al. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2002;176:149–158. doi: 10.1016/s0304-3835(01)00736-4. [DOI] [PubMed] [Google Scholar]
- 25.Piao Z, Choi Y, Park C, Lee WJ, Park JH, Kim H. Deletion of the M6P/IGF2r gene in primary hepatocellular carcinoma. Cancer Lett. 1997;120:39–43. doi: 10.1016/s0304-3835(97)00289-9. [DOI] [PubMed] [Google Scholar]
- 26.De Souza AT, Hankins GR, Washington MK, Fine RL, Orton TC, Jirtle RL. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725–1729. [PubMed] [Google Scholar]
- 27.De Souza AT, Hankins GR, Washington MK, Orton TC, Jirtle RL. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat Genet. 1995;11:447–449. doi: 10.1038/ng1295-447. [DOI] [PubMed] [Google Scholar]
- 28.Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, Rosenfeld R, Neely K, Hintz R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem. 1994;269:13779–13784. [PubMed] [Google Scholar]
- 29.Dong ZZ, Yao DF, Yao DB, Wu XH, Wu W, Qiu LW, Jiang DR, Zhu JH, Meng XY. Expression and alteration of insulin-like growth factor II—messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655–4660. doi: 10.3748/wjg.v11.i30.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YW, Paliwal S, Draheim K, Grossman SR, Lewis BC. p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res. 2008;68:476–482. doi: 10.1158/0008-5472.CAN-07-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YW, Klimstra DS, Mongeau ME, Tatem JL, Boyartchuk V, Lewis BC. Loss of p53 and Ink4a/Arf cooperate in a cell autonomous fashion to induce metastasis of hepatocellular carcinoma cells. Cancer Res. 2007;67:7589–7596. doi: 10.1158/0008-5472.CAN-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrizas M, Gazit A, Levitzki A, Wertheimer E, LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- 33.Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6:705–713. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Gibson SL, Byrne MA, Zhang J, White MF, Shaw LM. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol Cell Biol. 2006;26:9338–9351. doi: 10.1128/MCB.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagle JA, Ma Z, Byrne MA, White MF, Shaw LM. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol. 2004;24:9726–9735. doi: 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 37.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–R721. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Grzmil M, Hemmerlein B, Thelen P, Schweyer S, Burfeind P. Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Pathol. 2004;202:50–59. doi: 10.1002/path.1492. [DOI] [PubMed] [Google Scholar]
- 39.Long L, Navab R, Brodt P. Regulation of the Mr 72,000 type IV collagenase by the type I insulin-like growth factor receptor. Cancer Res. 1998;58:3243–3247. [PubMed] [Google Scholar]
- 40.Yoon A, Hurta RA. Insulin like growth factor-1 selectively regulates the expression of matrix metalloproteinase-2 in malignant H-ras transformed cells. Mol Cell Biochem. 2001;223:1–6. doi: 10.1023/a:1017549222677. [DOI] [PubMed] [Google Scholar]
- 41.Nussbaum T, Samarin J, Ehemann V, Bissinger M, Ryschich E, Khamidjanov A, Yu X, Gretz N, Schirmacher P, Breuhahn K, et al. Autocrine insulin-like growth factor-II stimulation of tumor cell migration is a progression step in human hepatocarcinogenesis. Hepatology. 2008;25:3787–3800. doi: 10.1002/hep.22297. [DOI] [PubMed] [Google Scholar]
- 42.Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, Baserga R, Barrett JC. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58:3353–3361. [PubMed] [Google Scholar]
- 43.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 44.Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–237. doi: 10.1016/j.cell.2007.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.