Abstract
Trichomonas vaginalis commonly causes vaginitis and perhaps cervicitis in women and urethritis in men and women. Macrophages are important immune cells in response to T. vaginalis infection. In this study, we investigated whether human macrophages could be involved in inflammation induced by T. vaginalis. Human monocyte-derived macrophages (HMDM) were co-cultured with T. vaginalis. Live, opsonized-live trichomonads, and T. vaginalis lysates increased proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 by HMDM. The involvement of nuclear factor (NF)-κB signaling pathway in cytokine production induced by T. vaginalis was confirmed by phosphorylation and nuclear translocation of p65 NF-κB. In addition, stimulation with live T. vaginalis induced marked augmentation of nitric oxide (NO) production and expression of inducible NO synthase (iNOS) levels in HMDM. However, trichomonad-induced NF-κB activation and TNF-α production in macrophages were significantly inhibited by inhibition of iNOS levels with L-NMMA (NO synthase inhibitor). Moreover, pretreatment with NF-κB inhibitors (PDTC or Bay11-7082) caused human macrophages to produce less TNF-α. These results suggest that T. vaginalis stimulates human macrophages to produce proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, and NO. In particular, we showed that T. vaginalis induced TNF-α production in macrophages through NO-dependent activation of NF-κB, which might be closely involved in inflammation caused by T. vaginalis.
Keywords: Trichomonas vaginalis, human monocyte-derived macrophage, proinflammatory cytokine, nitric oxide, iNOS, NF-κB
INTRODUCTION
Trichomonas vaginalis is a protozoan parasite that infects the urogenital tract of humans. It is one of the most common causes of non-viral sexually transmitted diseases in the world [1]. T. vaginalis infection typically elicits aggressive local cellular immune responses with inflammation of the vaginal epithelium and exocervix in women and the urethra of men [2]. This inflammatory response induces the recruitment of leukocytes, including HIV target cells, such as CD4+-bearing lymphocytes and macrophages to which HIV can bind and gain access [3-5].
However, few studies have reported how macrophages showed immune responses against T. vaginalis infection. Our previous experiments showed that natural cell-mediated cytotoxicity against T. vaginalis is mediated by macrophages in vitro, cytotoxicity by lymphokine-activated peritoneal macrophages increased more than non-activated peritoneal macrophages, and their nitric oxide (NO) plays an important role in the host defense against T. vaginalis [6-9].
Macrophages are expected to come to infection loci later than the first attacker, neutrophils, against extracellular parasites, including T. vaginalis. During the infection progress, a limited number of immune cells could not defend against proliferating trichomonads. Macrophages have been presumed to attack trichomonads remaining in the infection loci and to be involved in inflammatory reactions. However, it was not previously known whether macrophages have an influence on vaginal inflammation.
We suggest that human macrophages may be involved in vaginal inflammation by T. vaginalis via proinflammatory cytokine and NO production. In addition, trichomonad-triggered proinflammatory TNF-α production might be regulated by NO. In this study, we examined proinflammatory cytokine and NO production by human macrophages stimulated with T. vaginalis.
MATERIALS AND METHODS
Reagents
Histopaque 1077 and PDTC were purchased from Sigma (St. Louis, Missouri, USA). Dextran T500 was supplied by Pharmacia (Uppsala, Sweden). Fetal calf serum (FCS), horse serum, and Iscove's DMEM (IMDM) were bought from Gibco BRL (Gaithersburg, Maryland, USA). LPS was from List Biological Laboratories (Campbell, California, USA). Goat anti-mouse IgG and Cy3-conjugated anti-mouse antibodies (Ab) were from Jackson ImmunoResearch (Baltimore, Maryland, USA). M-CSF (recombinant human macrophage colony stimulating factor) (BioSource, Camarillo, California, USA), L-NMMA (NG-Monomethyl-L-arginine monoacetate salt) (Calbiochem, San Diego, California, USA), Bay11-7082 (BioMol, Plymouth Meeting, Pennsylvania, USA), rabbit anti-phospho NF-κB p65 Ab (Cell Signaling, Danvers, Massachusetts, USA), mouse anti-phospho NF-κB p65 Ab, mouse anti-inducible NO synthase (iNOS) Ab (Santa Cruz, California, USA), and Vectashield mounting medium with DAPI (Vecta Labs, Burlingame, California, USA) were used.
T. vaginalis culture and trichomonal lysate preparation
T. vaginalis isolate (T016) was grown in a complex trypticase-yeast extract-maltose (TYM) medium supplemented with 10% heat-inactivated horse serum. T. vaginalis lysates were prepared by harvesting during the log phase of T. vaginalis growth, sonicating in 0.1 M PBS, and centrifuging at 10,000 g for 1 hr at 4℃. The resulting supernatants were passed through a 0.22 µm filter [10]. To make opsonized trichomonads, live T. vaginalis was treated with 5% human plasma for 1 hr.
Human monocytes-derived macrophages (HMDM)
Human polymorphonuclear leucocytes (PMNs) and monocytes were isolated from peripheral venous blood drawn from healthy volunteers as previously described [10]. Briefly, mononuclear cells were separated by centrifugation on a Histopaque 1077 density gradient and plated at 5 × 106 cells/ml IMDM supplemented with 10% human and M-CSF (10 ng/ml). Non-adherent cells were removed after 2 hr, and macrophages were cultured for 3 days in IMDM containing 10% human plasma and M-CSF (10 ng/ml). The medium was changed every 3 days. For all experiments, cells were cultured for 6-8 days, and then used as HMDM. Cell viability was determined using the trypan blue exclusion test (> 99%). Monocytes were detached by scraping with a rubber policeman, and their purity (~95%) was examined by phenotypic analysis using flow cytometry (FACSCalibur, Becton Dickinson, San Jose, California, USA) after staining with fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 Ab.
Cytokine production by macrophages stimulated with T. vaginalis
Human macrophages were reacted with T. vaginalis (live trchomonads, lysate, opsonized trichomonads) for 6 hr or 24 hr. The culture supernatants were centrifuged to remove particle debris and stored in aliquots at -70℃. Cytokine concentrations of TNF-α, IL-6, and IL-1β in the culture supernatants were determined by ELISA using a BD TNF-α, IL-6, and IL-1β OptEIA set (BD Biosciences, San Diego, California, USA) according to the instructions provided by the manufacturers.
Western blot analysis for pNF-κB p65 and iNOS
After human macrophages were coincubated with live trichomonads (macrophages : trichomonads = 5 : 1) for 1 hr (iNOS) or 2 hr (NF-κB), cells (2 × 106) were lysed with ice-cold lysis buffer, i.e., 20 mM Tris-HCl (pH 7.5), 60 mM β-glycerophosphate, 10 mM EDTA, 10 mM MgCl2, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM APMSF, 1% NP-40, leupeptin (5 µg/ml), 2 mM levamisol, pepstatin A (10 µg/ml), 0.5 mM benzamidine, and 1 tablet of complete Mini® (protease inhibitor cocktail) (Roche, Indianapolis, Illinois, USA). Lysed samples were mixed with SDS-PAGE sample buffer and boiled at 100℃ for 5 min. The total cellular proteins were subjected to 15% SDS polyacrylamide gel and electrophoretically transferred to a PVDF membrane. The blots were incubated with rabbit anti-phospho NF-κB p65 monoclonal Ab (1 : 100) or mouse anti-iNOS monoclonal Ab (1 : 100) as a primary Ab, and anti-rabbit horseradish peroxidase-conjugated IgG or anti-mouse horseradish peroxidase-conjugated IgG (Amersham Pharmacia, Uppsala, Sweden) as a secondary Ab. The protein band detection was performed with ECL plus kits as described by the manufacturer (Amersham Pharmacia).
Immunofluorescent staining of NF-κB p65
The localization and translocation of NF-κB p65 in HMDM grown on coverslips was determined by immunofluorescence assay. After coincubation of HMDM and live trichomonads for 30 min, macrophages were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 10 min. Coverslips were then incubated overnight at room temperature in mouse anti-phospho NF-κB p65 monoclonal Ab (1 : 50). Cells were then washed and incubated in Cy3-conjugated anti-mouse Ab for 90 min. The cover slips were mounted in anti-fade mounting medium with DAPI to visualize the nucleus. Fluorescence was measured using the fluorescence microscope (Nikon, Tokyo, Japan).
Transient transfection and luciferase reporter gene assays
THP-1 cells cultured in RPMI medium in the presence of 10% FBS were induced to become an adherent, matured macrophage-like phenotype by the addition of 10 ng/ml phorbol 12-myristate 13-acetate (PMA) to the culture for 72 hr. PMA-treated adherent THP-1 cells were washed 3 times and cultured in fresh medium.
The PCH110 and PGL3 NF-κB promoter constructs were transfected into THP-1 cells with LipofectAmine 2000 (Invitrogen, Carlsbad, California, USA). Luciferase activity was assayed with a luciferase assay kit (Promega, Madison, Wisconsin, USA). Cell extracts obtained from THP-1 stimulated with live trichomonads for 1 hr were prepared in 500 µl of 1 × reporter lysis buffer. Lysates were centrifuged at 13,000 g for 5 min and supernatants were used to measure luciferase activity with a Luminometer (Turner Biosystems, Sunnyvale, California, USA).
Nitrite production
The concentration of NO in culture supernatants was determined as nitrite using Griess reagent as described previously [9].
Detection of TNF-α mRNA by RT-PCR
After HMDM coincubated with live T. vaginalis for 30 min, mRNA expressions of TNF-α was measured by RT-PCR [11]. Total RNA was extracted from cells using Trizol reagent (Invitrogen) according to the method described previously [11]. Primer sequences and PCR conditions used for amplification of β-actin and TNF-α were as follows: β-actin (sense) 5'-CCAGAGCAAGAGAGGTATCC-3', (antisense) 5'-CTGTGGTGGTGAAGCTGTAG-3'; PCR conditions of initial DNA denaturation at 95℃ for 10 min and 30 rounds of denaturation (95℃ for 45 sec), annealing (58℃ for 2.5 min), and extension (72℃ for 1 min). TNF-α (sense) 5'-ACT CTT CTG CCT GCT GCA CTT TGG-3', (antisense) 5'-GTT GAC CTT TGT CTG GTA GGA GAC GG-3'; PCR conditions of initial DNA denaturation at 95℃ for 2 min and 31 rounds of denaturation (94℃ for 30 sec), annealing (55℃ for 30 sec), and extension (72℃ for 1 min). The PCR products were electrophoresed in 2% agarose gel containing 0.5 µg/ml ethidium bromide and photographed under ultraviolet light. The band intensity was quantified using the Quantity One program (BioRad, Hercules, California, USA) [12].
Pretreatment of macrophages with pharmacological inhibitors
The HMDM and THP-1 cells were pretreated with NO synthase inhibitor (L-NMMA) or NF-κB inhibitors (PDTC, Bay11-7082) for 30 min or 60 min, and then reacted with live trichomonads. Expressions of TNF-α of the both macrophages were measured by ELISA and/or RT-PCR.
Statistical analysis
Data are expressed as means±SEM of 3-4 independent experiments and were analyzed with Mann-Whitney U-test. Significant difference was accepted at a P-value of < 0.05.
RESULTS
When T. vaginalis (T. vaginalis lysate, opsonized trichomonads, or live trichomonads) was coincubated with human monocyte-derived macrophage monolayer (HMDM) for 6 hr or 24 hr, TNF-α, 1L-1β, and IL-6 showed increased levels compared with untreated macrophages. The cytokine amount was generally increased with each trichomonads in dose- and time-dependent manner. The TNF-α concentration at 6 hr of coincubation showed increased levels than those of 24 hr. The live and opsonized trichomonads strongly stimulated HMDM to produce higher level of cytokines than T. vaginalis lysate. IL-6 showed increased levels regardless of the incubation time (Fig. 1).
Fig. 1.
Trichomonas vaginalis stimulates human monocyte-derived macrophages (HMDM) to produce proinflammatory cytokines. Human macrophages (1.25 × 106/well of 96-well culture plate) were incubated with live trichomonads (1, 5, and 10 × 104), opsonized trichomonads (1, 5, and 10 × 104) and T. vaginalis lysate (10 and 100 µg/ml). Cell culture supernatant was harvested after 6 hr and 24 hr of incubation, and analyzed for levels of TNF-α, IL-1β, and IL-6 using cytokine-specific ELISA. *P < 0.05 versus untreated macrophages.
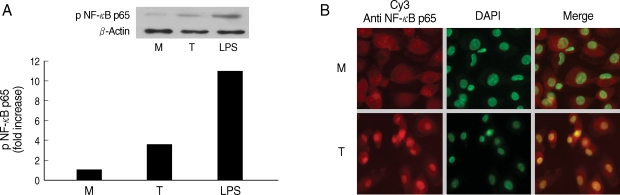
Next, we examined involvement of NF-κB in the cytokine production by HMDM. When human macrophages were stimulated with live T. vaginalis, increased phospho p65 NF-κB activity, and translocation of NF-κB p65 into nucleus were observed by western blot and fluorescent microscopy, respectively (Fig. 2).
Fig. 2.
NF-κB activation induced by Trichomonas vaginalis (T) in human macrophages (M). (A) Phosphorylation of NF-κB p65 expression in human macrophages reacted with T. vaginalis was determined by western blot. (B) Nuclear translocation of NF-κB p65 in human macrophages stimulated with T. vaginalis (low) compared with medium alone (up). Human macrophages (5 × 106/cover glass) were incubated with live T. vaginalis (1 × 106) for 30 min. Cells were then fixed and subjected to immunofluorescent microscopy after staining with anti-NF-κB p65 Ab (Red) and DAPI (Green) to visualize nuclei.
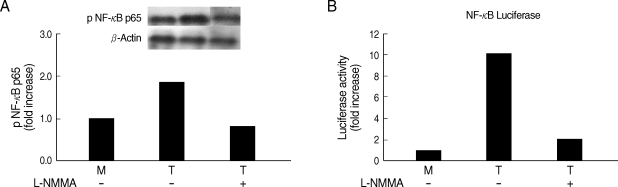
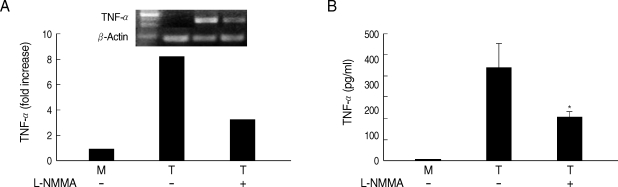
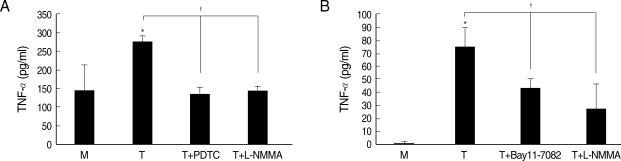
Whether NO is involved in cytokine production by HMDM stimulated with trichomonads, NO levels were measured by the Griess reagent. After 24 hr of coincubation, T. vaginalis, particularly live trichomonads, caused macrophages to produce significantly increased levels of NO. The iNOS expression of HMDM activated with live trichomonads was also increased 6 times more than untreated macrophages, and showed decreased levels by pretreatment with NO synthase inhibitor, L-NMMA by western blot (Fig. 3). Then, we investigated whether NO could influence NF-κB activity. When L-NMMA, NO synthase inhibitor-pretreated HMDM was coincubated with live trichomonads, decreased phospho NF-κB p65 activities, compared with untreated macrophages, were observed in HMDM or THP-1 by western blot and NF-κB luciferase assay, respectively (Fig. 4). Also, NO synthase inhibitor was expected to induce decreased production of cytokines by macrophages. TNF-α mRNA and protein expressions were significantly decreased by pretreatment with L-NMMA compared to the untreated group, examined by RT-PCR and ELISA, respectively (Fig. 5). Therefore, TNF-α production might be regulated by NO. Human macrophages (THP-1 and HMDM) showed decreased production of TNF-α on stimulation of live T. vaginalis by pretreatment with NF-κB inhibitor, such as PDTC or Bay11-7082, or NO synthase inhibitor, L-NMMA (Fig. 6).
Fig. 3.
NO production (A) and iNOS expression (B) by HMDM stimulated with T. vaginalis (T). Human macrophages (1.25 × 106/well of 96-well culture plate) were incubated with live trichomonads (1.25 × 105), opsonized trichomonads (1.25 × 105), or T. vaginalis lysate (100 µg/ml). NO was determined by the Griess reagent after 1 hr, 4 hr, and 24 hr of incubation (A). For identification of iNOS expression, human macrophages (1.25 × 106/well) were incubated with live trichomonads (1.25 × 105) for 1 hr. The iNOS expression was examined by western blot with anti-iNOS Ab (B). *P < 0.05 versus untreated macrophages.
Fig. 4.
Effects of L-NMMA (an iNOS inhibitor) on NF-κB activity of human macrophages induced by T. vaginalis. L-NMMA-preteated HMDM or THP-1 cells was stimulated with live T. vaginalis. NF-κB activities were determined by western blot (A) or luciferase assay (B). Human macrophages were coincubated with live trichomonads (macrophage : trichomonads = 5 : 1) for 2 hr, and then western blot was done with rabbit anti-phospho NF-κB p65 monoclonal Ab (A). The PCH110 and PGL3 NF-κB promoter constructs were transfected into THP-1 cells with LipofectAmine 2000. Luciferase activity was assayed with a luciferase assay kit. Cell extracts obtained from THP-1 cells stimulated with live trichomonads for 1 hr were prepared in 500 µl of 1 × reporter lysis buffer. The luciferase activity was measured with a Luminometer (B).
Fig. 5.
Effects of L-NMMA on TNF-α production in HMDM cells induced by live T. vaginalis. L-NMMA-pretreated macrophage suppressed T. vaginalis-triggered TNF-α expression, measured by RT-PCR (A) and ELISA (B), respectively. *P < 0.05 versus macrophages stimulated with T. vaginalis.
Fig. 6.
Effects of iNOS inhibitor or NF-κB inhibitor on TNF-α production in macrophages induced by T. vaginalis. Pretreatment of human macrophages (THP-1 or HMDM) with L-NMMA or NF-κB inhibitor (PDTC or Bay11-7082) showed significant reduction of trichomonad-induced TNF-α production in THP-1 (A) and HMDM (B). Protein levels of TNF-α were measured by ELISA. *P < 0.05 versus untreated macrophages. †P < 0.05 versus T. vaginalis-stimulated macrophages.
DISCUSSION
In this study, human macrophages were shown to be involved in vaginal inflammation by T. vaginalis via proinflammatory cytokine and NO production. In addition, TNF-α induced by live trichomonads in macrophages might be regulated through NO-dependent activation of NF-κB. Our results are consistent with previous reports in which macrophages infected with various protozoan parasites, such as Trypanosoma congolense, Eimeria bovis, Leishmania chagasi, and Leishmania donovani, showed increased expressions of many inflammatory mediators, such as cytokines, chemokines, and NO [13-16].
NO is gaseous water and lipid-soluble molecule that is one of the most widespread signaling molecules in mammals. NO is implicated in modulating a variety of physiological reactions, including vasodilation and smooth muscle relaxation associated with the circulation, functioning as a neurotransmitter in various neural processes, and regulation of immunological defense mechanisms [17]. The role of NO in inflammation and inflammatory responses remains unclear [18]. However, it is known that NO production in human macrophages differs from the rodent macrophages and the regulation of macrophage NO in humans appears to be a more selective and variable process than that seen in the rodent macrophages [19].
Human macrophage in the study showed increased levels of NO by stimulation with T. vaginalis after 24 hr of coincubation. The iNOS expression in HMDM by T. vaginalis was also about 6 times higher than the control group, however, trichomonad-induced iNOS levels were inhibited by pretreatment with NO synthase inhibitor, L-NMMA, suggesting that NO plays a significant role in immune responses by T. vaginalis.
It is widely known that NF-κB is normally present in the cytosol and exists as an inactive complex with a class of inhibitory proteins, known as inhibitor κB (IκB) proteins. Following an inflammatory stimulus, the phosphorylation of IκB triggers its degradation and the translocation of NF-κB to the nucleus, where it binds to promoter regions and induces the expression of a wide variety of genes involved in inflammation, including those encoding cytokines (such as IL-1, IL-6, and TNF-α), enzymes (including NO synthase), adhesion molecules, and acute-phase proteins [20]. Expectedly, in this study, we have demonstrated similar results that involvement of NF-κB activation in the production of cytokines, such as TNF-α, induced by T. vaginalis stimulation was identified by nuclear translocation and phosphorylation of NF-κB p65 in human macrophages (Fig. 2).
NO production is generally upregulated by some stimuli (including LPS) known to induce iNOS gene expression via a NF-κB-mediated pathway [21]. We found that human macrophages pretreated with an iNOS inhibitor, L-NMMA, showed decreased NF-κB activity and TNF-α production triggered by T. vaginalis (Figs. 2-5), although the inhibitory effects of NF-κB inhibitor on iNOS protein expression or NO production induced by T. vaginalis was not checked in this study. Therefore, it is speculated that decreased iNOS levels with L-NMMA may occur through suppressed NF-κB activities.
On the other hand, few reports have been shown about the cytokine production regulated by NO; NO production activates the release of cytokines, cytokine receptors, and adhesion molecules [22-24]. The effect of NO on expression of TNF-α is reported to be likely via activation of a TNF-α-converting enzyme (TACE), which is responsible for membrane-bound TNF and thereby activating release of this cytokine [22]. TNF-α production caused by T. vaginalis in the study was significantly decreased by pretreatment with L-NMMA in human macrophages, suggesting an important role of NO in T. vaginalis-induced TNF-α production. Our result is in line with a previous report by Huang et al. [25] in that NO inhibitors decreased TNF-α synthesis by an LPS-activated J774 macrophage cell line. In this study, we also found that L-NMMA-pretreated macrophages reduced NF-κB activity. Therefore, it is strongly suggested that T. vaginalis stimulates human macrophages to produce TNF-α through NO-dependent activation of NF-κB.
Neutrophil migration is a complex process, which results mainly from the release of neutrophil chemotactic factors by resident cells. Apart from its importance in host defense, the migration of neutrophils to the inflammatory site is, at least in part, responsible for tissue damage observed in several inflammatory diseases, such as rheumatoid arthritis, glomerulonephritis, and inflammatory bowel disease [26,27]. In trichomoniasis, neutrophils are known to be the predominant inflammatory cells found in the vaginal discharge of the patients. We previously indicated that T. vaginalis-induced recruitment of neutrophils may be mediated via CXC chemokine IL-8 [28]. IL-1β, TNF-α, C5a, and lipid mediators, such as platelet-activating factor 4 and leukotriene (LTB4), are known the main chemotactic mediators involved in neutrophil recruitment to the sites of inflammation [29]. Therefore, IL-1β and TNF-α produced by macrophages stimulated with T. vaginalis may play a crucial role in vivo as mediators of neutrophilic tissue recruitment at the infected sites in vaginal trichomoniasis in women.
Our results showed that T. vaginalis caused human monocyte-derived macrophage (HMDM) to produce proinflammatory cytokines, such as IL-1, IL-6, TNF-α, and NO, via NF-κB. It is suggested that human macrophages may be involved in inflammation caused by T. vaginalis via the cytokine and NO production.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-E00019) and Anti-Communicable Diseases Control Program of the National Institute of Health (NIH-348-6111-215), Ministry of Health and Welfare, Republic of Korea.
References
- 1.World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 2.Shafir SC, Sorvillo FJ, Smith L. Current issues and considerations regarding trichomoniasis and human immunodeficiency virus in African-Americans. Clin Microbiol Rev. 2009;22:37–45. doi: 10.1128/CMR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiviat NB, Paavonen JA, Brockway J, Critchlow CW, Brunham RC, Stevens CE, Stamm WE, Kuo CC, DeRouen T, Holmes KK. Cytological manifestations of cervical and vaginal infections. I. Epithelial and inflammatory cellular changes. JAMA. 1985;253:989–996. [PubMed] [Google Scholar]
- 4.Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, Farshy CE, Mitchell S, Talkington DF. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–174. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 5.Sardana S, Sodhani P, Agarwal SS, Sehgal A, Roy M, Singh V, Bhatnagar P, Murthy NS. Epidemiologic analysis of Trichomonas vaginalis infection in inflammatory smears. Acta Cytol. 1994;38:693–697. [PubMed] [Google Scholar]
- 6.Landolfo S, Martinotti MG, Martinetto P, Forni G. Natural cell-mediated cytotoxicity against Trichomonas vaginalis in the mouse. I. Tissue, strain, age distribution, and some characteristics of the effector cells. J Immunol. 1980;124:508–514. [PubMed] [Google Scholar]
- 7.Ryu JS, Ahn MH, Min DY. Cytotoxicity of resident and lymphokine-activated mouse peritoneal macrophage against Trichomonas vagianalis. Korean J Parasitol. 1990;28:85–89. doi: 10.3347/kjp.1990.28.2.85. [DOI] [PubMed] [Google Scholar]
- 8.Yoon K, Ryu JS, Min DY. Cytotoxicity of lymphokine activated peritoneal macrophages against Trichomonas vaginalis. Korean J Parasitol. 1991;29:381–388. doi: 10.3347/kjp.1991.29.4.381. [DOI] [PubMed] [Google Scholar]
- 9.Park GC, Ryu JS, Min DY. The role of nitric oxide as an effector of macrophage-mediated cytotoxicity against Trichomonas vaginalis. Korean J Parasitol. 1997;35:189–195. doi: 10.3347/kjp.1997.35.3.189. [DOI] [PubMed] [Google Scholar]
- 10.Ahn MH, Song HO, Ryu JS. Trichomonas vaginalis-induced neutrophil apoptosis causes anti-inflammatory cytokine production by human monocyte-derived macrophages. Parasite Immunol. 2008;30:410–416. doi: 10.1111/j.1365-3024.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Heo YM, Choi JH, Song HO, Ryu JS, Ahn MH. Suppressed production of pro-inflammatory cytokines by LPS-activated macrophages after treatment with Toxoplasma gondii lysate. Korean J Parasitol. 2008;46:145–151. doi: 10.3347/kjp.2008.46.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YS, Song HO, Choi IH, Park SJ, Ryu JS. Hydrogenosomal activity of Trichomonas vaginalis cultivated under different iron conditions. Korean J Parasitol. 2006;44:373–378. doi: 10.3347/kjp.2006.44.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Allie N, Jacobs M, Nedospasov S, Brombacher F, Ryffel B, De Baetselier P. Tumor necrosis factor (TNF) receptor-1 (TNFp55) signal transduction and macrophage-derived soluble TNF are crucial for nitric oxide-mediated Trypanosoma congolense parasite killing. J Infect Dis. 2007;196:954–962. doi: 10.1086/520815. [DOI] [PubMed] [Google Scholar]
- 14.Taubert A, Behrendt JH, Sühwold A, Zahner H, Hermosilla C. Monocyte-and macrophage-mediated immune reactions against Eimeria bovis. Vet Parasitol. 2009 doi: 10.1016/j.vetpar.2009.06.003. doi:10.1016/j.vetpar.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Gantt KR, Goldman TL, McCormick ML, Miller MA, Jeronimo SMB, Nascimento ET, Britigan BE, Wilson ME. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunology. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta B, Roychoudhury K, Ganguly S, Akbar MA, Das P, Roy S. Infection of human mononuclear phagocytes and macrophage-like THP1 cells with Leishmania donovani results in modulation of expression of a subset of chemokines and a chemokine receptor. Scand J Immunol. 2003;57:366–374. doi: 10.1046/j.1365-3083.2003.01227.x. [DOI] [PubMed] [Google Scholar]
- 17.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 18.Rogers JA, Fuseler JW. Regulation of NF-κB activation and nuclear translocation by exogenous nitric oxide (NO) donors in TNF-α activated vascular endothelial cells. Nitric Oxide. 2007;16:379–391. doi: 10.1016/j.niox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Thomassen MJ, Kavuru MS. Human alveolar macrophages and monocytes as a source and target for nitric oxide. Int Immunopharmacol. 2001;1:1479–1490. doi: 10.1016/s1567-5769(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 20.Jung WK, Choi IH, Lee DY, Yea SS, Choi YH, Kim MM, Park SG, Seo SK, Lee SW, Lee CM, Park YM, Choi IW. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-κB pathways. Int J Biochem Cell Biol. 2008;40:2572–2582. doi: 10.1016/j.biocel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 22.Zhang Z, Kolls JK, Oliver P, Good D, Schwarzenberger PO, Joshi MS, Ponthier JL, Lancaster JRJr. Activation of tumor necrosis factor-α-converting enzyme-mediated ectodomain shedding by nitric oxide. J Biol Chem. 2000;275:15839–15844. doi: 10.1074/jbc.M000604200. [DOI] [PubMed] [Google Scholar]
- 23.Stevanin TM, Laver JR, Poole RK, Moir JWB, Read RC. Metabolism of nitric oxide by Neisseria meningitidis modifies release of NO-regulated cytokines and chemokines by human macrophages. Microbes Infect. 2007;9:981–987. doi: 10.1016/j.micinf.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Wang S, Rafferty SP, Wesley RA, Danner RL. Endogenously produced nitric oxide increases tumor necrosis factor-α production in transfected human U937 cells. Blood. 1997;90:1160–1167. [PubMed] [Google Scholar]
- 25.Huang FP, Niedbala W, Wei XQ, Xu D, Feng GJ, Robinson JH, Lam C, Liew FY. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062–4070. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 27.Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka F, Maruyama Y, Matsushita I, Iwaoka Y, Saniabadi A. Correlation of serum soluble TNF-α receptors I and II levels with disease activity in patients with ulcerative colitis. Am J Gastroenterol. 2004;99:1532–1538. doi: 10.1111/j.1572-0241.2004.30432.x. [DOI] [PubMed] [Google Scholar]
- 28.Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of Interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–1332. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schröder JM. Chemoattractants as mediators of neutrophilic tissue recruitment. Clin Dermatol. 2000;18:245–263. doi: 10.1016/s0738-081x(99)00117-0. [DOI] [PubMed] [Google Scholar]