Abstract
Paragonimiasis typically results from the consumption of raw or improperly cooked crustacea, especially crabs and crayfish. Although previously endemic in Korea, the prevalence of this disease decreased in the early 1970s because of educational campaigns and fewer intermediate hosts as a result of ecological changes. Recently, we were presented with a family where all members were infected with Paragonimus after ingestion of Kejang (= drunken crab). The mother was hospitalized for general myalgia and weakness first, followed by the father, who was hospitalized for dyspnea 2 month later. After the parents were diagnosed with paragonimiasis, we recommended their daughter to visit our hospital for a checkup, because they all had eaten freshwater crabs soaked in soybean sauce. She complained of generalized myalgia, fever, and pleuritic pain, and was also diagnosed with paragonimiasis. Peripheral blood of the 3 patients revealed hypereosinophilia, and computed tomography (CT) scans of their chests showed pleural effusion. The results of antibody tests by ELISA were positive for paragonimiasis. We report here the case series of familial paragonimiasis in a modern urban city, rather than in a typical endemic area.
Keywords: Paragonimus, paragonimiasis, case report, eosinophilia, crab
INTRODUCTION
Human paragonimiasis results from eating raw freshwater crayfish or crabs containing metacercariae [1]. These metacercariae first enter the small intestine, and then pass through the intestinal wall, peritoneal cavity, diaphragm, and pleural cavity to the lung parenchyma, where they mature to adult flukes [1,2].
Paragonimiasis is common in certain areas of Asia (e.g., China, Korea, Japan, and Taiwan), where raw or undercooked freshwater crabs and crayfish are frequently consumed [2]. It also occurs in humans via ingestion of raw meat from wild boars or other paratenic hosts, particularly in Japan [2,3]. Recently, cases of paragonimiasis have been reported in almost every country in the world [4-7], because of increased numbers of immigrants [8] and overseas travelers, the popularization of ethnic dishes in developed countries [9], and the expansion of the international food trade [2,3].
Paragonimiais has been steadily decreasing in the Republic of Korea since the 1960s. An epidemiological survey in the 1990s revealed that the prevalence of paragonimiasis was about 1% of that seen in the early 1970s [10]. However, the disease remained a potential threat, as more than 10% of freshwater crabs sold in markets in Seoul were infected with the metacercariae of Paragonimus westermani in the early 1990s [11]. In fact, as Kejang (= drunken crab; a kind of Korean traditional food made with freshwater crabs soaked in soybean sauce) is expensive, it is much more sold in large cities rather than rural areas. Therefore, clinicians should include paragonimiasis in differential diagnoses of lung infiltration and peripheral blood eosinophilia even in metropolitan cities.
Unlike many cases of paragonimiasis worldwide, few literatures have reported the familial infection with paragonimiasis [12], even though many families continue to share meals of undercooked crabs. The purpose of this case report is to present the sequence of overt symptoms of paragonimiasis in a family who ate Kejang at the same time. Here, we report 3 cases of pulmonary paragonimiasis in a family residing in the metropolitan city of Seoul, Republic of Korea.
CASE REPORTS
Case 1
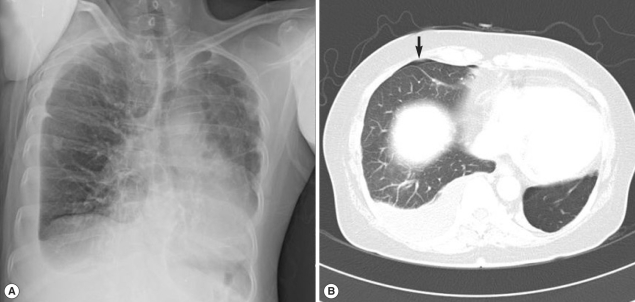
A 51-year-old woman visited our hospital to seek treatment for general myalgia and lower extremity weakness that had persisted for 1 month. She had previously undergone magnetic resonance imaging (MRI) examinations of her cervical and lumbar spines to determine the cause of her lower extremity weakness; however, the results provided no clear explanation. Her chief complaints had not been resolved by traditional rehabilitation therapy. She had eaten Kejang together with her family a few weeks before. Her past medical history and social history was uneventful. An auscultation examination revealed a normal cardiac sound, but decreased breathing in the right lower lung zone. Otherwise, a physical examination yielded no specific findings. A complete blood cell count (CBC) revealed WBC levels of 23,900/mm3, hemoglobin levels of 11.9 g/dl, platelet levels of 393,000/mm3, absolute eosinophil count (AEC) of 14,400/mm3, and erythrocyte sedimentation rate (ESR) of 74 mm/hr. The woman's chemistry battery, and electrolyte and urine analyses revealed no abnormal findings. A simple chest x-ray revealed right pleural effusion (Fig. 1A). Chest computed tomography (CT) revealed hydropneumothorax in the right hemithorax (Fig. 1B) and infiltrative lesions suggestive of parasitic infections in the liver and peritoneum. As the clinical findings were indicative of a parasitic infection, antibody test by ELISA and stool examination were performed. We could not observe parasite eggs in the stool specimen; however, the ELISA results indicated the presence of Paragonimus antibodies, even though the total serum IgE levels were 18.2 IU/ml (within normal range). The patient was treated with praziquantel (25 mg/kg, 3 times a day, for 2 days). A week after the medication, the patient's eosinophil count decreased from 14,400/mm3 to 4,250/mm3, her left pleural effusion was improved, and her chief complaints were resolved.
Fig. 1.
Simple chest x-ray and computed tomography (CT) findings in case 1. (A) Pleural effusion (in the right lung) with evident fluid shifting is recognized in a lateral decubitus view. (B) Ground-glass opacity in the subpleural portion of the right lung and right hydropneumothorax (arrow).
Case 2
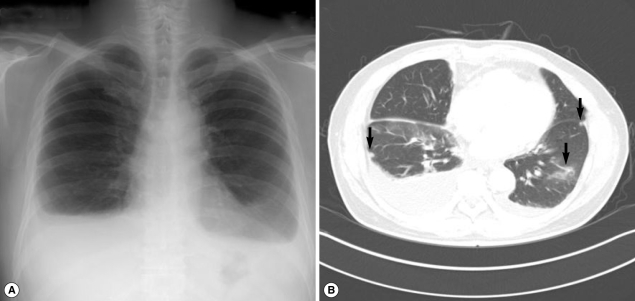
A 54-year-old man (the husband of case 1) visited our hospital to seek for treatment of sudden dyspnea accompanied by dry cough, 2 months after his wife had been treated for paragonimiasis. He had previously been healthy with no prior medical problems. However, his body weight decreased by 4 kg. He had eaten Kejang with her wife during the same period. On physical examination, his breathing sound was decreased in the lower lung zone. A CBC revealed WBC level of 21,800/mm3, hemoglobin level of 13.5 g/dl, platelet level of 258,000/mm3, and AEC of 13,580/mm3. Other laboratory tests revealed no abnormal findings. A simple chest x-ray revealed extensive pleural effusion in both hemithoraces (Fig. 2A). A chest CT also revealed extensive pleural effusion, as well as atelectasis and ground glass opacity in the lower lung and pleural nodules (Fig. 2B). According to the laboratory findings, image work-up, and recorded family history, stool examinations and ELISA tests were followed. As a result, Paragonimus eggs were observed in the stool sample, and ELISA test was positive for antibodies against Paragonimus westermani. The patient's total serum IgE level was 341 kU/L (cut-off level; below 100 kU/ml). On the basis of these findings, the patient was subsequently treated with praziquantel (25 mg/kg, 3 times daily for 2 days). The patient's symptoms of dyspnea were resolved after the treatment. A week after the medication, the patient's AEC decreased from 13,580/mm3 to 7,300/mm3, and an improvement in the left pleural effusion was observed.
Fig. 2.
Simple chest x-ray and CT findings in case 2. (A) Extensive pleural effusion is seen in both hemithoraces. (B) Atelectasis, ground-glass opacity in the lower lung and a few subpleural nodules (arrow) are observed in both lungs (arrow).
Case 3
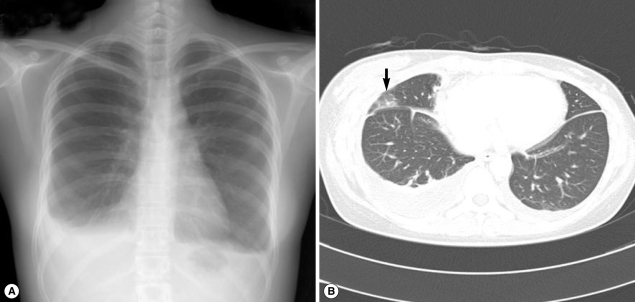
A 26-year-old woman (the daughter of cases 1 and 2) visited our hospital. The woman had previously been advised to undergo an examination because of her parents' diagnoses; however, because of time constraints, she was unable to visit our hospital until approximately 1 month after our initial recommendation. Recently, she had experienced general myalgia, vague night fever, and pleuritic pain. Approximately 4 to 5 days after her initial symptoms had developed, the woman noticed palpable subcutaneous nodules (5 to 10 cm in diameter) in her left flank. She said that she also had eaten Kejang with her parents. Auscultation revealed decreased breathing in the lower lung zone. A physical examination yielded no other specific findings. A CBC revealed WBC level of 28,000/mm3, hemoglobin level of 13.0 g/dl, platelet level of 313,000/mm3, AEC of 19,470/mm3, and ESR level of 90 mm/hr. Other laboratory tests revealed no abnormal findings. In simple chest x-ray, there was bilateral pleural effusion in both lung fields (Fig. 3A). In chest CT, bilateral pleural effusion with multiple patchy consolidations with focal bronchiectasis was observed (Fig. 3B). In abdominal CT, multiple low attenuated lesions in the liver and spleen were shown; they were suspected as eosinophilic abscesses caused by parasite infections. We performed stool examination and ELISA. We were unable to detect parasite eggs in the stool sample; however, ELISA results indicated the presence of Paragonimus and Clonorchis antibodies, and the total serum IgE level was 128 kU/L. The patient was also treated with praziquantel (25 mg/kg, 3 times daily for 2 days). All symptoms including pleuritic pain and subcutaneous nodules were resolved after the treatment, and the patient's AEC was decreased.
Fig. 3.
Simple chest x-ray and CT findings in case 3. (A) Bilateral pleural effusion with focal patchy areas of increased opacity is seen in the lower upper and middle lung. (B) Bilateral pleural effusion with multiple patchy consolidations is seen (arrow).
DISCUSSION
Although the incidence of paragonimiasis has steadily decreased in recent years owing to ecological changes, mass screening, and educational campaigns, this disease occasionally emerges as a public health problem. In Korea, Kejang was considered delicacies that often accompany bowls of rice [10]. Unfortunately, this traditional food is one of the primary sources of human paragonimiasis in Korea. Occasionally, patients diagnosed with paragonimiasis report that they had never eaten freshwater crabs or crayfish. It is also possible that they ate food contaminated with metacercariae from the fingers or cooking utensils of people who recently handled these crustaceans [3]. The metacercariae first enter the small intestine, and then pass through the intestinal wall, peritoneal cavity, diaphragm, and pleural cavity to the lung parenchyma, where they mature to adult flukes [1,2]. Thus, although the lungs are the primary site of paragonimiasis, ectopic infection can occur at unexpected locations within the body (e.g., the brain, subcutaneous tissue, muscle, omentum and mesentery, retroperitoneum, adrenal glands, ovary, epididymis, and liver) [1,3,13,14].
Paragonimiasis is diagnosed on the basis of eggs in the sputum or stool samples; however, this process is difficult and negative results are often unreliable, especially in the case of ectopic paragonimiasis. Therefore, immunological and serological methods have been developed to diagnose the disease [15]. These methods are relatively sensitive; however, crude worm extracts exhibit low specificity, with trematode cross-reactivity occurring in approximately 12.5% of samples [15-17]. Thus, ELISA is commonly used for the immunodiagnosis of paragonimiasis, as the overall sensitivity and specificity of the assay were reported to be 90.2% and 100%, respectively [1,3].
Peripheral blood eosinophilia and elevated serum total IgE levels are observed in approximately 80% of patients with paragonimiasis. However, patients often exhibit normal eosinophil counts when parenchymatous lesions are present. Total serum IgE levels, also an important indicator of a parasitic disease, unfortunately does not correlate with the clinical stage of paragonimiasis [3].
Among patients with pleuropulmonary paragonimiasis, the most common CT findings are pleural effusion, hydropneumothorax, pulmonary nodules or air-space consolidation, and occasionally cysts [18]. As rapid and reliable immunodiagnostic methods are available and treatment with praziquantel is quite successful, paragonimiasis should always be included in the differential diagnoses of lung lesions associated with eosinophilia or elevated IgE levels that do not respond to antibiotic therapy, for patients living even in non-endemic urban areas as well as endemic areas.
Paragonimiasis has been often considered a disease of the past or a disease limited to endemic regions. However, as paragonimiasis can be transmitted via incidental exposure even in large cities, educational and preventive campaigns are still warranted. We suggest that all family members should be evaluated for asymptomatic or pre-clinical stage of Paragonimus infection, even if there is documented infection only in 1 family member, because Paragonimus infection can occur from the same source of food in a household and family.
References
- 1.Choi DW. Paragonimus and paragonimiasis in Korea. Korean J Parasitol. 1990;28(suppl):79–102. doi: 10.3347/kjp.1990.28.suppl.79. [DOI] [PubMed] [Google Scholar]
- 2.Jeon K, Koh WJ, Kim H, Kwon OJ, Kim TS, Lee KS, Han J. Clinical features of recently diagnosed pulmonary paragonimiasis in Korea. Chest. 2005;128:1423–1430. doi: 10.1378/chest.128.3.1423. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura-Uchiyama F, Mukae H, Nawa Y. Paragonimiasis: a Japanese perspective. Clin Chest Med. 2002;23:409–420. doi: 10.1016/s0272-5231(01)00006-5. [DOI] [PubMed] [Google Scholar]
- 4.Vélez ID, Ortega JE, Velásquez LE. Paragonimiasis: a view from Columbia. Clin Chest Med. 2002;23:421–431. doi: 10.1016/s0272-5231(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 5.Lemos AC, Coelho JC, Matos ED, Montal G, Aguiar F, Badaró R. Paragonimiasis: first case reported in Brazil. Braz J Infect Dis. 2007;11:153–156. doi: 10.1590/s1413-86702007000100031. [DOI] [PubMed] [Google Scholar]
- 6.Procop GW, Marty AM, Scheck DN, Mease DR, Maw GM. North American paragonimiasis. A case report. Acta Cytol. 2000;44:75–80. doi: 10.1159/000326230. [DOI] [PubMed] [Google Scholar]
- 7.Boé DM, Schwarz MI. A 31-year-old man with chronic cough and hemoptysis. Chest. 2007;132:721–726. doi: 10.1378/chest.07-0712. [DOI] [PubMed] [Google Scholar]
- 8.Obara A, Nakamura-Uchiyama F, Hiromatsu K, Nawa Y. Paragonimiasis cases recently found among immigrants in Japan. Intern Med. 2004;43:388–392. doi: 10.2169/internalmedicine.43.388. [DOI] [PubMed] [Google Scholar]
- 9.Yumine K, Fujiwara H, Kamimori T, Tochino Y, Miki Y, Fujikawa T, Nakamura-Uchiyama F. Paragonimiasis westermani caused by ingestion of Chinese freshwater crabs. Nihon Kokyuki Gakkai Zasshi. 2003;41:186–190. [PubMed] [Google Scholar]
- 10.Cho SY, Kong Y, Kang SY. Epidemiology of paragonimiasis in korea. Southeast Asian J Trop Med Public Health. 1997;28(suppl 1):32–36. [PubMed] [Google Scholar]
- 11.Cho SY, Kang SY, Kong Y, Yang HJ. Metacercarial infections of Paragonimus westermani in freshwater crabs sold in markets in Seoul. Korean J Parasitol. 1991;29:189–191. doi: 10.3347/kjp.1991.29.2.189. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Choi YW, Kim SM, Lee TH, Im EH, Huh KC, Na MJ, Kang YW. Familial infestation of Paragonimus westermani with peritonitis and pleurisy. Korean J Gastroenterol. 2005;46:242–246. [PubMed] [Google Scholar]
- 13.Kim EA, Juhng SK, Kim HW, Kim GD, Lee YW, Cho HJ, Won JJ. Imaging findings of hepatic paragonimiasis: a case report. J Korean Med Sci. 2004;19:759–762. doi: 10.3346/jkms.2004.19.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Kang CM, Choi GH, Yang WI, Sim SB, Kwon JE, Kim KS, Choi JS, Lee WJ, Kim BR. Laparoscopic excision of intra-abdominal paragonimiasis. Surg Laparosc Endosc Percutan Tech. 2007;17:556–558. doi: 10.1097/SLE.0b013e31813738c7. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Lee J, Kim SH, Yong TS. Molecular cloning and characterization of a major egg antigen in Paragonimus westermani and its use in ELISA for the immunodiagnosis of paragonimiasis. Parasitol Res. 2007;100:677–681. doi: 10.1007/s00436-006-0324-7. [DOI] [PubMed] [Google Scholar]
- 16.Cho SY, Hong ST, Rho YH, Choi SY, Han YC. Application of micro-ELISA in serodiagnosis of human paragonimiasis. Korean J Parasitol. 1981;19:151–156. doi: 10.3347/kjp.1981.19.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Yong TS, Seo JH, Yeo IS. Serodiagnosis of human paragonimiasis by ELISA-inhibition test using monoclonal antibodies. Korean J Parasitol. 1993;31:141–147. doi: 10.3347/kjp.1993.31.2.141. [DOI] [PubMed] [Google Scholar]
- 18.Kim TS, Han J, Shim SS, Jeon K, Koh WJ, Lee I, Lee KS, Kwon OJ. Pleuropulmonary paragonimiasis: CT findings in 31 patients. AJR Am J Roentgenol. 2005;185:616–621. doi: 10.2214/ajr.185.3.01850616. [DOI] [PubMed] [Google Scholar]