Abstract
Quantitative fecal egg counts represented as the number of eggs per gram of feces (EPG) are generally a reliable parameter to estimate the worm burden of intestinal and hepatic parasitoses. Although Haplorchis taichui (Digenea: Heterophyidae) is one of the most common minute human intestinal flukes, little is known about the relationship between EPG and the actual worm burden in patients or the severity of the disease. In the present study, fecal samples were collected from 25 villagers in northern Thailand before and after praziquantel treatment. The EPG values of each participant were determined by the modified cellophane thick smear method, and adult worms were collected from the whole stool after the treatment. Eggs per day per worm (EPDPW) of H. taichui were estimated 82 from egg counts and expelled worms. The EPG was not well correlated with the worm burden, and a reverse correlation was observed between the EPDPW and the worm burden.
Keywords: Haplorchis taichui, eggs per gram of feces (EPG), eggs per day per worm (EPDPW), egg laying capacity
Haplorchis taichui, a minute intestinal fluke, is a digenean trematode widespread in Southeast Asian countries [1,2]. Metacercariae, the infective stage of the parasite, have been found in many species of freshwater fish [3-5]. Infection in humans occurs by eating infected fish in raw or undercooked condition. Infection with H. taichui is mostly asymptomatic unless heavily infected, therefore, haplorchiasis has been considered to be less important in public health view points. However, the precise status of H. taichui infection in humans has never been explored in detail even in heavy endemic areas.
In general, the number of eggs per gram of feces (EPG) is important to speculate the worm burden and intensity of the infection, and also is an indicator to evaluate the efficacy of anthelmintic drugs and thereby to design control programs. Herein, the number of eggs per day per worm (EPDPW) of H. taichui was estimated by the EPG and the number of discharged adult worms from individual participants. In addition, the correlation of the EPG and the worm burden was examined.
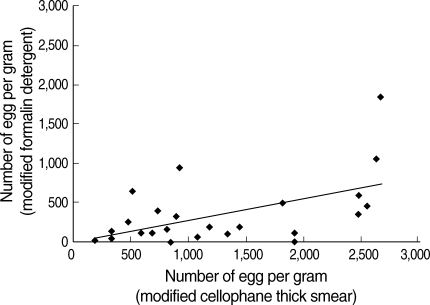
With the approval of the ethical committee of the Faculty of Tropical Medicine, Mahidol University (MUTM 2006-040), fecal egg examinations by the modified cellophane thick smear method were performed for 520 villagers of Ban Luang district, Nan province, north Thailand in September 2006. Fecal samples were examined by the modified cellophane thick smear method [6]. The kit used was manufactured by Department of Helminthology, Faculty of Tropical Medicine, Mahidol University. Approximately a 27.4 mg fecal sample was used for each specimen, and then the EPG was calculated by multiplying the number of eggs per slide with 37. The same fecal samples were examined also by the modified formalin detergent (mFD) method [7] to compare the sensitivity of the 2 methods. As shown in Fig. 1, the egg counts by 2 methods were correlated well with each other (P = 0.008). However, the sensitivity of the modified cellophane thick smear method was higher than the formalin method (t-test; P < 0.01). Therefore, the EPG data of the cellophane thick smear method were used for further analyses in this study.
Fig. 1.
Comparison of the eggs per gram of feces (EPG) obtained by the modified cellophane thick smear and modified formalin detergent methods.
Approximately 70% of the villagers were found to be parasite-egg positive by fecal examination. All egg-positive villagers were treated with praziquantel (Opticide-FC, Medicpharma Co., Bangkok, Thailand) 40 mg/kg. Among those small trematode egg-positives, 25 villagers agreed to submit their whole stool samples after the treatment. To those participants, 60 ml of saturated magnesium sulfate solution was given as the purgative 1 hr after the drug administration. The expelled worms were collected, brought back to the laboratory, identified and counted under a stereomicroscope. The number of eggs per day (EPD) was calculated by the EPG multiplied by the average daily fecal weight of adult humans, 150 g. Then, the EPDPW was calculated by the EPD divided by the number of worms expelled. Correlation was used to quantify the association between the worm burden, EPG, and EPDPW. The statistical analyses were carried out using Excel 2007 (Microsoft, Redmond, Washington, USA).
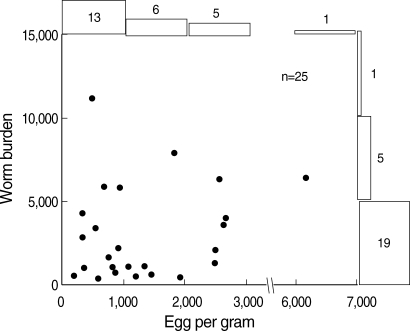
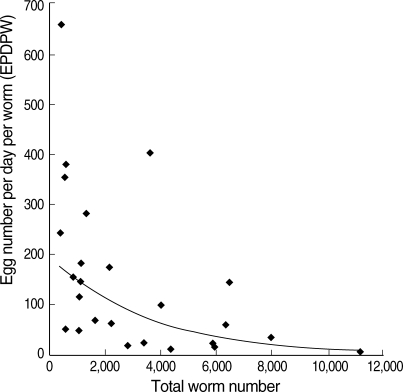
The EPG obtained by the modified cellophane thick smear method ranged from 185 to 6,171, and the number of worms expelled ranged from 364 to 11,172. When the EPG and worm burden of individual participants were plotted, no significant correlation was observed between those 2 parameters (Fig. 2) (r = 0.107, P > 0.50). In almost all (24 of 25) samples, the number of expelled worms were less than 10,000 and the EPG within 5,000 (Fig. 2). An overall EPDPW was calculated as 82. When the samples were divided into 3 groups by worm burdens (0-2,000, 2,001-6,000, and more than 6,000), the geometric means of EPDPW in each group was 168, 46, and 14, respectively, showing a possible reverse relationship between the worm burden and the EPDPW. To confirm this possibility, EPDPWs were calculated based on the EPG and the worm number of the individual participant. As shown in Fig. 3, EPDPW and the worm burden gave a strong negative correlation (r = -0.664, P < 0.001).
Fig. 2.
Marginal plots of EPG by modified cellophane thick smear and individual worm burden. Numbers of samples in each group are shown as flat columns and bars outside of the graph.
Fig. 3.
Correlation of the worm number and eggs per day per worm (EPDPW).
H. taichui is one of the commonest small intestinal flukes distributed mainly in Asia [1,2,8]. Although many people are estimated to be infected with this parasite, H. taichui has been considered to be essentially non-pathogenic or have low-pathogenicity for humans, so that the detailed biology or mode of infection has not been investigated in detail. However, pathogenicity of this species was recently proven by autopsy cases [9]. Since the severity of infection is primarily measured by EPG, the number of eggs produced per day per single worm (EPDPW) is the critical determinant for EPG. In this study, therefore, we intended to determine EPDPW of H. taichui. Surprisingly, however, our results showed a strong negative correlation between the worm burden and EPDPW. When the density of the worms was low, the egg production was high. Previously such a phenomenon was observed in hookworm infection and was reported as the density-dependent effects on parasites fecundity; when the worm burden was increased, individual parasites produced less number of eggs than usual [10]. Each parasite has a confidence limit until which the parasites may produce the eggs in a logarithmic liner manner. From the biological aspects, the competition for the survival among individual parasites might be low at the low density infection, so that worms may be able to produce more eggs than usual. On the other hand, an increased worm number may inhibit the egg productivity.
Since the EPDPW of H. taichui gave a negative correlation with the number of worms, the estimated EPDPW is high at low worm burden and is low at high worm burden cases. In this study, the EPDPW calculated from the overall data was 82. When the participants were divided into low, medium, and high worm burden groups, EPDPW of each group was calculated as 168, 46, and 14, respectively. Thus, maximum EPDPW of H. taichui is estimated at around 168. Concerning the EPDPW of trematodes, for example, Metagonimus yokogawai, which belongs to the same Heterophyidae family as H. taichui, was 280 [11]. The EPDPW of Echinostoma hortense ranged from 1,478 [12] to 3,882 [13], and the EPDPW of Pharyngostomum cordatum was 1,000 [14]. The EPDPW of O. viverrini was estimated to be 1,000-2,500 [15]. Considering these data and the size of adult worms, the EPDPW of 82 seems reasonable for H. taichui.
Mixed infections were observed in 8 samples, and the causative parasites included Enterobius vermicularis, hookworms, and Taenia spp. The EPG of H. taichui mono-infection and that of multiple parasite infections had no significant difference statistically. In mixed infections, the EPDPW may have been influenced. However, a different tendency may come out, when the parasites having similar life styles and similar parasitic mechanisms with H. taichui are the mixed infection agents.
The present results show that, in the case of H. taichui infection in humans, the EPG does not clearly reflect the worm burden or the severity of the disease. Pathogenicity of H. taichui to humans is evident [9], and a low EPG does not always mean a light infection; therefore, caution is required to evaluate the severity of haplorchiasis. Early diagnosis and treatment using praziquantel, in combination with proper health education, is strongly recommended.
ACKNOWLEDGEMENTS
We would like to thank all participants and health volunteers who helped the stool collection in Nan province, Thailand. We are also thankful to Dr. Yukifumi Nawa for editing the manuscript and Mr. Suporn Lungkee for technical help, Faculty of Tropical Medicine, Mahidol University. This study was partially funded by the Japan International Cooperation Agency (JICA) for Megumi Sato and by a grant from Faculty of Tropical Medicine, Mahidol University for Jitra Waikagul.
References
- 1.Belizario VY, Jr, de leon WU, Bersabe MJ, Purnomo, Baird JK, Bangs MJ. A focus of human infection by Haplorchis taichui (Trematoda: Heterophyidae) in the southern Philippines. J Parasitol. 2004;90:1165–1169. doi: 10.1645/GE-3304RN. [DOI] [PubMed] [Google Scholar]
- 2.Radomyos B, Wongsaroj T, Wilairatana P, Radomyos P, Praevanich R, Meesomboon V, Jongsuksuntikul P. Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J Trop Med Public Health. 1998;29:123–127. [PubMed] [Google Scholar]
- 3.Kumchoo K, Wongsawad C, Chai JY, Vanittanakom P, Rojanapaibul A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health. 2005;36:451–455. [PubMed] [Google Scholar]
- 4.Thien PC, Dalsgaard A, Thanh BN, Olsen A, Murrell KD. Prevalence of fishborne zoonotic parasites in important cultured fish species in the Mekong Delta, Vietnam. Parasitol Res. 2007;101:1277–1284. doi: 10.1007/s00436-007-0633-5. [DOI] [PubMed] [Google Scholar]
- 5.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 7.Waikagul J, Anantaphruti MT, Nuamtanong S, Sanguankait S. Hayashi, et al., editors. Evaluation of the modified formalin detergent technique for detection of intestinal parasites. Collected Papers on the Control of Soil-transmitted Helminthiases (by the APCO Research Group) 1997;6:5–11. [Google Scholar]
- 8.Chai JY, Park JH, Han ET, Guk SM, Shin EH, Lin A, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay B, Rim JH. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol. 2005;79:283–289. doi: 10.1079/joh2005302. [DOI] [PubMed] [Google Scholar]
- 9.Sukontason K, Unpunyo P, Sukontason KL, Piangjai S. Evidence of Haplorchis taichui infection as pathogenic parasite: three case reports. Scand J Infect Dis. 2005;37:388–390. doi: 10.1080/00365540510034473. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RM, Schad GA. Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans R Soc Trop Med Hyg. 1985;79:812–825. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y. Medical Zoology. 2nd ed. Tokyo, Japan: Nanzando; 1992. pp. 130–131. (in Japanese) [Google Scholar]
- 12.Lee SK, Chung NS, Ko IH, Sohn WM, Hong ST, Chai JY, Lee SH. An epidemiological survey of Echinostoma hortense infection in Chongsong-gun, Kyongbuk Province. Korean J Parasitol. 1988;26:199–206. doi: 10.3347/kjp.1988.26.3.199. [DOI] [PubMed] [Google Scholar]
- 13.Son WY, Huh S, Lee SU, Woo HC, Hong SJ. Intestinal trematode infections in the villagers in Koje-myon, Kochang-gun, Kyongsangnam-do, Korea. Korean J Parasitol. 1994;32:149–155. doi: 10.3347/kjp.1994.32.3.149. [DOI] [PubMed] [Google Scholar]
- 14.To M, Okuma H, Ishida Y, Imai S, Ishii T. Fecundity of Pharyngostomum cordatum parasitic in domestic cats. Jpn J Vet Sci. 1998;50:908–912. doi: 10.1292/jvms1939.50.908. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Control of foodborne trematode infections. WHO Technical Report Series. 1995;849:1–157. [PubMed] [Google Scholar]