Abstract
Background
Because secondary hemochromatosis is due to hereditary or acquired anemia, phlebotomy is not a suitable means of removing excess iron in this situation. Rather, the treatment is based on the targeted elimination of iron by means of iron chelators.
Methods
Selective review of the literature.
Results
Disorders causing secondary hemochromatosis (e.g., thalassemia) are characterized by ineffective erythropoiesis leading to increased duodenal uptake of iron. Most patients are also chronically transfusion-dependent and receive 200–250 mg of iron with each transfused unit of packed red blood cells. As the excess iron cannot be actively excreted, iron overload ensues, which can cause organ damage. Most patients with this condition in Germany are elderly persons with myelodysplastic syndromes (MDS). The standard treatment to date, parenterally administered deferoxamine, is often hampered by poor compliance. In September 2006, a new oral iron chelator, deferasirox, was approved for use in Germany. According to current findings, this medication is safe, except for a low risk of renal or hepatic failure.
Conclusions
Iron chelation is a treatment option not only for thalassemia patients, but also for those with lower-risk MDS who can be expected to need several years of transfusion therapy.
Keywords: hemochromatosis, anemia, chelators, treatment, myelodysplastic syndrome
Iron overload can cause major damage to the organs of the body. Homozygous patients with Mediterranean anemia (thalassemia major) who do not undergo intensive iron-chelation therapy die in their twenties of heart failure caused by iron deposition in the myocardium. In September 2006, the oral iron chelator deferasirox was approved for use in Europe. For patients who previously had to content themselves, for years or decades, with cumbersome parenteral deferoxamine treatment, the new medication is an important therapeutic advance. Thalassemia being rare in Germany, the problem of secondary hemochromatosis here mainly affects elderly patients with myelodysplastic syndromes (MDS). These diseases have a total incidence of about 4 per 100 000 persons per year. The indication for iron chelation in MDS should be determined only after careful consideration. This article, prepared on the basis of a selective literature review (PubMed and abstracts presented at the annual meetings of the American Society of Hematology), describes how secondary hemochromatosis comes about, and how it can be treated with chelators.
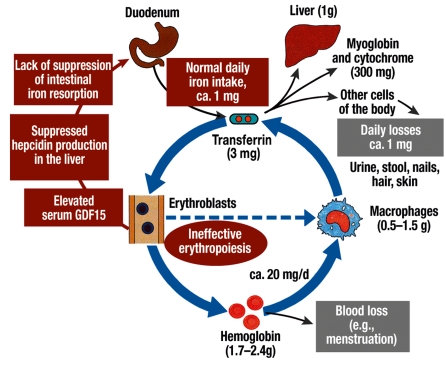
In both thalassemia and MDS, secondary hemochromatosis is caused by more than just transfusion treatment. Increased intestinal resorption of iron causes iron to accumulate even before the patient receives the first unit of transfused erythrocytes. (For more on pathophysiology, see the supplement that accompanies this article, and the diagram (efigure) found in the supplement.)
Increased iron resorption due to ineffective erythropoiesis.
The iron loading anemias are characterized by ineffective erythropoiesis, i.e., a high percentage of the erythropoietic precursor cells in the bone marrow do not survive. In patients with thalassemia who are homozygotic for the disease, ineffective erythropoiesis is mainly due to the precipitation of excessive (alpha or beta) globin chains, leading to oxidative stress. In the myelodysplastic syndromes, on the other hand, the cause of increased apoptosis remains unclear. Ineffective erythropoiesis leads to compensatory erythroid hyperplasia, as well as to chronically increased iron uptake in the duodenum. The mechanism of this increase in iron uptake was sought for years without success. Recent studies have shown that hepcidin and growth differentiation factor 15 (GDF15) play important roles in this process.
Hepcidin, the central regulator of iron metabolism, is synthesized in the liver. This peptide normally inhibits iron uptake in the duodenum as well as iron release from macrophages. GDF15 belongs to the family of the transforming growth factor ß cytokines; it is secreted by erythroblasts and suppresses the hepatic production of hepcidin. In thalassemia, the serum GDF15 level is extremely high, and suppression of hepatic hepcidin production is considered to be the cause of the increased intestinal iron uptake (1). Initial studies suggest that the same is true in MDS (2). Thus, the increased intestinal iron resorption accompanying increased, ineffective erythropoiesis is probably due to the lack of suppression by hepcidin (efigure).
eFigure.
The normal circuit of iron metabolism, including intake and physiological losses. Also: the influence of ineffective erythropoiesis on duodenal iron resorption
Thus, the concept of secondary hemochromatosis is a broad one, encompassing all cases of iron overload that are not due to a primary, hereditary disorder of iron metabolism (box 1). Such cases are usually caused, instead, by an inherited or acquired type of "iron-loading anemia"—in other words, by anemia accompanied by resorptive iron overloading.
Box 1.
Primary (hereditary) hemochromatosis is due to an inherited defect of iron metabolism.
Secondary hemochromatosis is almost always due to a hereditary or acquired disorder of erythropoiesis and/or the treatment of such a disorder with blood transfusions.
Iron overloading due to blood transfusions
The most important cause of secondary hemochromatosis is chronic transfusion therapy. Every unit of erythrocytes that is transfused contains about 200 to 250 mg of iron as a component of the red heme pigment (box 2). When the erythrocytes are broken down by the macrophage system, iron is freed from heme and stored in the body. As the normal daily loss of iron in sweat and in shed cutaneous and mucosal epithelial cells is only about 1 mg, a single unit of erythrocytes corresponds to about 200 daily rations of iron. Because excess iron cannot be eliminated from the body, it necessarily follows that chronic transfusion therapy brings the body’s iron balance very far out of equilibrium.
Box 2. Calculation of iron balance in transfusion therapy (example).
Donated blood contains about 0.5 mg iron per mL erythrocyte concentrate.
A 400 mL unit of donated erythrocytes contains about 200 mg of iron.
Transfusion of 100 units therefore involves loading with 20 g of iron.
Total body iron is normally 3 to 4 g.
Clinical consequences
The clinical and pathological findings of iron overload in secondary hemochromatosis are similar to those seen in hereditary hemochromatosis. Hepatomegaly and splenomegaly may be present, the latter due to increased sequestration of erythrocytes in the spleen. Hepatic function is usually still normal, or only mildly impaired, at the time of diagnosis. Patients with thalassemia often develop finely nodular hepatic cirrhosis three or four decades into the course of their illness. Further consequences of iron overload include decreased glucose tolerance or overt diabetes mellitus, as well as cardiac arrhythmias and heart failure (1). Patients with MDS may have such a short life expectancy because of their bone marrow disease that certain consequences of iron overload, such as hepatic cirrhosis, do not have time to become severe.
The pathophysiology of iron toxicity
The dictum "Only the dose keeps any substance from being a poison" (Paracelsus) is true of iron as well. Its toxicity in high doses comes from its ability to react with molecular oxygen, transferring electrons to it to create intermediate oxygen species, which, in turn, in the presence of iron, cause yet other highly reactive radicals to come into being. These can then attack lipids, proteins, and DNA, inducing cellular damage that ultimately becomes clinically manifest as organ dysfunction.
Iron is not dangerous if it is held in the storage molecule ferritin or bound to the transport protein transferrin. Once the storage and transport capacities of these molecules are exceeded, however, non-transferrin-bound iron (NTBI) begins to appear in the blood plasma. The redox-reactive part of NTBI, known as labile plasma iron (LPI), is rapidly taken up into cells by endocytosis. When the intracellular pool of LPI becomes too large, it can no longer be disposed of by the cell’s antioxidative mechanisms, and the formation of radicals ensues.
Diseases causing secondary hemochromatosis
The spectrum of iron-loading anemias encompasses both hereditary and acquired disorders of erythropoiesis. The congenital diseases in this category include the various types of thalassemia as well as sickle-cell anemia, pyruvate kinase deficiency, the various types of congenital dyserythropoietic anemia (CDA), hereditary spherocytosis, and X-linked sideroblastic anemia (XLSA) (box 3). Iron overloading is especially severe in homozygous ß-thalassemia.
Box 3. The most common causes of secondary hemochromatosis.
-
Hereditary diseases
Thalassemia
Sickle-cell anemia
Pyruvate kinase deficiency
Congenital dyserythropoietic anemia (CDA)
Diamond-Blackfan anemia
Hereditary spherocytosis
X-linked sideroblastic anemia (ALAS2 deficiency)
-
Acquired diseases
Acquired idiopathic sideroblastic anemia (AISA)
Other myelodysplastic syndromes (MDS)
Myelofibrosis
Intractable aplastic anemia
Excessive oral or parenteral iron loading (very rare)
Among the acquired causes, idiopathic sideroblastic anemia is found in the WHO classification among the myelodysplastic syndromes and is designated there as RARS (refractory anemia with ring sideroblasts). Idiopathic myelofibrosis (osteomyelofibrosis) is another potential cause of secondary hemochromatosis, as is treatment-resistant aplastic anemia. In the last-named condition, there is no secondary increase of intestinal iron uptake; chronic transfusion therapy is the sole cause of iron overload.
Myelodysplastic syndromes (MDS)
The indication for iron chelation in MDS should be determined only after careful consideration (2). Patients with "advanced MDS," i.e., those with a high percentage of blasts in the bone marrow or blood, generally do not live long enough to develop severe complications of iron overload. Thus, the use of iron chelators generally cannot help these patients.
In low-risk MDS, on the other hand, the situation is different. Here, ineffective erythropoiesis dominates the clinical picture. Patients with refractory anemia (RA), refractory anemia with ring sideroblasts (RARS), or the 5q syndrome are often treated with erythrocyte transfusions for years. According to Schafer et al. (3), regular transfusion therapy in adult patients can lead to glucose intolerance, focal portal hepatic fibrosis, and heart damage after less than four years of treatment. Iron chelation should be begun when the serum ferritin level exceeds 1000 ng/mL (2). Interestingly, the successful reduction of an iron overload can also improve bone marrow function, not just in MDS, but also in myelofibrosis (4, 5).
A benefit for iron chelation in MDS was long assumed, in analogy to its known benefit in thalassemia major. Study results are now available that document both the diminished life expectancy of MDS patients due to iron overloading (6) and improved survival after chelation therapy, particularly in low-risk MDS ([7] and Rose C et al.: Positive impact of iron chelation therapy on survival in regularly transfused MDS patients. A prospective analysis by the GFM. Blood 2007; 110 [11]: 80a.)
The diagnosis of iron overload
The laboratory test with the most favorable cost-benefit ratio is measurement of the serum ferritin level; this can, however, be elevated not just because of iron overload, but also in the setting of inflammation. A suspected diagnosis of secondary hemochromatosis should, therefore, be confirmed with imaging studies. This is particularly true because even a liver biopsy is not reliable as a gold standard for the diagnosis, as hepatic iron accumulation is markedly inhomogeneous. Furthermore, the biopsy procedure can be complicated by hemorrhage. After extensive development, magnetic resonance imaging (MRI) with so-called T2* weighting can now reliably and non-invasively quantify iron in the liver and heart. There is no linear correlation between hepatic and cardiac iron overload; severe iron overload of the myocardium arises only after the iron storage capacity of the liver has been exceeded (8, 9). The diagnosis of iron overload with MRI requires special expertise and is, unfortunately, not yet reimbursable in Germany.
Treatment with chelators
The most effective method of removing iron from the body is phlebotomy. Patients with secondary hemochromatosis, however, suffer from an inherited or acquired anemia, and therefore generally cannot be treated in this way. Chelators offer a different means of achieving a negative iron balance and tolerable iron concentrations in body tissues, and they can also neutralize deleterious NTBI (non-transferrin-bound iron). It is important to bear in mind that only a small percentage of the iron in the body is accessible to the chelator molecules because the majority of iron ions are tightly liganded to suitable storage or transport molecules; it follows that the chelator should be present continuously, or as nearly so as possible. The intermittent administration of high doses is not efficient.
Three iron chelators are now available for clinical use: deferoxamine, deferiprone, and deferasirox. The properties and side effects of these substances are summarized in the Table.
Table. Properties of iron chelators that have been approved for medical use (25).
| Deferoxamine (DFO) | Deferiprone (DFP) | Deferasirox (DFX) | |
| Chelator molecule: iron atom | 1:1 | 3:1 | 2:1 |
| Usual dose | 25–40(–50) mg/kg | 75–90 mg/kg | (10–)20–30 mg/kg |
| Administration | Subcutaneous, intravenous (8–12 hrs.), 5 days per week | Oral, b.i.d.–t.i.d. | Oral, once daily |
| Plasma half-life | 20–30 minutes | 3–4 hours | 12–16 hours |
| Elimination | Renal and biliary | Renal (biliary) | Biliary excretion |
| Side effects | Local inflammatory reactions, visual and auditory disturbances, disturbance of bone growth, allergic reactions, pulmonary, renal, and neurological manifestations (rare, usually only at high doses) | Gastrointestinal manifestations, agranulocytosis/neutropenia, arthralgia, elevated liver enzymes | Gastrointestinal manifestations, skin exanthem, rise in serum creatinine level, visual and auditory disturbances (rare) |
| Approval | Approved in thalassemia and other types of anemia requiring transfusion (SCA, DBA, PK deficiency, MDS, etc.) | Approved in thalassemia major if the use of DFO is contraindicated or inappropriate | Approved for all patients with thalassemia major over age 6 years, also for younger thalassemia major patients and patients with other types of anemia if deferoxamine therapy is contraindicated or inappropriate |
SCA, sickle-cell anemia; DBA, Diamond-Blackfan anemia; PK, pyruvate kinase; MDS, myelodysplastic syndrome
Deferoxamine (desferrioxamine, DFO)
Deferoxamine has been the standard therapy for about three decades. The medication must be given parenterally and has a very short plasma half-life (20 minutes). It is usually administered subcutaneously. In thalassemia, and probably in other types of iron-loading anemia as well, a neutral iron balance can be achieved despite continuing transfusion therapy by the administration of deferoxamine in a dose of 40 mg per kg of body weight (30 to 50 mg/kg), as an infusion lasting 8 to 10 hours, five nights per week. Several research groups have shown that subcutaneous bolus injections given twice daily (as slowly as possible, over several minutes) can produce a similar degree of iron excretion in the urine as a 10-hour infusion (10, 11). In cases of extreme iron overload, deferoxamine can also be given in higher doses of 50 to 60 mg/kg/day by continuous intravenous infusion through a port system.
Most patients develop local irritation at the sites of infusion or injection, with redness, induration, and mild pain. Further side effects are listed in the Table. The adverse effects are generally reversible if diagnosed early. Allergic reactions to deferoxamine (independent of the dose) are rare, but can range in severity all the way to anaphylaxis (12).
Poor compliance is a major problem besetting treatment with DFO, mainly because of the inconvenient long-lasting parenteral infusions and their local side effects. This, in turn, can affect the patient’s prognosis adversely. Brittenham et al. (13) showed that thalassemia patients who do not undergo a 12-hour Desferal infusion at least 250 times a year have a markedly shorter survival than those who steadily maintained their iron chelation therapy (12% of the former patients survived to the age of 30, compared with 95% of the latter).
Deferiprone (DFP, L1)
Deferiprone is resorbed in the gastrointestinal tract. In order to achieve a negative iron balance, it must be given in a total dose of 75 mg/kg/day; because of the short half-life of the drug (about 1.5 hours), this amount must be given in three divided doses. In a randomized comparison of DFP and DFO involving 144 patients with thalassemia major who had a serum ferritin level between 1500 and 3000 ng/mL, no significant difference between the two groups was found after one year of treatment with respect to the decline of the ferritin level, the iron content of the liver as determined by biopsy, or the iron loading of the liver and heart as determined by MRI (14). Multiple studies now indicate that deferiprone prevents or reduces cardiac iron overload better than deferoxamine (15– 17).
The most important side effect is agranulocytosis, with granulocyte counts below 500 per microliter; this complication is, however, quite rare (0.5%, or 0.2 cases per 100 patient-years). Mild neutropenia is more common (8.5%, or 2.8 cases per 100 patient-years) (16). Frequent (weekly) checking of the complete blood count is mandatory, so that the treatment can be stopped in time in case the granulocyte count drops. Deferiprone has been approved in Europe, but not in the USA.
The combination of deferoxamine (DFO) and deferiprone (DFP)
In the last few years, studies have addressed the question of the possible benefit of a combination of DFO and DFP (18). Such a benefit does seem to exist. Patients stand to benefit from combination therapy particularly when cardiac iron overload has not adequately responded to DFO treatment alone (19).
Deferasirox (DFX, ICL670)
Deferasirox is an iron chelator that can be given orally. It was licensed in the USA in late 2004, and in Europe in 2006. It is licensed for use not only in thalassemia, but also to treat chronic, transfusion-induced iron overload in patients with other types of anemia, when deferoxamine therapy is contraindicated or inappropriate (20). DFX needs only to be swallowed in liquid form once a day; the tablets are dissolved in water or juice before they are consumed.
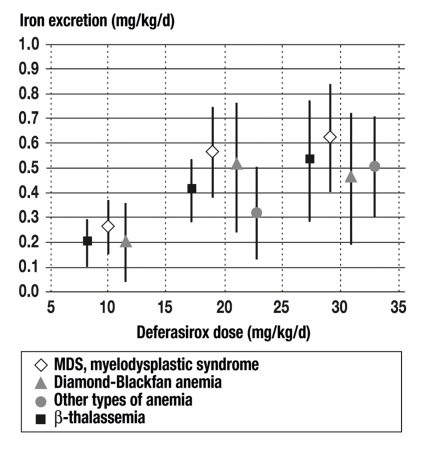
In a randomized, Phase III trial involving 591 thalassemia patients who were treated with either DFO or DFX for one year, it was found that DFX in a dose of 20 or 30 mg/kg/day resulted in an equivalent reduction of hepatic iron, and of ferritin values, to that produced by DFO (21). Deferasirox increased the elimination of iron in dose-dependent fashion, not only in patients with ß-thalassemia (n = 85), but also to a comparable extent in 184 patients with secondary hemochromatosis due to various types of anemia, including MDS (n = 47), Diamond-Blackfan anemia (n = 30), and other types (n = 22) (22). In a further trial in the USA, the serum ferritin level of MDS patients treated by iron chelation with deferasirox for one year declined by about 800 µg/L. Moreover, the labile plasma iron (LPI) normalized in all patients (List A et al.: ICL670 [Exjade®] reduces serum ferritin [SF] and labile plasma iron [LPI] in patients with myelodysplastic syndromes. Blood 2007; 110 [11]: 440a) (figure).
Figure.
Iron excretion as a function of the type of disease and the dose of the iron chelator deferasirox. There is an evident dose-response effect. The efficacy of deferasirox does not vary to any significant degree among the various diseases requiring chronic transfusion therapy (23).
The common side effects of DFX are abdominal symptoms (usually diarrhea), skin exanthems, and elevation of the serum creatinine level. Diarrhea can usually be brought under control by reducing the DFX dose and giving appropriate symptomatic treatment. In case of a skin exanthem, treatment with DFX can be temporarily interrupted and then begun again in a slowly rising dose, possibly accompanied by short-term protection with corticosteroids. In clinical trials, a rise in the serum creatinine level was found in 36% of patients; in 33%, it exceeded the upper limit of normal in two consecutive visits (23). These creatinine elevations, however, did not progress in 2.5 years of further follow-up. In the American MDS study, the serum creatinine level rose in 25% of the cases in which it had initially been normal, to a maximum value of 2.2 mg/dL. When the initial value was elevated, it rose by more than one-third in 8% of cases (List et al., 2007). Since deferasirox was approved, acute renal failure (defined as elevation of the serum creatinine level over 3 mg/dL) has been observed in a few patients. There have been cases with a fatal outcome, but the cause of death was multifactorial in all cases; according to the manufacturer’s assessment, death was mainly due to complications of the underlying disease, rather than the treatment (Novartis Safety Database, 2007). In some cases of reversible renal failure, DFX could not be ruled out as a contributory cause. It is, therefore, recommended that patients with renal risk factors should have their serum creatinine levels checked weekly in the first month of treatment, and monthly thereafter. A rise of more than one-third in the serum creatinine level should be followed by a reduction of the dose by (initially) 10 mg/kg/day.
Pancytopenia has been observed as well, but only in patients whose bone marrow disease provided a possible explanation for it. Agranulocytosis was apparently not induced by DFX.
As with any new medication, physicians sometimes become aware of rare, but severe side effects only after the medication has been in use for years or decades. After 37 000 patients had been treated with deferasirox around the world up to October 2007, the information for physicians was supplemented in July 2008 with the mention of rare cases of liver dysfunction and liver failure (occasionally fatal), rare cases of renal tubulopathy, and rare cases of esophagitis, ulceration, and hemorrhage in the upper gastrointestinal tract. Most of the reports of liver failure concerned patients with severe disease, including pre-existing hepatic cirrhosis and multi-organ failure. Liver failure never arose in patients whose initial liver function tests were normal and who suffered from no other life-threatening complications of the underlying disease. The revised information for physicians also include a recommendation to monitor the serum levels of the hepatic transaminases, bilirubin, and alkaline phosphatase.
The evidence to date indicates that deferasirox is well tolerated. Its initial dose should be chosen with the aim of achieving a neutral or negative iron balance. In patients who regularly receive blood transfusions, iron overload can usually be prevented with a deferasirox dose of 20 mg/kg/day, while the regression of an iron overload that is already present requires a dose of 30 mg/kg/day.
The author considers deferasirox to be a breakthrough in the treatment of secondary hemochromatosis. Likewise, David Nathan of Harvard Medical School, who is widely known as the "grand old man" of thalassemia and iron overload, summarized the situation as follows in early 2008 (24): "Although the old rules have not changed, the advent of an oral chelator has made a huge difference for patients who require chronic transfusions. This is surely a boon for patients and for physicians, nurses, and parents who have struggled to achieve compliance with an unpleasant subcutaneous treatment regimen."
Key messages.
Secondary hemochromatosis is due in part to ineffective erythropoiesis leading to increased iron resorption in the small intestine.
The main cause of secondary hemochromatosis is iron overloading from blood transfusions. With each unit of erythrocyte concentrate, 200 to 250 mg of iron are transfused; this corresponds to the total normal intestinal intake of iron over a period of about 6 months.
Iron overload can cause organ damage, mainly affecting the pancreas (diabetes mellitus), liver (cirrhosis), and heart (heart failure due to cardiomyopathy).
The most common primary diseases causing hemochromatosis are thalassemia (in the Mediterranean area) and myelodysplastic syndrome (MDS). In patients with MDS, the indication for iron chelation is determined both by the extent of iron overload and by the prognosis of the bone marrow disease.
Three iron chelators are available for iron depletion. Each has its own advantages and disadvantages.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor Gattermann has received lecture honoraria, reimbursement of travel expenses, and study support from Novartis Pharma GmbH.
References
- 1.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 2.Gattermann N, Porter JB, Lopes LF, Seymour J. Consensus statement on iron overload in myelodysplastic syndromes. Hematology/Oncology Clinics of North America. 2005;19(Suppl 1):18–25. [Google Scholar]
- 3.Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304:319–324. doi: 10.1056/NEJM198102053040603. [DOI] [PubMed] [Google Scholar]
- 4.Jensen PD, Heickendorff L, Pedersen B, Bendix-Hansen K, Jensen FT, Christensen T, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94:288–299. doi: 10.1046/j.1365-2141.1996.d01-1795.x. [DOI] [PubMed] [Google Scholar]
- 5.di Tucci AA, Murru R, Alberti D, Rabault B, Deplano S, Angelucci E. Correction of anemia in a transfusion-dependent patient with primary myelofibrosis receiving iron chelation therapy with deferasirox (Exjade, ICL670) Eur J Haematol. 2007;78:540–542. doi: 10.1111/j.1600-0609.2007.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcovati L, Della Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 7.Leitch H. Improving clinical outcome in patients wtih myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res. 2007;31(Suppl 3):7–9. doi: 10.1016/S0145-2126(07)70460-5. [DOI] [PubMed] [Google Scholar]
- 8.Jensen PD, Jensen FT, Christensen T, Eiskjaer H, Baandrup U, Nielsen JL. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. 2003;101:4632–4639. doi: 10.1182/blood-2002-09-2754. [DOI] [PubMed] [Google Scholar]
- 9.di Tucci AA, Matta G, Deplano S, Gabbas A, Depau C, Derudas D, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93:1385–1388. doi: 10.3324/haematol.12759. [DOI] [PubMed] [Google Scholar]
- 10.Borgna-Pignatti C, Cohen A. Evaluation of a new method of administration of the iron chelating agent deferoxamine. J Pediatr. 1997;130:86–88. doi: 10.1016/s0022-3476(97)70314-7. [DOI] [PubMed] [Google Scholar]
- 11.Franchini M, Gandini G, de Gironcoli M, Vassanelli A, Borgna-Pignatti C, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload. Blood. 2000;95:2776–2779. [PubMed] [Google Scholar]
- 12.Bousquet J, Navarro M, Robert G, Aye P, Michel FB. Rapid desensitisation for desferrioxamine anaphylactoid reaction. Lancet. 1983;2:859–860. doi: 10.1016/s0140-6736(83)90785-7. [DOI] [PubMed] [Google Scholar]
- 13.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 14.Ceci A, Baiardi P, Felisi M, Cappellini MD, Carnelli V, De Sanctis V, et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br J Haematol. 2002;118 doi: 10.1046/j.1365-2141.2002.03554.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–520. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AR, Galanello R, Piga A, De Sanctis V, Tricta F. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood. 2003;102:1583–1587. doi: 10.1182/blood-2002-10-3280. [DOI] [PubMed] [Google Scholar]
- 17.Pennell DJ. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–3744. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 18.Hoffbrand AV, Cohen A, Hershko O. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17–24. doi: 10.1182/blood-2002-06-1867. [DOI] [PubMed] [Google Scholar]
- 19.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 20.Gattermann N, Musch A. Deferasirox. Orale Therapie bei transfusionsbedingter Eisenüberladung. Arzneimitteltherapie. 2007;25:240–247. [Google Scholar]
- 21.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2005;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 22.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anemias to deferasirox (ICL670): a 1-year prospective study. Eur J Haematol. 2008;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DG. Oral iron chelation: new drug, old rules. Blood. 2008;111:483–484. [Google Scholar]
- 25.Cario H, Janka-Schaub G, Janssen G, Jarisch A, Strauss G, Kohne E. Recent developments in iron chelator therapy. Klin Pädiatr. 2007;219:158–165. doi: 10.1055/s-2007-973845. [DOI] [PubMed] [Google Scholar]
- 1.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 2.Winder A, Lefkowitz R, Ghoti H, Leiba M, Ganz T, Nemeth E, et al. Urinary hepcidin excretion in patients with myelodysplastic syndrome and myelofibrosis. Br J Haematol. 2008;142:669–671. doi: 10.1111/j.1365-2141.2008.07225.x. [DOI] [PubMed] [Google Scholar]