Abstract
OBJECTIVES
To estimate the frequency of ambulatory-care-sensitive hospitalizations (ACSH) and to examine risk of ACSH among breast cancer survivors living in high (compared with low) poverty areas.
DESIGN
Prospective, multilevel study.
SETTING
We analyzed the national, population-based 1991-1999 National Cancer Institute’s Surveillance, Epidemiology, and End Results Program data linked with Medicare claims data throughout the United States.
PARTICIPANTS
Breast cancer survivors age 66 or older.
MEASUREMENTS
ACSH was classified according to diagnosis at hospitalization. The percentage of the population living below the US federal poverty line was calculated at the census-tract level. Potential confounders included demographic characteristics, comorbidity, tumor and treatment factors, and availability of medical care.
RESULTS
13.3% of 47,643 women had at least one ACSH. Women who lived in high-poverty census tracts (≥ 30% poverty rate) were 1.52 times (95% confidence interval [CI]: 1.34-1.72) more likely to have at least one ACSH after diagnosis than women who lived in low-poverty census tracts (< 10% poverty rate). After adjusting for most confounders, results remained unchanged. After adjustment for comorbidity, the hazard ratio (HR) was reduced to 1.34 (95% CI: 1.18-1.52), but adjusting for all variables did not further reduce the risk of ACSH associated with poverty rate beyond adjustment for comorbidity (HR: 1.37; 95% CI: 1.19-1.58).
CONCLUSION
Elderly breast cancer survivors who lived in high-poverty census tracts may be at increased risk of reduced post-treatment follow-up care, preventive care, or symptom management as a result of not having adequate, timely, and high-quality ambulatory primary care as suggested by ACSH.
Keywords: poverty rate, elderly, preventable hospitalization, breast cancer, geography
INTRODUCTION
An estimated one million women age 65 or older live with breast cancer.1,2 This segment of the population is expected to continue to grow. Older breast cancer survivors may be vulnerable to undertreatment3 and particularly in need of primary care after diagnosis and completion of treatment in order to reduce their risk of adverse health outcomes.4
Primary care physicians play an important role in the care of breast cancer survivors by providing adequate, timely, efficient, and high-quality ambulatory care after diagnosis. These physicians perhaps play a more important role among breast cancer survivors than among the general population because of the additional medical needs among breast cancer survivors. First, primary care physicians provide effective post-treatment follow-up care, such as screening for contralateral or ipsilateral recurrence.5,6 Second, primary care physicians often manage symptoms after breast cancer treatment is completed.4 Third, primary care physicians can play a key role in prevention of non-breast-cancer-related diseases. Breast cancer survivors are more likely to receive preventive care when a primary care physician is involved.7,8
While timely access to primary care is clearly important after the diagnosis of breast cancer, in some instances there is a breakdown in access to, or in the processes of, care. One indicator of this breakdown is preventable hospitalizations, also known as ambulatory-care-sensitive hospitalizations (ACSH). Risk factors for ACSH in the general population include being older, African American, in poor health, and having co-existing conditions and low availability of primary care.9-12 Additionally, persons in the general population who reside in areas with higher poverty rates have a higher likelihood of ACSH in cross-sectional ecological analysis.13-16 However, unlike in a multilevel approach, ecological studies that use area-level factors and the rate of ACSH may be subject to the ecological fallacy and cannot examine the effect of area-level characteristics on individual health outcomes.
An emerging body of literature shows that women residing in areas with higher poverty rates have worse breast-cancer-related outcomes, including increased late-stage disease and reduced survival, possibly due to suboptimal screening, diagnostic follow-up, and treatment.17-20 There is a paucity of similar research regarding ACSH in breast cancer survivors, a large and growing population, despite the need for primary care after diagnosis and completion of treatment in order to reduce their risk of adverse health outcomes. Therefore, we estimated the overall frequency of ACSH and examined whether breast cancer survivors living in high poverty areas have a higher risk of ACSH relative to survivors living in low poverty areas over and above other factors associated with ACSH using a prospective, multilevel approach.
METHODS
Cohort definition
The cohort for the study was obtained from a database linking breast cancer patients from the 1992-1999 National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program with 1991-1999 Medicare claims files from the Centers for Medicare and Medicaid. The study population included women age 66 or older with a first primary breast cancer diagnosis (n=71,523) from 1992 through 1999. We excluded 23,880 women who were: 1) enrolled in a health maintenance organization (HMO) at any point from 1991 through 1999, since claims data were not available, 2) not covered by Medicare Parts A and B during the time between their first primary breast cancer diagnosis and study end point (date of death or December 31, 1999), 3) identified by death certificate only, 4) age 65 at diagnosis, which would preclude retrieval of comorbidity information from the Medicare file for the year prior to a patient’s breast cancer diagnosis, or 5) were diagnosed with stage IV breast cancer. This left 47,643 breast cancer survivors available for inclusion in the study. Data from 1991 Medicare were used to obtain comorbidity data for women diagnosed with breast cancer during 1992 as described later.
The study was conceptualized in terms of three-level models in which breast cancer survivors (level 1) were nested within census tracts (level 2), which, in turn, were nested within health-service areas (level 3). A health-service area (HSA) is defined as one or more counties that are relatively self-contained with respect to the provision of routine medical care and are used by the federal government for planning purposes.21 Residents are more likely to seek hospital care inside the HSA in which they reside than to travel outside it. HSAs that are comprised of more than one county were classified based on travel between the counties for routine hospital care. There are 802 HSAs in the United States, 76 of which are contained in the SEER data.
Ambulatory care sensitive hospitalizations
Similar to other studies, we used Medicare hospitalization claims data to identify ACSH.10,11 The International Classification of Disease (ICD)-9-CM codes reported as a first or primary diagnosis for each hospitalization were used to determine if a hospitalization could be classified as ACSH.10,22 Diagnoses for which a hospitalization was classified as an ACSH included angina, asthma, cellulitis, chronic obstructive pulmonary disease, congestive heart failure, dehydration, diabetes, gastroenteritis, grand mal and epileptic convulsions, hypertension, hypoglycemia, kidney/urinary tract infections, pneumonia, and severe ear, nose, and throat infections.10,22 Effective outpatient care can help to reduce the risks of ACSH by preventing the onset of an illness or condition, controlling an acute episodic illness or condition, or managing a chronic disease or condition.
Area-level poverty rate
We used the percentage of the population living below the US federal poverty line from the 1990 census to calculate the area’s socioeconomic position at the census-tract level. Addresses of all breast cancer patients were matched by Geographic Data Technology to identify the census tract and determine the census-tract poverty rate using the 1990 census data. The poverty rate measure is robust across various diseases and levels of geography; it is linked to possible policy implications and is comparable over time.23,24 We grouped poverty rate into four levels — less than 10 percent (low-poverty rate), 10-19%, 20-29%, and 30% or greater (high-poverty rate) — to allow for nonlinear effects.
Covariates
We examined several factors that may account for any observed association between census-tract poverty rates and ACSH including demographic characteristics, comorbidity, tumor and treatment factors, and availability of medical care.
First, demographic characteristics consisted of age group (66-69, 70-74, 75-79, 80-84, 85+), marital status (never married, married, separated, divorced, widowed, unknown), and race (White, African American, and Other). These three variables were obtained from the SEER data at the time of diagnosis.
Second, we used the Deyo adaptation of the Charlson comorbidity index to measure comorbidity.25,26 We searched all available ICD-9 CM codes in the Medicare files (inpatient, outpatient, physician claims) to identify women who had received treatment for the following conditions from 365 days before to 120 days after their breast cancer diagnosis: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild to severe liver disease, diabetes with or without end-organ damage, hemiplegia, moderate or severe renal disease, or AIDS. Each category was weighted according to its prognostic importance.25,26 We compared women with no comorbidity with women who had one comorbid condition, two or more conditions, or unknown comorbidity. Women who had no Medicare claims during this period were categorized as having unknown comorbidity; women who had Medicare claims during this period for conditions not listed above were categorized as having no comorbidity.
Third, tumor and treatment factors consisted of stage at diagnosis, type of surgery (none, breast conserving, mastectomy, or unknown), receipt of radiation therapy (yes, no, or unknown), and receipt of chemotherapy. Stage at diagnosis, type of surgery, and receipt of radiation therapy were obtained from SEER data. Chemotherapy use was obtained from the Medicare claims data, which were shown to be of adequate validity and completeness.27 The following codes were used to determine use of chemotherapy: ICD-9 CM procedure code 9925 for a hospital inpatient or outpatient facility claim of chemotherapy, the Common Procedure Terminology codes 96400-96549, J9000-J9999, and Q0083-Q0085 for a physician or outpatient claim, revenue center codes 0331, 0332, and 0335 for an outpatient claim, and the ICD-9-CM V codes V58.1, V66.2, or V67.2.28 Women were considered to have received chemotherapy for breast cancer if at least one claim were recorded after the date of diagnosis. Women were classified as having received chemotherapy within six months of diagnosis, more than six months after diagnosis, or not having received chemotherapy after diagnosis when there was no such claim recorded. Year of breast cancer diagnosis was included in our analysis to account for any temporal trends in medical care.
Fourth, availability of medical care considered the availability of primary care physicians (office-based MDs for general practice, family medicine, and general internal medicine), specialists, having an office visit with a primary care physician, and having a visit with an oncologist. The number of primary care physicians for each county was obtained from the Area Resource File, which was aggregated to the HSA level.29 The supply of primary care physicians was operationalized as living in a HSA where the population was higher than 2500 persons per primary care physician versus ≤2500 per primary care physician during the years after breast cancer diagnosis until ACSH (if any), death, or censoring (whichever came first).30 We also obtained from the Area Resource File the number of specialists per 1000 population at the HSA level, which was previously found to be negatively correlated with ACSH in the general population.30,31
We used the provider’s specialty code in Medicare to categorize breast cancer survivors’ visits to primary care and oncology physicians following their diagnosis at the individual level.7 To be broadly inclusive, the following provider specialty codes were used to denote primary care visits: 01, general practice; 11, internal medicine; 08, family medicine; 16, obstetrics/gynecology; 38, geriatric medicine; and 70, multispecialty group practices. Women who had at least one visit to any of these types of primary care providers prior to their ACSH (if any) were compared with women who did not. Oncology specialists were defined as subspecialists in medical oncology or hematology-oncology (HCFA specialty codes 83 or 90, respectively), surgeons (code 02, general surgery; codes 49 and 92, surgical oncology), or radiation oncologists (code 92). Women who had at least one visit to any of these types of oncology providers prior to their ACSH (if any) were compared with women who did not.
Statistical analysis
Our three-level survival models used restricted iterative generalized least squares32 and first-order marginal quasi-likelihood estimation.33 These methods are approximations to full maximum likelihood estimation that produce accurate estimates of the random and fixed parts of the models.32 Survival time consisted of the number of months between the date of diagnosis and until first ACSH (if any), death, or end of the study period (December, 1999). Women who died or reached the end of the study period without ACSH were censored at that time. Women who had an ACSH were compared to women who were censored.
We calculated the geographic variation in ACSH between HSAs and between census tracts as follows. The variation between HSAs is the percentage of the total variance between HSAs, namely VHSA/[VHSA + VCT + Vi] × 100, where VHSA = HSA variance, VCT =census tract variance, and Vi = π2 / 3.34 A high value indicates large geographic differences between HSAs. The variation between census tracts is the percentage of the total variance between census tracts, namely VCT/[VHSA + VCT + Vi] × 100.
Models were developed and fitted using the multilevel-modeling software MLwiN, Version 2.0.2.35 Parameters in the fixed part and the random part of the survival models were tested with the Wald test.36
Sensitivity analysis
Because some factors previously found to be associated with ACSH in the general population were not available in the SEER-Medicare data, we conducted a sensitivity analysis to determine the extent to which an unmeasured binary confounder might explain any association between census-tract poverty rate and the risk of ACSH.37 Of the independent risk factors for ACSH among persons age 65 or older identified in prior studies, self-rated health was one of the factors consistently associated with ACSH for which we did not have data. Persons age 65 or older in the general population who rated their health as poor were 1.4 times more likely to have an ACSH than those persons who rated their health better.12 Thus, we performed the sensitivity analysis using the 1.4 times increased risk of ACSH due to this unmeasured binary confounder (self-rated health) and the prevalence of this confounder according to census-tract poverty rate (high-poverty rate vs. low-poverty rate). We used SAS software, version 9.1, for this analysis. This study was approved by the Washington University Institutional Review Board.
RESULTS
A total of 47,643 breast cancer survivors with known census tract were at subsequent risk of having at least one ACSH during the study period. There were a total of 11,367 ACSH among 6,355 women (13.3 percent of 47,643 breast cancer survivors) following their diagnosis. As shown in Figure 1, the most frequent ACSH were for congestive heart failure (n=3,893), chronic obstructive pulmonary disease (n=1,771), and kidney or urinary tract infection (n=1298). On average, 26.1 months had passed after diagnosis until first ACSH (median=21.0 months, range: 0.0-96.0 months). Average time between date of diagnosis and date of death or end of the study period (December 31, 1999) was 47.0 months (median: 46.0 months; range: 0.0-96.0 months).
Figure 1.
Frequency of the number of ambulatory-care sensitivty hospitalization and frequency of first ambulatory-care-sensitive hospitalization, 1992-1999.
Table 1 shows individual-level characteristics for each of the four levels of census-tract poverty rate. With increasing poverty rate, there was an increase in the percentage of women who were African American, not married, had two or more comorbidities, had stage III breast cancer, had no surgery, had no radiotherapy, lived in HSAs where the population per primary care physician was at least 2500, and did not visit an oncologist after diagnosis.
Table 1.
Percentage of the Study Population by Census-Tract Poverty Rate and Multivariable Association with Risk of ACSH among Breast Cancer Survivors.
| Census-tract poverty rate | Multivariable adjusted hazard ratio (95% CI) |
||||
|---|---|---|---|---|---|
| Characteristics | <10% | 10-19% | 20-29% | 30+% | |
| Number of breast cancer survivors |
32,346 (100.0) |
10,403 (100.0) |
2,819 (100.0) |
2,070 (100.0) |
|
| Demographic characteristics | |||||
| Race† | |||||
| White | 94.1 | 89.4 | 70.1 | 45.7 | 1.00 |
| African American | 1.8 | 6.5 | 23.6 | 50.5 | 0.95 (0.83 – 1.08) |
| Other | 3.7 | 3.7 | 6.0 | 3.5 | 0.64 (0.53 – 0.78) |
| Unknown | 0.5 | 0.4 | 0.4 | 0.3 | 0.53 (0.30 – 0.95) |
| Age† | |||||
| 66-69 | 21.7 | 20.5 | 20.2 | 20.5 | 1.00 |
| 70-74 | 29.1 | 26.9 | 27.1 | 27.4 | 1.18 (1.07 – 1.29) |
| 75-79 | 23.7 | 23.3 | 24.8 | 24.6 | 1.25 (1.14 – 1.38) |
| 80-84 | 15.2 | 16.6 | 16.0 | 15.5 | 1.59 (1.43 – 1.76) |
| 85+ | 10.4 | 12.7 | 11.9 | 12.0 | 1.57 (1.45 – 1.83) |
| Marital status† | |||||
| Never married | 6.2 | 7.9 | 10.9 | 14.9 | 0.93 (0.82 – 1.05) |
| Married | 45.5 | 39.1 | 31.9 | 28.3 | 1.00 |
| Separated | 0.2 | 0.3 | 0.5 | 0.6 | 1.12 (0.60 – 2.09) |
| Divorced | 5.2 | 6.0 | 7.7 | 7.5 | 1.28 (1.13 – 1.46) |
| Widowed | 40.7 | 44.3 | 46.3 | 43.8 | 1.25 (1.17 – 1.34) |
| Unknown | 2.3 | 2.5 | 2.6 | 5.0 | 1.30 (1.08 – 1.56) |
| Comorbidity† | |||||
| None | 56.2 | 53.4 | 48.7 | 42.0 | 1.00 |
| One | 26.5 | 26.9 | 25.5 | 29.3 | 1.95 (1.81 – 2.09) |
| Two or more | 17.3 | 19.7 | 25.8 | 28.7 | 3.35 (3.12 – 3.61) |
| Tumor and treatment factors | |||||
| Stage† | |||||
| In situ | 5.5 | 6.6 | 7.8 | 8.7 | 1.00 |
| I | 30.8 | 33.0 | 36.5 | 37.6 | 1.08 (0.96 – 1.22) |
| II | 50.2 | 48.5 | 43.1 | 38.9 | 1.17 (1.03 – 1.33) |
| III | 13.6 | 11.9 | 12.6 | 14.9 | 0.97 (0.83 – 1.13) |
| Surgery† | |||||
| None | 1.0 | 1.0 | 1.3 | 2.8 | 0.43 (0.33 – 0.57) |
| Mastectomy | 45.6 | 55.1 | 52.2 | 51.5 | 1.00 |
| Breast-conserving surgery | 53.3 | 43.9 | 46.3 | 45.6 | 0.82 (0.76 – 0.88) |
| Unknown | 0.1 | 0.0 | 0.2 | 0.1 | 1.09 (0.42 – 1.20) |
| Radiotherapy | |||||
| Yes | 37.4 | 29.5 | 30.2 | 26.3 | 0.79 (0.73 – 0.86) |
| No | 61.2 | 69.5 | 68.4 | 71.5 | 1.00 |
| Unknown | 1.4 | 1.0 | 1.4 | 2.2 | 1.09 (0.87 – 1.38) |
| Chemotherapy | |||||
| No | 87.5 | 87.0 | 87.5 | 85.6 | 1.00 |
| Within 6 months | 9.4 | 9.6 | 9.2 | 11.0 | 1.23 (1.10 – 1.37) |
| After 6 months | 3.1 | 3.4 | 3.3 | 3.4 | 2.23 (1.93 – 2.59) |
| Access to medical care | |||||
| Population per primary care physician † |
|||||
| ≤2500 per physician | 69.5 | 61.6 | 63.2 | 44.6 | 1.00 |
| >2500 per physician | 30.5 | 38.4 | 36.8 | 55.4 | 1.02 (0.90 – 1.14) |
| Mean number of specialists per population (s.d.) |
8.6 (3.4) | 6.3 (3.7) | 7.5 (3.6) | 7.4 (2.5) | 1.00 (0.99 – 1.02) |
| Primary care visits† ‡ | |||||
| General practice | 18.3 | 24.1 | 23.5 | 27.9 | 0.94 (0.85 – 1.04) |
| Internal medicine | 57.1 | 48.8 | 49.3 | 50.3 | 0.59 (0.54 – 0.65) |
| Family practice | 8.6 | 12.0 | 8.3 | 5.1 | 0.22 (0.19 – 0.26) |
| Obstetrics/gynecologist | 2.2 | 1.2 | 1.1 | 1.0 | 0.18 (0.12 – 0.26) |
| Geriatric medicine | 0.1 | 0.1 | 0.2 | 0.2 | 0.09 (0.08 – 0.37) |
| Multispecialty group practice | 2.8 | 1.5 | 2.1 | 3.0 | 0.18 (0.13 – 0.24) |
| One or more oncologist visits† | |||||
| Yes | 87.3 | 86.3 | 85.0 | 83.2 | 1.02 (0.93 – 1.11) |
| No | 12.7 | 13.8 | 15.0 | 16.8 | 1.00 |
Percentages may not add to 100 due to rounding.
p<0.05
each type versus not
The 47,643 women were nested within 1,476 census tracts, which, in turn, were nested within 76 HSAs. There were, on average, 19.7 census tracts per HSA (range 1-1476 census tracts) and 32.3 women per census tract (range 1-88 women). Of the total variance in ACSH, 12.2% (p<0.05) was between HSAs and 0.5% (p>0.05) was between census tracts in an empty model. The negligible variance between census tracts suggests that these areas are not useful targets in an effort to reduce the risk of ACSH. In contrast, a much larger percentage of the total variance lied between HSA, suggesting that these areas may be useful targets in an effort to reduce the risk of ACSH.
In a model adjusted for all variables, women with the following characteristics were at increased risk for ACSH: increasing age, being widowed or divorced, having at least one comorbid condition, stage II breast cancer, and chemotherapy. Women with the following characteristics were at reduced risk of ACSH: other race, not having had surgery, and having had breast-conserving surgery, radiotherapy, and at least one office visit to an internal medicine physician, family practitioner, obstetrician/gynecologist, geriatrician, or multispecialty group practice.
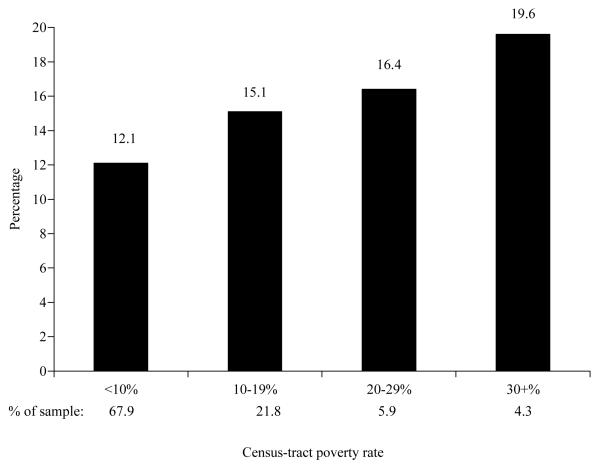
The percentage of women who had at least one ACSH increased with increasing poverty rate (Figure 2). Women who lived in census tracts with at least 10% poverty rate were more likely to have ACSH following their breast cancer diagnosis than women who lived in census tracts with < 10% poverty rates in unadjusted analysis (Table 2). Women who lived in census tracts with at least 30% poverty rate were 1.52 times more likely to have at least one ACSH after diagnosis than women who lived in census tracts with < 10% poverty rate (Model 1). The hazard ratio for each of the poverty rates remained about the same compared to unadjusted analysis after adjustments for demographic factors (Model 2), tumor and treatment factors (Model 3), and access to medical care (Model 4). The largest reduction in hazard ratio from unadjusted analysis was seen after adjustment for comorbidity (Model 5). Adjusting for all variables (Model 6) did not further reduce the hazard ratios associated with poverty rate beyond inclusion of comorbidity. After adjustment for all factors, women living in census tracts with poverty rates of at least 10% were more likely to have at least one ACSH relative to those residing in census tracts with < 10% poverty rate. We also found that women who resided in areas with poverty rates of at least 30% were equally likely to have at least one ACSH as women who resided in areas with poverty rates of 20-29% (chi-square=2.13, p= 0.1444) or 10-19% (chi-square=1.485; p=0.2230) in the fully adjusted model, suggesting that there was no dose-response relationship between census-tract poverty rate and ACSH..
Figure 2.
Unadjusted percentage of women with at least one ambulatory-care-sensitive hospitalization by census-tract poverty rate.
Table 2.
Association (Hazard Ratio; 95% Confidence Interval) of Census-Tract Poverty Rate with First ACSH by Examining the Cumulative Effect of Four Potential Pathways among Breast Cancer Survivors Age 66 or Older, 1992-1999.
| Census-tract poverty rate |
||||
|---|---|---|---|---|
| Model | < 10% | 10-19% | 20-29% | 30+% |
| 1. Unadjusted | 1.00 | 1.29 (1.20 – 1.38) | 1.33 (1.18 – 1.49) | 1.52 (1.34 – 1.72) |
| 2. Adjusted for demographic factors | 1.00 | 1.25 (1.16 – 1.34) | 1.29 (1.15 – 1.45) | 1.49 (1.29 – 1.71) |
| 3. Adjusted for tumor and treatment factors | 1.00 | 1.25 (1.16 – 1.35) | 1.28 (1.14 – 1.44) | 1.47 (1.29 – 1.67) |
| 4. Adjusted for access to medical care | 1.00 | 1.28 (1.19 – 1.38) | 1.34 (1.20 – 1.51) | 1.50 (1.31 – 1.70) |
| 5. Adjusted for comorbidity | 1.00 | 1.21 (1.13 – 1.30) | 1.19 (1.06 – 1.33) | 1.34 (1.18 – 1.52) |
| 6. Adjusted for all factors | 1.00 | 1.19 (1.10 – 1.28) | 1.20 (1.06 – 1.36) | 1.37 (1.19 – 1.58) |
Demographic factors: age group, race, marital status
Tumor and treatment factors: stage at diagnosis, type of surgery, receipt of radiation therapy, and receipt of chemotherapy
Comorbidity: Deyo adaptation of the Charlson comorbidity index
Access to medical care: population per primary care physician, specialists per population, type of primary care physician visit, oncologist visit
As part of sensitivity analyses, we estimated the effect that an unmeasured binary confounder (e.g., proportion of women reporting poor self-rated health) would have on the risk of at least one ACSH when living in census tracts with at least 30% poverty rate relative to living in census tracts with < 10% poverty rate (hazard ratio from Model 6: 1.37; 95% CI: 1.19 – 1.58 from Table 2). As Table 3 shows, an unmeasured binary confounder was unlikely to account for this observed association unless the difference in prevalence between women who lived in census tracts with at least 30% poverty rates and women who lived in census tracts with <10% poverty rates was at least 60 percent. For example, in Table 3, if the prevalence of poor self-rated health was 60% in women who lived in census tracts with least 30% poverty rates and 0% in women who lived in census tracts with <10% poverty rates, then the observed association between census-tract poverty rate and increased risk of ACSH would become non-significant (hazard ratio: 1.10; 95% CI: 0.96 – 1.27) since the 95% confidence interval includes the value of 1.0.
Table 3.
Effects of an Unmeasured Binary Confounder on the Risk of ACSH when Living in High-Poverty vs. Low-Poverty Census Tracts (i.e., At Least 30% Vs. < 10% Poverty Rate).
| Prevalence of UBC in high- poverty census tracts, % |
Adjusted hazard ratio (95% confidence interval) for census-tract poverty rate by prevalence of UBC in low-poverty census tracts, % |
||
|---|---|---|---|
| 0 | 20 | 40 | |
| 20 | 1.27 (1.10 – 1.46) | 1.37 (1.19 – 1.58) | Not plausible |
| 40 | 1.18 (1.03 – 1.36) | 1.28 (1.11 – 1.47) | 1.37 (1.19 – 1.58) |
| 60 | 1.10 (0.96 – 1.27) | 1.19 (1.04 – 1.38) | 1.28 (1.11 – 1.48) |
| 80 | 1.04 (0.90 – 1.20) | 1.12 (0.97 – 1.29) | 1.20 (1.05 – 1.39) |
UBC: Unmeasured binary confounder. UBC risk of self-rated health status with ACSH=1.441.
DISCUSSION
The purpose of the current study was to examine the effect of census-tract poverty rate on subsequent risk of ACSH among older breast cancer survivors. Our results show that 13.3 percent of women had at least one ACSH during the study period. Similar to other studies, we generally found relatively low levels of associations between the individual-level factors and ACSH.10,11,31 Breast cancer survivors who lived in census tracts with poverty rates of at least 10 percent were at increased risk for ACSH compared with survivors who lived in census tracts with < 10 percent poverty rate over and above individual factors associated with ACSH. While the risk of ACSH associated with census tracts with at least 10% poverty rate is modest, one of every three breast cancer survivors age 66 or older in the 1992-1999 SEER data is affected since they live in census tracts with at least 10 percent poverty rate. Our study confirms prior ecological studies of ACSH in the general population that showed that increasing poverty rate is associated with increasing ACSH.13,15,38,39 However, our study went beyond these ecological studies by using a prospective, multilevel design, thereby providing additional evidence for a possible relationship between census-tract poverty rate and ACSH above and beyond the confounding influence of individual-level factors, particularly among the growing population of women with breast cancer. To our knowledge, our study is the first to examine ACSH among breast cancer survivors.
The association between poverty rates and ACSH may be due to deficiencies in (1) the accessibility to care (the presence of access barriers) and/or (2) the appropriateness of care.13 Accessibility to care may have played a role in our study even though the vast majority of women had at least one office visit to a primary care physician or oncologist after diagnosis. Barriers may still exist at the patient level and could have prevented timely primary care and thereby increased the risk of ACSH. Deficiencies in appropriateness of care may mean that there is inadequate emphasis on prevention and patient education, which may lead to noncompliance with treatment regimens, preventive health protocols, and pertinent health behavior.13 Lack of continuity of care with the same medical provider has been found to be positively associated with chronic ACSH40 and may play a role in the poverty rate-ACSH association found in our study.
Studies also have suggested that the presence of medical comorbidities or poor health increased the risk of ACSH9 and might explain the increased risk of ACSH among those living in areas with high poverty rates in the general population.15,16 In our study, comorbidity was the only factor that was able to explain in part the association between poverty rate and ACSH. Medicare beneficiaries in the general population who were in fair or poor health also were more likely to have ACSH.10,41 Sensitivity analysis showed that our results can only be explained if the difference in prevalence of poor self-rated health between high (>30%) and low poverty (<10%) census tracts was at least 60 percent. Since differences in self-rated health by poverty rate among the general population were typically smaller,42,43 it is unlikely that our observed association between poverty rate and ACSH is entirely due to differences in health status and disability. This sensitivity analysis also applies to any other factors that might increase the risk of ACSH by 1.4 times and were not available in the SEER-Medicare data, such as lack of continuity of care described above, although it unclear if there is a 60% difference in such care between women living in high versus low poverty areas.
Household income was not available in the SEER-Medicare data of breast cancer survivors. Because income was shown not to be associated with ACSH among Medicare beneficiaries in the general population,10,11,39 it is unlikely to account for the observed association between poverty rate and ACSH among breast cancer survivors. The difference in the associations between ACSH and each of area-level and individual-level socioeconomic position shows that area-level socioeconomic position may play a more important role than individual-level income. Although the percentage of African Americans was much higher in areas with increased poverty rate, being African American was not independently associated with the risk of ACSH and would therefore not explain our findings. In contrast, elderly African Americans were at increased risk of ACSH in the general population 10.
This study was limited by our analysis of women participating in the Medicare program from SEER-program registries and the limited number of variables available from the SEER and Medicare data. Our findings can only be generalized to women who met the eligibility criteria and none of the exclusion criteria. Therefore, our findings cannot be generalized to women age 65 or younger, who resided elsewhere, who were enrolled in a health maintenance organization, and who only had Medicare Part A coverage. Also, using administrative claims data precluded us from examining some factors that were not available and that may confound the relation between census-tract poverty rates and ACSH. However, in sensitivity analysis, we showed that an unmeasured binary confounder was unlikely to account for our findings. Also, we did not have information about possible migration of the study population into different areas after diagnosis, and therefore could not determine whether patients who moved to different areas were more or less likely to have ACSH compared with patients who did not move. That being said, strengths of this study include the use of the population-based SEER-Medicare linked data for these SEER registries, the prospective multilevel design, while most previous studies have been cross-sectional and/or ecological in nature, and our focus on this rapidly growing population of older breast cancer survivors.
In summary, elderly breast cancer survivors who lived in census tracts with at least a 10% poverty rate were more likely to have had at least one ACSH following their diagnosis compared with survivors living in census tracts with < 10% poverty rate. As a result, breast cancer survivors who lived in these higher poverty areas also may have been at increased risk of reduced post-treatment follow-up care, preventive care, or symptom management, which could have been provided by adequate, timely, efficient, and high-quality ambulatory primary care. Despite the need for continued primary care, breast cancer survivors remain vulnerable to inadequate ambulatory primary care, particularly when living in areas with poverty rates of at least 10 percent.
ACKNOWLEDGMENTS
We thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior and Outreach Core, especially James Struthers and Yan Yan, for data management and selected statistical services. This research was supported in part by grants from the National Cancer Institute (CA100760; CA9184206).
Sponsor’s Role: The sponsor had no role in the design, methods, recruitment, data collection, analysis, or preparation of this manuscript.
Contributor Information
Mario Schootman, Washington University School of Medicine Departments of Medicine and Pediatrics Division of Health Behavior Research, Box 8504 4444 Forest Park Blvd, Box 8504 Saint Louis, MO 63108 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
Donna B. Jeffe, Washington University School of Medicine Departments of Medicine and Pediatrics Division of Health Behavior Research Saint Louis, MO 63108 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
Min Lian, Washington University School of Medicine Departments of Medicine and Pediatrics Division of Health Behavior Research Saint Louis, MO 63108
Anjali D. Deshpande, Saint Louis University Department of Community Health Saint Louis, MO 63104 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
William E. Gillanders, Washington University School of Medicine Department of Surgery Saint Louis, MO 63110 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
Rebecca Aft, Washington University School of Medicine Department of Surgery Saint Louis, MO 63110 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110 and John Cochran VA Medical Center, Saint Louis, MO
Walton Sumner, Washington University School of Medicine Department of Medicine Division of General Medical Sciences Saint Louis, MO 63108 and Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, Saint Louis, MO 63110
REFERENCES
- 1.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17(3):1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN practice guidelines in oncology. 2005.
- 3.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Winer EP. Primary care for survivors of breast cancer. N Engl J Med. 2000;343(15):1086–1094. doi: 10.1056/NEJM200010123431506. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24(6):848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld E, Mant D, Yudkin P, et al. Routine follow up of breast cancer in primary care: Randomised trial. BMJ. 1996;313(7058):665–669. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21(8):1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Etim A, Schellhase K, Sparapani R, et al. Effect of model of care delivery on mammography use among elderly breast cancer survivors. Breast Cancer Res Treat. 2006;V96(3):293–299. doi: 10.1007/s10549-005-9141-4. [DOI] [PubMed] [Google Scholar]
- 9.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–311. [PubMed] [Google Scholar]
- 10.Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804–817. doi: 10.1097/00005650-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Parchman ML, Culler SD. Preventable hospitalizations in primary care shortage area. An analysis of vulnerable Medicare beneficiaries. Arch Fam Med. 1999;8:487–491. doi: 10.1001/archfami.8.6.487. [DOI] [PubMed] [Google Scholar]
- 12.Laditka JN, Laditka SB, Mastanduno MP. Hospital utilization for ambulatory care sensitive conditions: Health outcome disparities associated with race and ethnicity. Soc Sci Med. 2003;57(8):1429–1441. doi: 10.1016/s0277-9536(02)00539-7. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber S, Zielinski T. The meaning of ambulatory care sensitive admissions: Urban and rural perspectives. J Rural Health. 1997;13(4):276–284. doi: 10.1111/j.1748-0361.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Samuels ME, Pease M, et al. Patient characteristics associated with hospitalizations for ambulatory care sensitive conditions in South Carolina. South Med J. 1999;92(10):989–998. doi: 10.1097/00007611-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Billings J, Anderson GM, Newman LS. Recent findings on preventable hospitalizations. Health Aff. 1996;15(3):239–249. doi: 10.1377/hlthaff.15.3.239. [DOI] [PubMed] [Google Scholar]
- 16.DeLia D. Distributional issues in the analysis of preventable hospitalizations. Health Serv Res. 2003;38(6 Pt 2):1761–1779. doi: 10.1111/j.1475-6773.2003.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry J, Breen N. The importance of place of residence in predicting late-stage diagnosis of breast or cervical cancer. Health Place. 2005;11(1):15–29. doi: 10.1016/j.healthplace.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Schootman M, Jeffe D, Gillanders W, et al. Geographic clustering of adequate diagnostic follow-up after abnormal screening results for breast cancer among low-income women in Missouri. Ann Epidemiol. 2007;17(9):704–712. doi: 10.1016/j.annepidem.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin JS, Freeman JL, Mahnken JD, et al. Geographic variations in breast cancer survival among older women: implications for quality of breast cancer care. J Gerontol A Biol Sci Med Sci. 2002;57(6):M401–M406. doi: 10.1093/gerona/57.6.m401. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds P, Hurley SE, Quach A-T, et al. Regional variations in breast cancer incidence among California women, 1988–1997. Cancer Causes Control. 2005;16(2):139–150. doi: 10.1007/s10552-004-2616-5. [DOI] [PubMed] [Google Scholar]
- 21.Makuc DM, Haglund B, Ingram DD, et al. Health service areas for the United States. Vital Health Stat. 1991;2(112):1–102. [PubMed] [Google Scholar]
- 22.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA. 1992;268(17):2388–2394. [PubMed] [Google Scholar]
- 23.Singh G, Miller B, Hankey B, et al. Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. National Cancer Institute; Bethesda, MD: 2003. [Google Scholar]
- 24.Krieger N, Chen J, Waterman P, et al. Geocoding and monitoring of socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter? Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 28.Du X, Goodwin JS. Increase of chemotherapy use in older women with breast carcinoma from 1991 to 1996. Cancer. 2001;92:730–737. doi: 10.1002/1097-0142(20010815)92:4<730::aid-cncr1376>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services. Health Resources and Services Administration. Bureau of Health Professions et al. Area resource file. Information for health resources planning and research. 2003 [Google Scholar]
- 30.Zhan C, Miller MR, Wong H, et al. The effects of HMO penetration on preventable hospitalizations. Health Serv Res. 2004;39(2):345–361. doi: 10.1111/j.1475-6773.2004.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu J, Friedman B, Burstin H. Primary care, HMO enrollment, and hospitalization for ambulatory care sensitive conditions: a new approach. Med Care. 2002;40(12):1260–1269. doi: 10.1097/00005650-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein H. Restricted unbiased iterative generalized least-squares estimation. Biometrika. 1989;76(3):622–623. [Google Scholar]
- 33.Goldstein H. Multilevel statistical models. 2nd Ed Edward Arnold Publishers; London, UK: 1995. [Google Scholar]
- 34.Snijders TAB, Bosker RJ. An Introduction to Basic and Advanced Multilevel Modeling. Sage Publications; London, England: 1999. Multilevel Analysis. [Google Scholar]
- 35.Rasbash J, Browne W, Healy M, et al. MLwiN Beta Version 2.0. Multilevel Models Project, Institute of Education, University of London; London: 2003. [Google Scholar]
- 36.Hox JJ. Multilevel analysis of grouped and longitudinal data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling Longitudinal and Multilevel Data. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 15–32. [Google Scholar]
- 37.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–963. [PubMed] [Google Scholar]
- 38.Pappas G, Hadden WC, Kozak LJ, et al. Potentially avoidable hospitalizations: inequalities in rates between US socioeconomic groups. Am J Public Health. 1997;87(5):811–816. doi: 10.2105/ajph.87.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billings J, Zeitel L, Lukomnik J, et al. Impact of socioeconomic status on hospital use in New York City. Health Aff. 1993;12(1):162–173. doi: 10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- 40.Gill JM, Mainous AG. The role of provider continuity in preventing hospitalizations. Arch Fam Med. 1998;7(4):352–357. doi: 10.1001/archfami.7.4.352. [DOI] [PubMed] [Google Scholar]
- 41.Laditka JN. Hazards of hospitalization for ambulatory care sensitive conditions among older women: Evidence of greater risks for African Americans and Hispanics. Med Care Res Rev. 2003;60(4):468–495. doi: 10.1177/1077558703257369. [DOI] [PubMed] [Google Scholar]
- 42.Brown AF, Ang A, Pebley AR. The relationship between neighborhood characteristics and self-rated health for adults with chronic conditions. Am J Public Health. 2007;97(5):926–932. doi: 10.2105/AJPH.2005.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: Neighborhood economic structure and its implications for health. Soc Sci Med. 2003;57(5):843–860. doi: 10.1016/s0277-9536(02)00457-4. [DOI] [PubMed] [Google Scholar]