Abstract
We report herein a simple device for rapid biosensing consisting of a single microfluidic channel made from poly(dimethylsiloxane) (PDMS) coupled to an injector, and incorporating a biocatalytic sensing electrode, reference and counter electrodes. The sensing electrode was a gold wire coated with 5 nm glutathione-decorated gold nanoparticles (AuNPs). Sensitive detection of H2O2 based on direct bioelectrocatalysis by horseradish peroxidase (HRP) was used for evaluation. HRP was covalently linked the glutathione-AuNPs. This electrode presented quasi-reversible cyclic voltammetry peaks at −0.01 V vs Ag/AgCl at pH 6.5 for the HRP heme FeIII/FeII couple. Direct electrochemical activity of HRP was used to detect H2O2 at high sensitivity with a detection limit of 5 nM in an unmediated system.
Keywords: microfluidics, biosensor, H2O2, electrocatalysis, direct electron transfer
1. Introduction
Microfluidic devices have the ability to analyze very small quantities of sample, limit reagent use, and do analyses at high resolution and sensitivity, low cost, and in short times [1]. They have applications in biology, chemistry and medicine for measurements including diffusion coefficients [2–5], fluid viscosity [6], binding constants [2], DNA analysis [7–9], cell separation [10], cell patterning [11,12] capillary electrophoresis [13,14], and immunoassays [15–19].
Implementing biosensors in microfluidic format provides a potentially more efficient approach to control and automate sample introduction and steps such as washing and reagent addition. By coupling with 6microfluidics, immunoassay procedures could potentially be made fast enough for point-of-care diagnostics without sacrificing sensitivity [20].
Microfluidic devices are commonly fabricated in glass, silicon, or polymers, with polymers finding considerable recent attention. Poly(dimethylsiloxane) (PDMS) is used extensively to fabricate these microfluidic devices using photolithography or more simply by polymer deposition onto molds [21,22]. Recently, an electrochemical sensor was constructed by integrating injection-molded electrodes into a polystyrene micro-flow channel [23].
In this communication, we report fabrication by mold deposition of a simple microfluidic device for electrochemical biosensing, and validate it by sensitive detection of hydrogen peroxide. The device features a single microfluidic channel made from poly(dimethylsiloxane) (PDMS) coupled to a fixed volume injector, and incorporates a biocatalytic sensing electrode, Ag/AgCl reference and Pt wire counter electrodes (Fig. 1). PDMS was chosen because it is easily moldable, soft and readily integrated with outside components [24], and effective protocols exist to inhibit biomolecular contamination [25,26]. The sensor was a gold wire coated with 5 nm glutathione-decorated gold nanoparticles (AuNPs). Horseradish peroxidase (HRP) was covalently linked to the glutathione-AuNPs. Highly sensitive amperometric measurement of H2O2 was achieved with an unprecedented detection limit of 5 nM for unmediated biocatalysis.
Fig. 1.
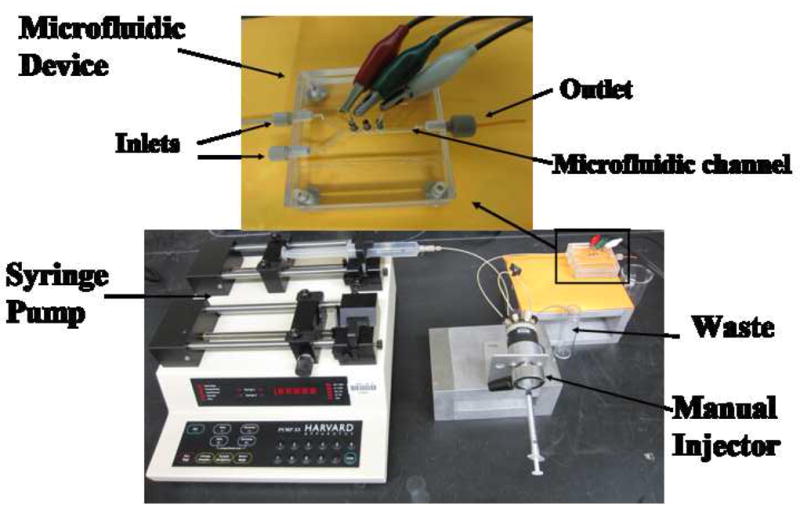
Photographs of microfluidic chip and accessories.
2. Experimental Section
2.1. Chemicals
Horseradish peroxidase (HRP, MW 44000, 250–330 unit mg−1), 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide hydrochloride (EDC), L-Gluthathione reduced (GSH, 99%), HAuCl4·3H2O (99.9%), sodium borohydride (99%) and poly(diallydimethylammonium chloride) (PDDA, MW 20%) was from Sigma. Hydrogen peroxide (H2O2, 30%) was from Fisher. The poly(dimethoxy)silane (PDMS) kit was from Dow Corning. 3-mercapto-1-propanesulfonic acid (MPA) was from Aldrich.
2.2. Fabrication of the microfluidic channel and synthesis Au nanoparticles
Using the PDMS kit, PDMS base material and curing agent were mixed in 10:1 ratio, stirred vigorously for 5 min, and then degassed for 30 min under dynamic vacuum to remove all air bubbles. The clear solution was poured onto a Y-shaped negative mold and heated at 85 °C for 2.5 h [27]. After cooling, the Y-shape PDMS was pealed off the mold, then placed between two flat, machined poly(methylmethacrylate) (PMMA) plates to provide a microfluidic channel (Fig. 1). The channel is ~1 mm wide, 3.6 cm long and 60 μL volume. The top PMMA plate was machined with two inlets and an outlet and equipped with female ports (4 mm diameter) for screwing in standard plastic fittings (1.5 mm i.d., Upchurch) to hold connecting 0.2 mm i.d. tubing (PEEK). Samples were injected by using a syringe pump (Harvard, no. 55-3333) connected to one inlet via an injector valve (Rheodyne, no. 9725i) via 0.2 mm i.d. tubing (Fig. 1). The second inlet was not used, but is included for use in more complex protocols. The top PMMA substrate is equipped with three holes (1 mm diameter) directly above the microfluidic channel for plugging in sensor, Ag/AgCl reference and Pt counter electrodes.
Glutathione-decorated gold nanoparticles (AuNPs) with diameter 5.0 ± 1.4 nm (TEM) were prepared by the reduction of gold salt using sodium borohydride in the presence of glutathione as reported earlier [28]. Nanoparticles were characterized by UV-Vis and IR spectroscopy and atomic force microscopy confirmed nearly complete coverage of the electrode surface after deposition [29] described below.
2.3. Preparation of HRP electrodes
Gold wires (0.5 mm diameter) were first with piranha solution for 5 min., washed with pure ethanol and water, then immersed in 4 mM ethanolic MPA for 12 h to chemisorb a MPA [30]. These electrodes were alternately immersed in cationic PDDA solutions (2 mg mL−1, 0.05 M NaCl) and AuNP solutions (2 mg mL−1 in 20 mM HEPES pH 8.0) for 30 min with intermediate water washing, to form Au/PDDA/AuNP electrodes [29]. HRP was attached onto the carboxylated gold nanoparticles by amidization between the carboxyl-terminated gold nanoparticles and HRP lysines [29] using freshly prepared 5 mg mL−1 EDC and 3 mg mL−1 HRP in pH 6.5 buffer (PBS) at volume ratio 1:2 for 4 h at room temperature. They were then washed with pH 6.5 buffer. When inserted into the microfluidic channel, 1 mm of this Au/PDDA/AuNP/HRP electrode contacts the solution, with an active area of 1.4 × 10−2 cm2 estimated using the Randles-Sevcik equation and the slope of peak current vs. square root of scan rate for soluble 1 mM ferrocyanide in 0.1 M KCl.
2.4. Instruments and procedures
Cyclic voltammetry (CV) and amperometry utilized a CHI 660 potentiostat, a Ag/AgCl reference, a platinum wire auxiliary, and the Au/PDDA/AuNP/HRP electrode within the microfluidic device (Fig. 1). Flow rates between 50 and 500 μL min−1 were evaluated for the amperometric detection using 50 μL and 100 μL injector sample loops. The largest, narrowest peaks were at 200 μL min−1 and 50 μL sample loop. Amperometry gave optimum response at −0.2 V. Thus, amperometry was done at 22 ± 2 °C, 200 μL min−1, −0.2 V vs. Ag/AgCl with 50 μL injections of H2O2 samples. Buffers and hydrogen peroxide solutions were deoxygenized by bubbling with purified nitrogen.
3. Results and Discussion
3.1. Cyclic voltammetry (CV) of AuNP/HRP electrodes
The sensing Au/PDDA/AuNP/HRP electrode transferred to the microfluidic channel filled with pH 6.5 buffer, then cyclic voltammetry (CV) was done. A well-defined, nearly reversible reduction-oxidation peak pair occurred at −0.01 V vs Ag/AgCl (Fig. 2Ac), characteristic of the HRP heme FeIII/FeII couple [31,32] Bare Au and Au/PDDA/AuNP electrodes without HRP showed very small oxidation peaks that may be due to residual surface oxidations (Fig. 2A, a and b). The AuNPs resulted in an increase in charging current consistent with an increase in surface area compared to bare Au wire. The electrochemical surface area increased 35% for Au/PDDA/AuNP electrode compared to the bare Au.
Fig. 2.
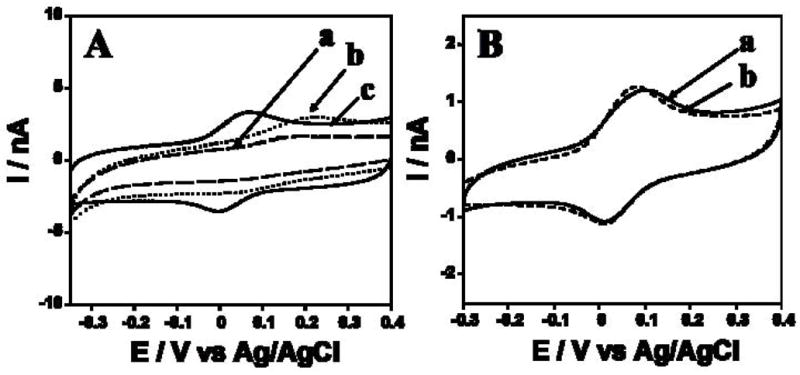
(A) CVs at 100 mV s−1 in pH 6.5 phosphate buffer of (a) Au/PDDA/AuNP/HRP, (b) Au/PDDA/AuNP and (c) bare Au wire. (B) CVs at 50 mV s−1 in pH 6.5 phosphate buffer of Au/PDDA/AuNP/HRP electrode (a) within the microfluidic channel (b) outside microfluidic channel in a conventional electrochemical cell, unstirred solution.
CVs of Au/PDDA/AuNP/HRP electrodes (Fig. 2Ac) showed nearly equal heights of reduction-oxidation peaks. Peak currents increased linearly with scan rate (25–300 mV s−1). These results are indicative of non-ideal surface-confined voltammetry characteristic of immobilized iron heme proteins like peroxidases [31,33]. Integration of CV reduction peaks gave total charge for reduction of the peroxidase, gave an estimated the surface concentration of HRP on Au/PDDA/AuNP/HRP of 0.43 ± 0.05 nmol cm−2. Addition of 0.1 mM H2O2 to the buffer caused disappearance of the HRP oxidation peak and catalytic increase in the reduction peak (not shown), characteristic of peroxidase-catalyzed electrochemical reduction of H2O2 [31,33]. Qualitatively similar results were found for HRP on PDDA/AuNP layers on pyrolytic graphite electrodes [29]. CVs of the Au/PDDA/AuNP/HRP electrode in a conventional cell were similar to those in the microfluidic channel (Fig. 2B), suggesting that little additional cell resistance is contributed by the microfluidic system.
3.2. Detection of H2O2 using the microfluidic system
Amperometry using Au/PDDA/AuNP/HRP sensors at optimized conditions −0.2 V vs Ag/AgCl and 50 μL injections of H2O2 samples into the microfluidic gave sharp reduction peaks from catalytic reduction of H2O2 (Fig. 3A). Peak height increased linearly with H2O2 concentration from 10 nM to 1 μM (Fig. 3B). The detection limit (DL) measured as 3 times the average noise was 5 nM H2O2 (Fig. 4A, inset) with sensitivity 1.46 nA nM−1 cm−2 (electrochemical surface area (Ae) is 1.4 × 10−2 cm−2). DL of the microfluidic sensor was better than that found in a previous study of rotating disc PDDA/AuNP/HRP biosensors at 3000 rpm in a bulk electrochemical cell which gave DL 20 nM, but similar sensitivity of 1.37 nA nM−1 cm−2 (Ae =0.2 cm2) [29]. This improvement in DL may be related to better control of mass transport in the microfluidic system compared to the rotating disc, leading to better signal-to-noise.
Fig. 3.
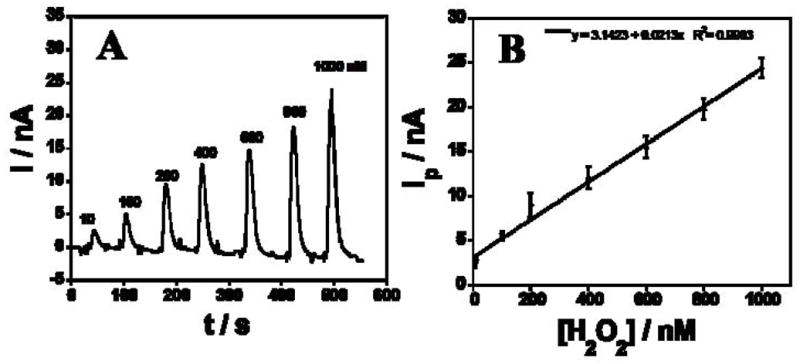
(A) Amperometric response of Au/PDDA/AuNP/HRP sensors in microfluidic device for different H2O2 concentrations at −0.2 V in pH 6.5 buffer. (B) Calibration curve showing peak current (Ip) vs H2O2 concentration.
Fig. 4.
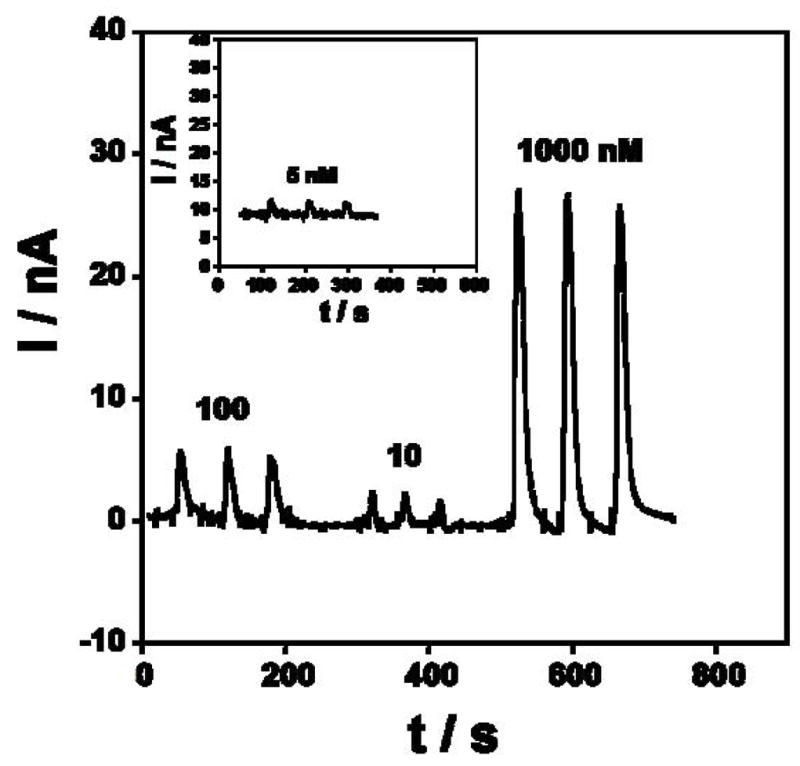
Reproducibility of amperometric responses of Au/PDDA/AuNP/HRP sensors in microfluidic device at −0.2 V in pH 6.5 phosphate buffer.
No peaks were observed for peroxide injections using a bare Au or Au/PDDA/AuNP electrode in the microfluidic system. No change in amperometric current was seen in control experiments injecting buffers containing no H2O2. PDMS is a gas-permeable polymer. and oxygen leaking into the flow chamber may increase the background signal. However, the sensitivity of our peroxide biosensor in the microfluidic system is similar to that in a conventional cell with oxygen-free electrolyte, and the detection limit is considerably better [29]. Thus, oxygen porosity does not seem to create significant problems in the microfluidic system.
To test the reproducibility, several concentrations of H2O2 were repetitively injected under the same conditions. Fig. 4 shows the amperometric response for three 50 μL injections of 5, 10, 100 and 1000 nM H2O2, respectively. Repeatability of peak height for the electrode was 2–5% for peroxide injections.
The microfluidic biosensor showed good stability and reproducibility. The Au/PDDA/AuNP/HRP electrodes were stored either in pH 6.5 buffers or in air at 5 °C and tested periodically. With both storage methods, after one week, CV peak currents and amperometric peaks for 1 μM H2O2 maintained 90% of initial values.
3.3. Summary
A low cost microfluidic biosensor system was fabricated from PDMS and standard accessories, and tested for rapid detection of H2O2 using Au/PDDA/AuNP/HRP sensors. These sensors presented the expected redox and biocatalytic activity of the enzyme, and were used in mediatorless detection of H2O2 at high sensitivity with an unprecedented detection limit for an unmediated enzyme electrode. This microfluidic device is a general bioelectronic platform, and applications can be fitted simply by changing the sensing electrode and using appropriate experimental protocols. Different biosensing electrodes can be fabricated using amidization chemistry to link virtually any biomolecule onto the carboxylated AuNP outer layer. We are currently using this device to develop point-of-care diagnostic assays for cancer biomarkers, by attaching capture antibodies to the sensing electrode and including appropriate washing and detection steps similar to those used in established electrochemical immunoassays [20,29].
Acknowledgments
This research was supported by PHS Grant ES013557 from NIEHS/NIH. JFR is grateful to Science Foundation Ireland for a Walton Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Ludi H, Widmer H. J Chromatogr. 1992;593:253. [Google Scholar]
- 2.Kamholz AE, Weigl BH, Finlayson BA, Yager P. Anal Chem. 1999;71:5340. doi: 10.1021/ac990504j. [DOI] [PubMed] [Google Scholar]
- 3.Kamholz AE, Schilling EA, Yager P. Biophys Journal. 2001;80:1967. doi: 10.1016/S0006-3495(01)76166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culbertson CT, Jacobson SC, Ramsey JM. Talanta. 2002;56:365. doi: 10.1016/s0039-9140(01)00602-6. [DOI] [PubMed] [Google Scholar]
- 5.Pappaert K, Biesemans J, Clicq D, Vankrunkelsven S, Desmet G. Lab on a Chip. 2005;5:1104. doi: 10.1039/b505122c. [DOI] [PubMed] [Google Scholar]
- 6.Avram AM, Avram M, Bragaru A, Vasilco R, Iliescu C. J Phys: Conf Ser. 2006;34:82. [Google Scholar]
- 7.Koutny L, Schmalzing D, Solano OS, Difrawy SE, Adourian A, Buonocore S, Abbey K, McEwan P, Matsudaira P, Ehrlich D. Anal Chem. 2000;72:3388. doi: 10.1021/ac9913614. [DOI] [PubMed] [Google Scholar]
- 8.Jung J, Chen L, Lee S, Kim S, Seong GH, Choo J, Lee EK, Oh CH, Lee S. Anal Bioanal Chem. 2007;387:2609. doi: 10.1007/s00216-007-1158-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Gale BK. Lab on a Chip. 2008;8:1516. doi: 10.1039/b804624g. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Liu AQ, Hjort K. J Micromech Microeng. 2007;17:1992. [Google Scholar]
- 11.Rhee SW, Taylor AM, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Lab on a Chip. 2004;5:102. doi: 10.1039/b403091e. [DOI] [PubMed] [Google Scholar]
- 12.Yen MH, Cheng JY, Wei CW, Chuang YC, Young TH. J Micromech Microeng. 2006;16:1143. [Google Scholar]
- 13.Xue Q, Wainright A, Gangakhedkar S, Gibbons I. Electrophoresis. 2001;22:4000. doi: 10.1002/1522-2683(200110)22:18<4000::AID-ELPS4000>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Tsai YC, Jen HP, Lin KW, Hsieh YZ. J Chromatogr A. 2006;1111:267. doi: 10.1016/j.chroma.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Rossier JS, Girault HH. Lab on a Chip. 2001;1:153. doi: 10.1039/b104772h. [DOI] [PubMed] [Google Scholar]
- 16.Yakovleva J, Davidsson R, Lobanova A, Bengtsson M, Eremin S, Laurell T, Emneus J. Anal Chem. 2002;74:2994. doi: 10.1021/ac015645b. [DOI] [PubMed] [Google Scholar]
- 17.Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. Proc Natl Acad Sci USA. 2007;104:5268. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nashida N, Satoh W, Fukuda J, Suzuki H. Biosens Bioelectron. 2007;22:3167. doi: 10.1016/j.bios.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Meng S, Guo K, Liu Y, Yang P, Zhong W, Liu B. Electrochem Commun. 2008;10:447. [Google Scholar]
- 20.Bange A, Halsall HB, Heineman WR. Biosens Bioelectron. 2005;20:2488. doi: 10.1016/j.bios.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Annu Rev Biomed Eng. 2001;3:335. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Hirdes D, Folch A. Proc Natl Acad Sci USA. 2003;100:1499. doi: 10.1073/pnas.0435755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naseri NG, Baldock SJ, Economou A, Goddard NJ, Fielden PR. Electroanalysis. 2008;20:448. [Google Scholar]
- 24.Ng JMK, Gitlin I, Stroock AD, Whitesides GM. Electrophoresis. 2002;23:3461. doi: 10.1002/1522-2683(200210)23:20<3461::AID-ELPS3461>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Jiang H, Wang E. Electrophoresis. 2007;28:4597. doi: 10.1002/elps.200700261. [DOI] [PubMed] [Google Scholar]
- 26.Boxshall K, Wu MH, Cui Z, Cui ZF, Watts JF, Baker MA. Surf Interface Anal. 2006;38:198. [Google Scholar]
- 27.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal Chem. 1998;70:4974. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M, Huang X. J Am Chem Soc. 2004;126:12047. doi: 10.1021/ja047029d. [DOI] [PubMed] [Google Scholar]
- 29.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF. ACS Nano. doi: 10.1021/nn800863w. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Rusling JF. Anal Chem. 2001;73:4780. doi: 10.1021/ac0105639. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Chouchane S, Magliozzo RS, Rusling JF. Anal Chem. 2002;74:163. doi: 10.1021/ac010701u. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Lin Y, Ostatna V, Wang J. Chem Commun. 2005:3481. doi: 10.1039/b504943a. [DOI] [PubMed] [Google Scholar]
- 33.Rusling JF, Zhang Z. In: Biomolecular Films. Rusling JF, editor. Marcel Dekker; N. Y: 2003. pp. 1–64. [Google Scholar]


