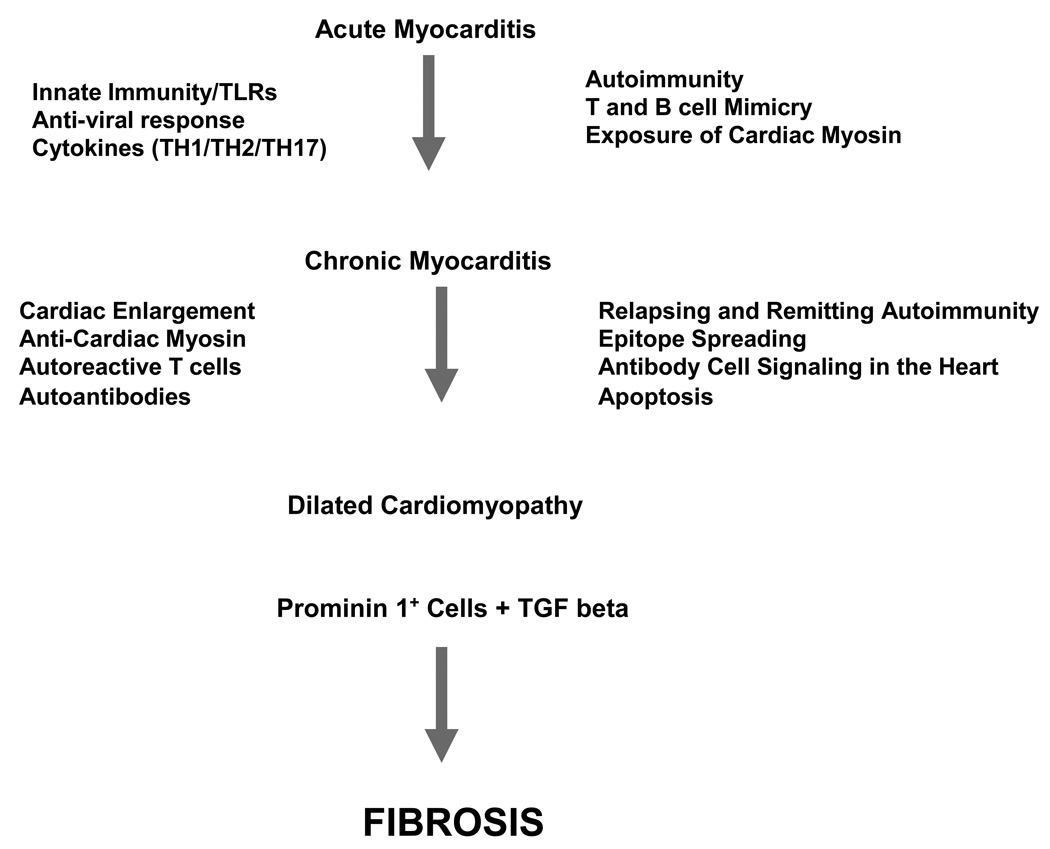
Myocarditis is an acute inflammatory disease of the heart and a precursor of dilated cardiomyopathy1–8. Myocarditis is often characterized by a cellular infiltrate, and if inflammation of the myocardium does not resolve during the acute stage, the heart may be compromised due to necrosis and direct loss of myocytes9, injury from granulomatous inflammation10, 11, or fibrosis due to proliferation of fibroblasts and collagen deposition12, 13. Once the myocardium becomes fibrotic, there may be loss of function. In this issue of Circulation Research, Kania et al describe in their article, “Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of TGF-beta mediated cardiac fibrosis in experimental autoimmune myocarditis”, a novel system to study fibrosis in myocarditis and the origins of the fibrosis in a mouse model14. Mice expressing Enhanced Green Flourescent Protein(EGFP+) were used as donors of prominin 1+ cells which may directly lead to fibrosis during the development of the chronic disease state in myocarditis and cardiomyopathy. Prominin 1+ cells are precursors of fibroblasts in the bone marrow and once injected into hearts were shown to develop into fibroblasts and produce collagen in the presence of transforming growth factor beta (TGF beta). Fibrosis could be blocked with anti-TGF beta treatment14. These studies are directly applicable to human disease. TGF beta is a potential turning point where therapy may prevent the chronic and destructive progression to irreversible end stage dilated cardiomyopathy with lowered ejection fraction and loss of function in the heart.
Dilated cardiomyopathy is a more chronic disease which is characterized by ventricular hypertrophy and which may be a direct result of myocarditis leading to heart failure1–3, 15. The heterogeneous nature of myocarditis in humans makes diagnosis and treatment decisions difficult16. It is well established that cardiac myosin is an autoantigen in autoimmune myocarditis1, 17. In support of this hypothesis, patients with myocarditis and dilated cardiomyopathy have elevated antibodies against cardiac myosin1 and immunosuppressive or immunoabsorption therapy can improve heart function in myocarditis or dilated cardiomyopathy patients18–20. However, to prevent fibrotic changes in the heart, the study published in this issue of Circulation Research by Kania et al describe how anti-TGF-beta may lead to prevention of the fibrosis14. Anti-TGF beta most likely affected the prominin 1+ cells and prevented them from being transformed into fibroblasts by TGF-beta, and therefore, producing fibrosis in the heart. Control of the fibrosis could be a turning point in preventing loss of function and end stage heart disease.
Although Kania et al induce myocarditis with a cardiac myosin peptide14, various agents cause myocarditis in humans, including bacteria, chlamydia, viruses, protozoa and chemicals2, 8, 21, 22, a significant proportion of disease in the western world is due to viral etiology, with enteroviruses implicated8, 23, 24. Coxsackieviruses are frequently reported to be the infectious agent leading to acute myocarditis8, 25, 26. In viral myocarditis, the disease occurs in either acute or chronic forms and may result in progressive weakening of the heart muscle leading to dilated cardiomyopathy27. Little is known why most patients with acute myocarditis resolve while approximately one-third progress to either chronic myocarditis or dilated cardiomyopathy. Myocarditis becomes autoimmune and inflammatory in part because host immune responses are activated by viral infections and then directed against heart tissue epitopes(See Figure 1). Adaptive immunity, including T cells and antibodies against both viral and myocardial proteins could play an important role in myocardial damage. Cardiomyocytes release cardiac myosin during lytic viral infections, and the host recognizes cardiac myosin as a foreign antigen and responds by an adaptive immune response against the heart28, 29.
Figure 1.
Potential Mechanisms in Progression of Myocarditis to Cardiomyopathy
In a recent review article by Cooper, he states that most people who develop acute dilated cardiomyopathy have relatively mild disease that resolves with few sequelae30. Cooper points out that the duration of illness over more than several months to years of illness may lead to increased risk of loss of cardiac function. Complications may be a direct result of irreversible fibrosis after longer periods of inflammation when end stage disease may be more likely to develop. Chronic inflammation potentially may result from release of cardiac myosin and its exposure to and recognition by the innate immune system31 either directly or in immune complexes. It is well known that adaptive immunity against cardiac myosin leads to autoimmunity in myocarditis and cardiomyopathy32. The importance of linking adaptive and innate immunity in myocarditis has been appreciated33. Most studies are performed in animal models of viral or cardiac myosin-induced myocarditis and which provide clues to human disease. Studies in human translational research will further sort out our knowledge regarding the treatments and diagnostic recognition of the progressive stages in myocarditis and cardiomyopathies.
Experimental autoimmune myocarditis (EAM) is known primarily as a CD4+ T cell mediated disease34. Although Th1 or Th2 cell mediated immunity have often been blamed for disease9, 12, 13, 35–37, myocarditis has been reported to develop independently of TH1 and TH2 mechanisms involving TH17 cells and local production of IL-17 with infiltration of neutrophils in acute inflammation38. IL-17 is important in mobilization and recruitment of neutrophils and development of inflammation39, 40. Since the identification of the T helper subset of Th17 cells 41–43, the factors controlling their differentiation and function have been defined. In mice, differentiation of TH17 cells requires a combination of IL-6 and TGF-beta and Th17 transcription factor RORγt44. Kania et al in this issue of Circulation Reearch demonstrate that during inflammation, prominin 1+ cells are recruited to the heart and can be transformed into fibroblasts secreting collagen after exposure to TGF beta14. Kania et al show that prominin 1+ cells cultured with TGF beta in vitro revealed phosphorylation of SMAD2 protein indicating the upregulation of the TGF beta signaling pathway14.
TGF-beta family members (Beta 1, 2 and 3 isoforms) are highly pleiotrophic cytokines with functions in cell differentiation, extracellular matrix formation, inflammation and apoptosis. Dysregulation of TGF beta can lead to a number of pathological states including fibrosis and autoimmunity45. TGF beta has far reaching affects on the immune inflammatory response and protective effects on the vascular system. The role of TGF-beta in controlling immune responses is of great importance in the heart as shown in this issue of Circulation Research by Kania et al. where the development of fibrosis in the heart was linked to TGF-beta and its affects on the prominin 1+ cell which developed into a fibroblast in the presence of TGF-beta14.
Upregulation of TGF beta may be involved in several inflammatory disorders where restoration of normal control of TGF beta signaling by inhibition without impairing its beneficial protective effects may be a potential therapy. Kania et al show that treatment with anti-TGF beta alleviates the fibrosis in the heart by preventing prominin 1+ cells from becoming collagen secreting fibroblasts14. Clearly, animal models, such as the novel EGFP+ myocarditis animal model provide many clues to the pathogenesis of diseases such as autoimmune myocarditis, which can be used in guiding studies in human translational research.
Acknowledgments
Sources of Funding: MWC is funded by the National Heart Lung and Blood Institute by grants HL35280 and HL56267 and is the recipient of an NHLBI Merit Award.
Footnotes
Disclosures: MWC has no disclosures or conflicts of interest
References
- 1.Caforio ALP, Goldman JH, Haven AJ, et al. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54:157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- 2.Brown CA, O'Connell JB. Myocarditis and Idiopathic dilated cardiomyopathy. Amer. J. Med. 1995;99:309–314. doi: 10.1016/S0002-9343(99)80164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felker GM, Hu W, Hare JM, et al. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine. 1999;78:270–283. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18(6):619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 5.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3–14. [PubMed] [Google Scholar]
- 6.Maisch B, Deeg P, Liebau G, et al. Diagnostic relevance of humoral and cytotoxic immune reactions in primary and secondary dilated cardiomyopathy. Am J Cardiol. 1983;52(8):1072–1078. doi: 10.1016/0002-9149(83)90535-0. [DOI] [PubMed] [Google Scholar]
- 7.Maisch B, Trostel-Soeder R, Stechemesser E, et al. Diagnostic relevance of humoral and cell-mediated immune reactions in patients with acute viral myocarditis. Clin Exp Immunol. 1982;48(3):533–545. [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff JF. Viral myocarditis- a review. American Journal of Pathology. 1980;101:425–466. [PMC free article] [PubMed] [Google Scholar]
- 9.Huber SA, Job LP, Woodruff JF. Lysis of infected myofibers by coxsackievirus B3 immune lymphocytes. American Journal of Pathology. 1980;98:681–694. [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper LT., Jr Giant cell myocarditis: diagnosis and treatment. Herz. 2000 May;25(3):291–298. doi: 10.1007/s000590050023. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997 Jun 26;336(26):1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004 Dec;165(6):1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005 Jan 1;174(1):261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 14.Kania G, Blyszczuk P, Sokrates-Stein M, et al. Heart-Infiltrating prominin 1+/CD133+ progenitor cells represent the cellular source of TGF-beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.196287. [DOI] [PubMed] [Google Scholar]
- 15.Towbin JA, Bowles KR, Bowles NE. Etiologies of cardiomyopathy and heart failure. Nature Medicine. 1999;5:266–267. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LT, Virmani R, Chapman NM, et al. National Institutes of Health-sponsored workshop on inflammation and immunity in dilated cardiomyopathy. Mayo Clin Proc. 2006 Feb;81(2):199–204. doi: 10.4065/81.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Caforio AL, Grazzini M, Mann JM, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992 May;85(5):1734–1742. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 18.Cooper LT, Jr, Shabetai R. Immunosuppressive therapy for myocarditis. N Engl J Med. 1995 Dec 21;333(25):1713–1714. [PubMed] [Google Scholar]
- 19.Cooper LT, Belohlavek M, Korinek J, et al. A pilot study to assess the use of protein A immunoadsorption for chronic dilated cardiomyopathy. J Clin Apheresis. 2007 doi: 10.1002/jca.20130. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Burgstaler EA, Cooper LT, Winters JL. Treatment of chronic dilated cardiomyopathy with immunoadsorption using the staphylococcal A-agarose column: a comparison of immunoglobulin reduction using two different techniques. J Clin Apheresis. 2007;22:224–232. doi: 10.1002/jca.20137. [DOI] [PubMed] [Google Scholar]
- 21.Bachmaier K, Neu N, de la Maza L, et al. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 22.Zabriskie JB. Rheumatic fever: the interplay between host, genetics and microbe. Circulation. 1985;71:1077–1086. doi: 10.1161/01.cir.71.6.1077. [DOI] [PubMed] [Google Scholar]
- 23.McManus BM, Chow LH, Wilson JE, et al. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993;68(2):159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- 24.Bowles NE, Richardson PJ, Olsen EG, et al. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 25.Herskowitz A, Wolfgram LJ, Rose NR, et al. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987;9(6):1311–1319. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- 26.Wolfgram LJ, Beisel KW, Herskowitz A, et al. Variations in the susceptibility to coxsackievirus B3-ionduced myocarditis among different strains of mice. J. Immunol. 1986;136:1846–1852. [PubMed] [Google Scholar]
- 27.Abelmann WH. Classification and natural history of primary myocardial disease. Prog Cardiovasc Dis. 1984;27(2):73–94. doi: 10.1016/0033-0620(84)90020-3. [DOI] [PubMed] [Google Scholar]
- 28.Rose NR. Viral damage or 'molecular mimicry'--placing the blame in myocarditis. Nature Medicine. 2000;6:631–632. doi: 10.1038/76199. [DOI] [PubMed] [Google Scholar]
- 29.Neu N, Rose NR, Beisel KW, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. Journal of Immunology. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 30.Cooper LT. Myocarditis. New England Journal of Medicine. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Cox CJ, Alvarez KM, et al. Cutting Edge: Cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascaro-Blanco A, Alvarez K, Yu X, et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008 Sep;41(6):442–453. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson U, Ricci R, Hunziker L, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003 Dec;9(12):1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 34.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell mediated disease. Journal of Immunology. 1991;147:2141–2147. [PubMed] [Google Scholar]
- 35.Fairweather D, Frisancho-Kiss S, Gatewood S, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004 Mar;37(2):131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 36.Kodama M, Matsumoto Y, Fujiwara M, et al. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clinical Immunology and Immunopathology. 1991;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 37.Kodama M, Hanawa H, Saeki M, et al. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ Res. 1994;75(2):278–284. doi: 10.1161/01.res.75.2.278. [DOI] [PubMed] [Google Scholar]
- 38.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006 Aug 7;203(8):2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF alpha. Journal of Immunology. 1999;162:494–502. [PubMed] [Google Scholar]
- 40.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 41.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005 Nov;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 42.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006 Jun;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005 Nov;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]