Summary
OBJECTIVE
To investigate the nutritional status of individuals from a rural area of Brazil, and associations with helminth infections in an age-stratified sample.
METHOD
A total of 1113 individuals aged from 6 months to 83 years from the rural community of Americaninhas in Minas Gerais were investigated. Assessments comprised anthropometric measurements of weight, height and body composition, examining faecal samples for helminth eggs, and peripheral blood assays for albumin, haemoglobin and ferritin concentrations.
RESULTS
Ten percent of the participants were underweight, 12.8% were overweight and 28.3% of the children and adolescents were stunted. 11.6% had low lean body mass and 28.8% had low fat body mass. Hypoalbuminaemia was seen in 5.5%, anaemia in 12.5% and iron deficiency in 13.1%, although the prevalence of these two indices increased with age. Multivariate analysis showed that, after controlling for age, sex and socio-economic status, stunting was significantly associated with Ascaris lumbricoides infection among children and adolescents, whereas low body mass was significantly associated with hookworm infection among adults and the elderly.
CONCLUSIONS
Helminth infections are associated with undernutrition in endemic populations, with important differences between the effects of hookworm and A. lumbricoides on age-related nutritional status.
Keywords: undernutrition, anaemia, iron stores, body composition, intestinal helminths
Introduction
Intestinal parasitic infections remain serious public health problems, especially in poor and developing countries (Bethony et al. 2006). Although investigators have reached various conclusions regarding the age groups at greatest risk and the effect of helminths on nutrition, the relationship between malnutrition and intestinal helminth infection has been well established (Stoltzfus et al. 1997; Stephenson et al. 2000). This relationship is complex and depends on determinants such as the social, economic and physical environment in which an individual lives (Assis et al. 2004). Different types of helminth infection may affect growth in different ways [e.g. nutrient absorption, voluntary intake of food and degree of mucosal damage (Stephenson et al. 2000)]. The nutritional status of the host may be impaired by the increased nutrient demands of the parasite itself or by specific actions such as blocking the absorbing surface of the mucosa by adult Ascaris lumbricoides worms (Stephenson et al. 2000), ongoing blood loss by hookworm infections (Stoltzfus et al. 1997) and/or schistosomiasis (Friedman et al. 2005).
Previous studies have found A. lumbricoides, Schistosoma mansoni and Trichuris trichiura infections to be associated with stunting in pre-school and school children (Tshikuka et al. 1997; Saldiva et al. 1999; Assis et al. 2004). But most studies of intestinal parasites and malnutrition have been limited because they have only studied children and by their focus on helminths (Beltrame et al. 2002; Muniz et al. 2002; Hughes et al. 2004; Quihui-Cota et al. 2004; Ulukanligil & Seyrek 2004). Ours is one of the few studies to investigate the impact of different helminths on specific nutritional deficiencies including body composition and biochemical parameters, and it is the first to investigate individuals from all age groups including the elderly. Therefore, this study aimed to describe the nutritional patterns of a population in a helminth endemic area and to assess the relationships between helminth species and anthropometric parameters.
Materials and methods
Study area and population
This is a substudy of an immuno-epidemiological survey of helminth infection and iron status in a community in the northeast part of Minas Gerais State, Brazil (an area being developed for the testing of experimental hookworm vaccines). Prior to the beginning of the study, a household census was conducted which enumerated 1496 residents in the urban municipal centre of Americaninhas and the surrounding rural areas. The area has an agrarian-based economy that consists of cattle ranching and growing manioc, coffee and beans. Houses are predominantly made from concrete, or a combination of wood and mud, and have either tiles or corrugated iron sheets for roofing. Approximately half of the homes have a latrine, and people commonly collect their water from local springs.
The study was carried out between July and December, 2004, and was approved by the ethics committees of the Instituto René Rachou-FIOCRUZ (Brazil), The George Washington University (USA), the London School of Hygiene and Tropical Medicine (UK) and by the National Brazilian Committee for Ethics in Research (CONEP, Brazil). Individuals were enrolled after written consent was obtained from all adult subjects and from parents or guardians of minors.
Parasitological methods
Helminth infections were diagnosed by the formalin–ether sedimentation technique (Carli 2001). Egg-positive individuals were asked to collect two more faecal samples from different days which were examined by the Kato-Katz method (Katz et al. 1972). Intensity of infection was estimated indirectly by counting the mean number of eggs per gram of faeces (epg), as described elsewhere (Fleming et al. 2006; Brooker et al. 2007), and categorized using thresholds recommended by the World Health Organization (WHO 2002): for hookworm infection, light = 1–1999 epg, moderate = 2000–3999 epg, and severe ≥4000 epg; for A. lumbricoides, light = 1–4999 epg, moderate = 5000–9999 epg, and severe ≥10000 epg; and for S. mansoni, light = 1–99 epg, moderate = 100–399 epg and severe ≥400 epg. Quality control of egg counts was assured by having every tenth faecal sample read by a second trained technician (Fleming et al. 2006).
Anthropometric measurements
Weight, height, mid-upper arm circumference (MUAC) and triceps skinfold thickness (TST) were measured according to anthropometric standardization (Jellife 1966). Height was measured using an anthropometer with up to 0.5 cm precision, weight with a platform scale for up to 150 kg and 100 g precision, MUAC using a regular metric tape with 1.0 mm precision and TST using a Lange skinfold caliper with up to 1.0 mm precision.
The study sample was divided into four age groups: children (0–9 years), adolescents (10–19 years), adults (20–59 years) and the elderly (≥60 years). For additional analyses, children were subdivided into infants (0–1 year), pre-school (2–5 years) and school-aged (6–9 years) children. Nutritional indices were calculated as follows: children were assessed by calculating the height-for-age (HAZ), weight-for-age (WAZ) and weight-for-height (WHZ) scores, which are the differences from the mean in standard deviation (SD) units or z-scores. For the purposes of descriptive analyses, individuals were classified as stunted, underweight and/or wasted if the HAZ, WAZ and WHZ scores were >2 SD below the National Center for Health Statistics (NCHS) mean, respectively (CDC 2002).
The weight status of adolescents was evaluated by calculating the body mass index (BMI)-for-age percentile in comparison to a reference population and was classified as follows: eutrophic (5–85th percentile), under-weight (<5th), or overweight (>85th) (WHO 1995). The nutritional status of adults and the elderly was determined using the absolute BMI and classified as eutrophic (18.5–24.9 kg/m2), underweight (<18.5) or overweight (≥25) (WHO 1995).
Body composition was evaluated by the TST and MUAC measurements and estimating arm fat (F) and arm muscle (M) areas, according to age (Frisancho 1981). It was assumed that the TST indicates the calorie reserves stored in the form of fat, whereas the arm muscle size reflects the levels of circulating protein (Jellife 1966; Frisancho 1981). Therefore, depleted calorie and protein reserves were defined as F and M < 5th percentile, respectively, and a high calorie reserve was defined as F > 95th percentile. Depleted calorie reserves were represented by low fat body mass while low protein reserves were indicated by low lean body mass.
Biochemical measurements
Serum albumin concentration was measured as an approximation of the visceral protein status (Frisancho 1981). Low visceral protein was defined as serum albumin1 concentration below 3.5 mg/dl (Blackburn et al. 1977). Iron status and anaemia were assessed by measuring serum ferritin (SF) and haemoglobin (Hb) concentrations. In children younger than 6 years, Hb levels were determined using fingerprick blood and a portable, battery-operated haemoglobinometer (HemoCue®). Older individuals gave venous blood in the fasting state and had their Hb concentration measured using a Coulter Model S Counter (Coulter MAXM). Anaemia was defined as haemoglobin levels <11.0 mg/dl for children aged 6–59 months, 11.5 mg/dl for children aged 5–11 years, 12.0 mg/dl for children aged 12–14 years and for women, and <13.0 mg/dl for men (>15 years of age) (WHO 2001).
Serum ferritin concentration was determined for individuals aged ≥6 years by the chemiluminescent immunoassay detection method. Iron deficiency was defined as SF <15 μg/l and severe risk of iron overload as SF >200 μg/l for males and >150 μg/l for females (WHO 2001).
Socioeconomic survey
A pre-tested standardized questionnaire was used to record details of household socio-economic characteristics including: household construction, water and sanitation, parental education and ownership of selected household assets. Information on household ownership, characteristics of the dwelling and land ownership were used to construct a wealth index, using the method of Filmer and Pritchett (2001). This method reliably predicts economic status without the necessity of direct income or expenditure information.
The resultant index was divided into quintiles, so that each household could be classified in terms of relative socio-economic status. These quintiles were considered categorical socio-economic variables. Levels of maternal and parental formal education and possession of a radio, refrigerator or land were selected to give an overview of the educational and economic status of the population.
Statistical analyses
Proportions were used as the statistical parameter in the descriptive analysis. Chi-square tests were used to test for differences in nutritional status and prevalence of helminth infection among the age groups. The anthropometric indices HAZ, WAZ and BMI-for-age percentile were expressed as mean and SD to describe the patterns of nutrition during the pre-school, school and adolescence-aged years.
Logistic regression was used to test for an association between nutritional factors and helminth infection. Because sex, age, maternal level of education and socioeconomic status have previously been associated with nutritional status and helminth infection (Drachler et al. 2003; Quihui et al. 2006), they were considered potential confounders of the principal associations being studied. To study each nutritional factor as the dependent variable, univariate logistic regression was initially performed to assess associations with: age and helminth infection status (continuous variables), sex, maternal level of education and socio-economic status (categorical variables). For the regression analyses, BMI-for-age index was used to evaluate underweight and overweight for children and adolescents. Associations with a significance level below 20% (P < 0.2) in univariate analysis were included in a multivariate model that was analysed by backward stepwise regression with variables removed if the association had a level of significance of ≥5% (P > 0.049). Due to the large amplitude of variation in egg counts for A. lumbricoides and hookworm among the studied individuals, these scales were arbitrarily adjusted dividing them by 10 000 prior to conducting the regression analyses. Models were adjusted to account for the non-independence of observations clustered within households (Ridout et al. 1999). Analysis was undertaken using spss 12.0.
Results
Parasitological surveys were performed on 1113 (537 males and 576 females) individuals (Table 1), of whom 1105 had anthropometric indices measured; 758 of these also agreed to have blood collected by venipuncture or finger prick. The percentages within each age group were as follows: children 29.2%, adolescents 24.7%, adults 35.5% and the elderly 10.6%. Among the children between 0 and 9 years, 20.9% were infants, 31.7% pre-schoolers and 47.4% schoolchildren. Level of maternal education was classified as either illiterate (63.6%), primary school level (31.9%), or post-primary school level (4.5%). Level of paternal education was classified as illiterate (65.7%), primary school level (30.9%) or post-primary school level (3.4%). A radio was present in 30.2% of households and a refrigerator in 24.3%. Possession of land was reported by 38.6% of participants (Table 1).
Table 1.
Description of socioeconomic status and other variables in the study population
| Variables | n | % |
|---|---|---|
| Gender | ||
| Male | 535 | 48.4 |
| Female | 570 | 51.6 |
| Age group (years) | ||
| 0–9 | 323 | 29.2 |
| 10–19 | 275 | 24.9 |
| 20–59 | 390 | 35.3 |
| >60 | 117 | 10.6 |
| Level of maternal formal education | ||
| Illiterate | 672 | 63.6 |
| Primary school | 337 | 31.9 |
| Post-primary school | 48 | 4.5 |
| Level of paternal formal education | ||
| Illiterate | 687 | 65.7 |
| Primary school | 323 | 30.9 |
| Post-primary school | 35 | 3.4 |
| Possession of radio | 310 | 29.5 |
| Possession of refrigerator | 260 | 24.7 |
| Possession of land | 406 | 38.7 |
We observed a high prevalence of hookworm (69.8%), A. lumbricoides (50.8%) and S. mansoni (45.2%) infection, with the majority of individuals harbouring infections of low intensity (Table 2). The prevalence of Strongyloides stercoralis was 8.1% and the prevalences of other helminth species were negligible [T. trichiura (1.1%), Enterobius vermicularis (0.8%), Hymenolepis nana (0.2%) and Taenia spp. (0.2%)]. The prevalence of hookworm was significantly greater in males than females (74.9% vs. 65.1%, P < 0.001). Children had lower prevalences of hookworm and S. mansoni infection but higher prevalence of A. lumbricoides (P < 0.001) than the other age groups (Table 2).
Table 2.
Intensity of helminth infection by age group and gender in the study population
| Intensity of helminth infection† |
Sex |
P-value* | Age (years) |
P-value* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Male |
Female |
0–9 |
10–19 |
20–59 |
≥60 |
||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |||
| Hookworm | <0.001 | <0.001 | ||||||||||||||
| Not infected |
336 | 30.2 | 135 | 25.1 | 201 | 34.9 | 131 | 40.3 | 65 | 23.6 | 105 | 26.6 | 35 | 29.7 | ||
| Light | 598 | 53.7 | 316 | 58.8 | 282 | 49.0 | 161 | 49.5 | 161 | 58.5 | 224 | 56.7 | 52 | 44.1 | ||
| Moderate | 75 | 6.7 | 44 | 8.2 | 31 | 5.4 | 14 | 4.3 | 24 | 8.7 | 29 | 7.3 | 8 | 6.8 | ||
| Severe | 104 | 9.3 | 42 | 7.8 | 62 | 10.8 | 19 | 5.8 | 25 | 9.1 | 37 | 9.4 | 23 | 19.5 | ||
| Ascaris lumbricoides | 0.181 | <0.001 | ||||||||||||||
| Not infected |
548 | 49.2 | 273 | 50.8 | 275 | 47.7 | 112 | 34.5 | 116 | 42.2 | 248 | 62.8 | 72 | 61.0 | ||
| Light | 254 | 22.8 | 115 | 21.4 | 139 | 24.1 | 64 | 19.7 | 68 | 24.7 | 92 | 23.3 | 30 | 25.4 | ||
| Moderate | 109 | 9.8 | 60 | 11.2 | 49 | 8.5 | 47 | 14.5 | 34 | 12.4 | 22 | 5.6 | 6 | 5.1 | ||
| Severe | 202 | 18.1 | 89 | 16.6 | 113 | 19.6 | 102 | 31.4 | 57 | 20.7 | 33 | 8.4 | 10 | 8.5 | ||
| Schistosoma mansoni | 0.947 | <0.001 | ||||||||||||||
| Not infected |
610 | 54.8 | 294 | 54.7 | 316 | 54.9 | 234 | 72.0 | 130 | 47.3 | 170 | 43.0 | 76 | 64.4 | ||
| Light | 252 | 22.6 | 120 | 22.3 | 132 | 22.9 | 51 | 15.7 | 49 | 17.8 | 122 | 30.9 | 30 | 25.4 | ||
| Moderate | 149 | 13.4 | 71 | 13.2 | 78 | 13.5 | 21 | 6.5 | 49 | 17.8 | 69 | 17.5 | 10 | 8.5 | ||
| Severe | 102 | 9.2 | 52 | 9.7 | 50 | 8.7 | 19 | 5.8 | 47 | 17.1 | 34 | 8.6 | 2 | 1.7 | ||
Chi-square test statistic reported.
Classification of helminth intensities of infection, according to eggs per gram of faeces (epg). Hookworm — light: 1–1999 epg, moderate: 2000–3999 epg, severe: ≥4000 epg. Ascaris lumbricoides — light: 1–4999 epg, moderate: 5000–9999 epg, severe: ≥10000 epg. Schistosoma mansoni - light: 1–99 epg, moderate: 100–399 epg, severe: ≥ 400 epg (WHO, 2002).
Anthropometric measurements
Overall, the prevalence of stunting was 28.3%, underweight 10.0% and overweight was 12.8% (Table 3). Wasting was observed in 2.4% of children. The prevalence of stunting did not differ by sex. Being underweight was more prevalent in adolescent males than in females (9.7% vs. 0.7%, P = 0.001) but more prevalent in adult females than in males (8.3% vs. 4.6%, P = 0.046).
Table 3.
Alterations in nutritional parameters by age group in the study population
| Variable/age group (years) |
Overall |
0–9 |
10–19 |
20–59 |
≥60 |
P-value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Body mass† | <0.001 | ||||||||||
| Underweight | 111 | 10.0 | 56 | 17.3 | 14 | 5.1 | 26 | 6.7 | 15 | 12.8 | |
| Overweight | 141 | 12.8 | 10 | 3.1 | 14 | 5.1 | 97 | 24.9 | 20 | 17.1 | |
| Height/stunting | 169 | 28.3 | 101 | 31.3 | 68 | 24.7 | 0.077 | ||||
| Low lean body mass/Low protein reserves | 124 | 11.6 | 25 | 8.1 | 39 | 14.2 | 45 | 11.6 | 15 | 15.5 | 0.072 |
| Fat body mass | 0.002 | ||||||||||
| Low calorie reserves |
307 | 28.8 | 75 | 24.8 | 62 | 22.5 | 134 | 34.4 | 36 | 37.1 | |
| Excess calorie reserves |
13 | 1.2 | 2 | 0.7 | 4 | 1.5 | 7 | 1.8 | 0 | 0 | |
| Haemoglobin/anaemia | 95 | 12.5 | 65 | 24.5 | 10 | 5.6 | 9 | 3.8 | 11 | 14.7 | <0.001 |
| Serum ferritin | 0.049 | ||||||||||
| Depleted iron stores | 54 | 13.1 | 10 | 17.2 | 16 | 12.9 | 19 | 10.4 | 9 | 19.1 | |
| Risk of iron overload | 9 | 2.2 | 0 | 0 | 0 | 0 | 6 | 3.3 | 3 | 6.4 | |
| Serum albumin/depleted visceral proteins | 23 | 5.5 | 4 | 6.8 | 5 | 4.0 | 9 | 4.8 | 5 | 10.2 | 0.394 |
Chi-square test statistic reported.
Expressed as weight-for-age for children, body mass index percentile for adolescents and body mass index for adults and elderly.
Overweight was less prevalent among children (3.1%) than the other age groups, and increased with age until 60 years, then it decreased. Underweight was more prevalent in children (17.3%) and the elderly than in adults (12.8%; P < 0.001). Among children, underweight was more prevalent in infants than in pre-school or school-children (25.0% vs. 15.8% and 15.3% respectively, P = 0.003). The proportion of those who were stunted was the same for children and adolescents, although among children, infants showed a higher prevalence of stunting than pre-school or school children (42.3% vs. 31.4% and 26.1% respectively, P = 0.050). In this population, the mean heights of older children were low, being below the 10th percentile for boys and below the 5th percentile for girls, compared to a reference population (166.1 ± 4.5 cm and 152.8 ± 5.3 cm for 18-year-old boys and girls respectively). Final adult height was also low (163.5 ± 6.7 cm in males and 151.1 ± 5.6 cm in females). Only two individuals (aged 2 and 3 years respectively) had a kwashiorkor-like appearance (both were treated with nutritional supplementation and anthelmintic drugs).
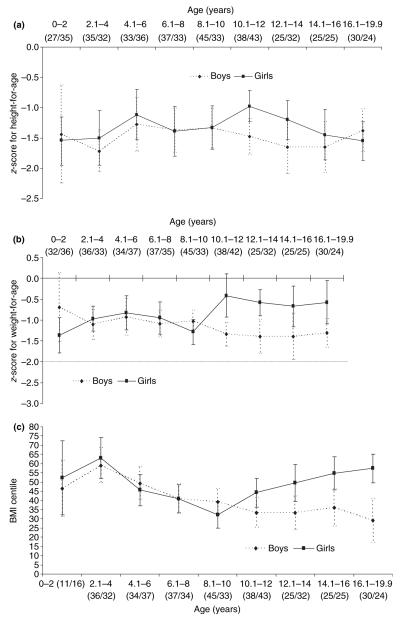
Figure 1 shows the mean HAZ and WAZ scores and the percentiles of BMI by age and sex. The curves for HAZ and WAZ showed little difference between the sexes in terms of growth pattern between the ages of 0 and 10 years, with both remaining relatively flat. But after 10 years of age, the growth lines diverged between sexes. Girls showed compensatory growth from 10 to 12 years in contrast to boys who showed a decline in growth until 16 years of age, after which a slight increase was observed (Figure 1a). The compensatory growth spurt seen in girls seemed to occur only between the ages of 10 and 12 years, after which it stopped. The pattern of divergence between the sexes after 10 years was also apparent in the mean WAZ scores and BMI-for-age percentile (Figures 1b,c), although the increase was not present after 16 years among boys.
Figure 1.
Mean z-scores of (a) height-for-age, (b) weight-for-age and (c) body mass index percentiles of boys and girls. Vertical bars indicate 95% confidence levels. Sample sizes in parentheses (boys/girls).
11.6% and 28.8% of individuals had low lean body mass and low fat body mass respectively (Table 3). Adults and the elderly showed lower fat mass than children and adolescents (34.4% and 37.1% vs. 24.8% and 22.5%, P = 0.002) (Table 3). Low fat body mass prevalence did not differ between sexes, but excess in fat body mass was higher in females than males (2.2% vs. 0.2%, P = 0.010).
Biochemical markers of nutritional status
Overall, the prevalence of anaemia was 12.5% and was more evident in children (24.5%) and the elderly (14.7%, P < 0.001) than in adults. The prevalence of anaemia did not differ according to sex. The overall prevalences of iron deficiency and iron overload were 13.1% and 2.2% respectively. The risk of iron overload was observed only among adults and the elderly (Table 3). The prevalence of anaemia decreased with age until 60 years, after which it increased (infants 50%, pre-school children 21.3%, school children 13.8%, adolescents 5.6%, adults 3.8%, elderly 14.7%; P < 0.0001). A similar pattern was observed for the prevalence of iron deficiency (Table 3).
Hypoalbuminaemia was detected in 5.5% of those who provided blood samples. Only one individual (aged 22 years) had severe hypoalbuminaemia (<2.8 mg/dl).
Effect of intestinal helminths on nutritional status
The relationships between nutritional status and helminth infection among children/adolescents and adults/elderly are presented in Table 4. Multiple regression analysis showed that, after adjusting for age, sex and socioeconomic status, there was a significant positive association between undernutrition and A. lumbricoides infection among children and adolescents. An increase in the faecal A. lumbricoides egg count of 10 000 epg increased the odds of having stunting among children and adolescents by 47% [adjusted odds ratio (AOR) = 1.47, 95% CI = 1.00–2.16], low lean body mass by 93% (AOR = 1.93, 95% CI = 1.11–3.35) and low fat body mass by 68% (AOR = 1.68, 95% CI = 1.12–2.51). For adults and the elderly, hookworm infection was negatively associated with overweight and positively associated with low fat mass, such that an increase of 10 000 epg of hookworm reduced the odds of being overweight by 35% (AOR = 0.65, 95% CI = 0.06–0.66) and increased the odds of having low fat body mass by 91% (AOR = 1.91, 95% CI = 1.08–3.35). An increase in 10 000 epg of A. lumbricoides increased the odds of having low fat body mass by 87% (95% CI = 1.07–3.28) in this age group. In this analysis, the odds ratios of having low lean body mass of males were 8.71 (3.93–19.32) and 13.8 (5.82–32.84) greater than females in children/adolescents and adults/elderly respectively. The odds ratio of being overweight of males was 0.64 (0.26–0.64) within the adults and elderly group. No significant association was found between nutritional status and infection with S. mansoni in this population.
Table 4.
Odds ratio for association between nutritional status and intensity of helminth infection between children/adolescents and adults/elderly
| Nutritional status | Association | Odds ratio* |
95% CI | P-value |
|---|---|---|---|---|
| Children and adolescents | ||||
| Stunting | Ascaris lumbricoides | 1.47 | 1.00–2.16 | 0.048 |
| Low lean mass | A. lumbricoides | 1.93 | 1.11–3.35 | 0.019 |
| Low fat mass | A. lumbricoides | 1.68 | 1.12–2.51 | 0.012 |
| Adults and elderly | ||||
| Overweight | Hookworm | 0.65 | 0.06–0.66 | 0.008 |
| Low fat mass | Hookworm | 1.91 | 1.08–3.35 | 0.025 |
| A. lumbricoides | 1.87 | 1.07–3.28 | 0.029 | |
Adjusted for age group, sex and socio-economic status, and clustering by household. Odds ratios represent the odds associated with a change in faecal egg count of 10 000.
Discussion
In assessing the relationship between nutritional status and helminth infection, we have shown that A. lumbricoides infection is more likely to influence the nutritional status of children and adolescents, whereas hookworm infection has a greater impact on adults and the elderly. Stunting was associated with A. lumbricoides infection in infancy, which confirms the studies conducted elsewhere (Tshikuka et al. 1997; Saldiva et al. 1999). In children, no significant association was found between hookworm infection and alterations in anthropometric parameters; although previously in this population, infection with hookworm was significantly associated with anaemia (Brooker et al. 2007). Regarding body mass status, we found no association between underweight and helminth infection, but we did find lower odds of being overweight in adulthood, which was, in general, more prevalent in this population.
Potential reasons for the observed positive association between low fat body mass and hookworm infection might be impairment of the host’s nutritional status by disrupted nutrient absorption and reduced appetite, both of which are mechanisms described in animal models (Albonico & Savioli 1997). Therefore, although some studies have concluded that hookworm rarely contributes to malnutrition (other than the important exception of iron deficiency anaemia), the impairment in fat body mass observed in this study reveals an important association between hookworm infection and malnutrition among the age groups with the highest prevalence of infection. Similarly, infection with A. lumbricoides was associated with reduced growth profile and protein-calorie body reserves in children and adolescents. This is probably due to reduced intestinal absorption and luminal obstruction, which can lead to anorexia and blocking of the absorbing surface (Stephenson et al. 2000). It is interesting to note the link found in this study between specific nutritional deficiencies in different age groups and specific parasites (i.e. the association between hookworm and weight, and between A. lumbricoides and height, lean and fat body mass). Previously these specific deficiencies have been reported in few cases and most studies refer to only a general association between helminth infection and malnutrition. In this population, S. mansoni infection was not associated with malnutrition, in contrast to other studies that have shown an association with childhood stunting (Parraga et al. 1996; Assis et al. 2004).
In this study, overweight was more prevalent than underweight and there was a trend towards increasing overweight with age (but the prevalence of overweight decreased in the elderly). This is similar to previous nutritional surveys conducted in Brazil that have indicated a nutritional transition to a higher prevalence of overweight than underweight (POF 2004). But the prevalence of underweight in the studied population was higher and the prevalence of overweight was lower than in Brazilian adults and the elderly overall (POF 2004). An almost threefold higher prevalence of stunting was observed in the studied infants than the overall prevalence in Brazil (POF 2004). Children, especially younger ones, and the elderly, showed a tendency to be more undernourished for both sexes. Crispim et al. (2004) reported similar findings in a descriptive nutritional study in rural Brazil, in which children and the elderly were more underweight than adolescents and adults. In the current study, boys within the adolescent group showed more susceptibility to being underweight than girls [which corresponds to other studies conducted in Southeast and Northeast Brazil (Pinto et al. 2005)]. Among adults, women were dystrophic (either underweight or overweight) more often than men, which corresponds to prior nutritional surveys conducted in a rural area of Southeast Brazil (POF 2004).
The HAZ, WAZ and BMI-for-age curves showed relatively flat growth patterns until the age of 8 years for both sexes; then all the three indices increased for girls until the age of 12 years. Indices for boys remained flat until the age of 14 years when an increase was seen that, in the case of HAZ, surpassed the mean score for girls. The decrease in the mean HAZ score observed after the age of 12 years is very different from the growth pattern seen in a similar study conducted in Tanzania, where HAZ progressively increased throughout adolescence, likely representing a more prolonged compensatory growth spurt than was seen in the girls of the current study (Lwambo et al. 2000). A difference was also observed for WAZ in girls: a plateau observed after a brief spurt between the ages of 8 and 12 years. This was again in contrast to Tanzanian girls who had a progressive increase in this parameter throughout adolescence. It is apparent that compensatory growth in height begins to occur at 10 years among girls and at 14 years among boys in the population studied. Lwambo et al. (2000) found that compensatory growth began at 16 years for boys and 12 years for girls in Tanzania, whereas in a study of South African children, compensatory growth occurred at 15.5 years in males and 13.5 years in females (Cameron et al. 1994).
These findings suggest earlier compensatory growth among the studied girls (at 10 years) relative to other malnourished populations. This earlier compensatory growth phenomenon may explain the low mean heights observed in older girls in our study (mean heights of female adolescents were low, being below the 5th percentile, compared to a reference population) and the short final adult height. Some authors have stressed that normal adult height can still be attained among those who have delayed maturation (Cameron 1991; Cameron et al. 1994); but this was not observed in Americaninhas females, who experienced a normal age at menarche of 12.9 ± 1.4 years (data not shown) compared to Brazilian girls living in a satisfactory socioeconomic environment (12.8 years) (Picanço 1995). Thus, our findings suggest that earlier compensatory growth in stunted girls who experience a normal age at menarche could compromise their final height. But longitudinal studies are required to investigate this hypothesis further.
The high rate of anaemia (50%) in the infants from Americaninhas is similar to that found in other regions of Brazil (Torres et al. 1994; Neves et al. 2005) and remains an important public health problem. Studies from São Paulo State found infant anaemia prevalence ranging from 47.8% to 68.7% (Monteiro et al. 2000). In other regions of the country, such as the North, Northeast and South, the anaemia prevalence in this age group was 66.6%, 82.8% and 54.0% respectively (Neves et al. 2005). The anaemia prevalence variation by age group in our study is in4 accordance with other studies (Monteiro et al. 2000), showing higher prevalence of anaemia in the first years of life followed by a decrease with age. The prevalence of anaemia in the elderly was higher than the estimated percentages in industrialized countries, although it was lower than that of non-industrialized countries (WHO 1995). As shown by Brooker et al. (2007), the increase of prevalence in anaemia might be associated with helminth infection in this group.
In conclusion, stunting was the most prevalent form of undernutrition observed in this study. It has an impact on growth and perhaps on being overweight in adulthood, the most prevalent nutritional abnormality observed in adults. These data suggest that chronic undernutrition in the first years of life in this population may lead to the low adult stature that contributes to the development of5 overweight (Sawaya & Roberts 2003). The most vulnerable age groups for malnutrition were children and the elderly and, in general, males were more susceptible to having reduced body mass. A significant association between helminth infection and age-related nutritional status was found. Ascaris lumbricoides was associated with impaired growth patterns in childhood and hookworm infection associated with impaired body mass (with regard to both BMI and fat body mass) in adulthood. These associations may result in adverse consequences for children in terms of mortality, morbidity, growth and cognitive performance that eventually translate into reduced physical and work capacities in adulthood (WHO 1995). This may negatively affect the economic situation of this population where agricultural work is the dominant economic activity.
The association between parasitic infection and malnutrition found in this study could be due either to the direct effect of intestinal parasites on nutritional status (Stephenson et al. 2000) or to the effect of undernourishment on the immune response leading to an increased susceptibility to infection (Bundy & Medley 1992). Prospective studies will be conducted to confirm the effect of intestinal parasites on nutrition.
Acknowledgements
Our warm thanks to the inhabitants of Americaninhas for their participation and co-operation. We also thank the field staff for their untiring and dedicated work during field trips. Thanks are also given to Dr. Neal Alexander and Anna Carolina Lustosa for the statistical support. This work was financially supported by the Human Hookworm Vaccine Initiative (HHVI) of the Albert B. Sabin Vaccine Institute (Washington, DC). SB is supported by a Wellcome Trust Research Career Development Fellowship (081673). DJD is an employee of the Albert B. Sabin Vaccine Institute. No conflicting interests arise from these funding sources.
References
- Albonico M, Savioli L. Hookworm infection and disease: advances for control. Annali Dell Istituto Superiore Di Sanita. 1997;33:567–579. [PubMed] [Google Scholar]
- Assis AMO, Prado MS, Reis ML, et al. Childhood stunting in northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. European Journal of Clinical Nutrition. 2004;58:1022–1029. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- Beltrame A, Scolari C, Torti C, et al. Soil transmitted helminth (STH) infections in an indigenous community in Ortigueira, Paraná, Brazil and relationship with nutritional status. Parassitologia. 2002;44:137–139. [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Blackburn GL, Bistriam BR, Maini BS, et al. Nutritional and metabolic assesment of the hospitalized patient. Journal of Parenteral and Enteral Nutrition. 1977;1:11–22. doi: 10.1177/014860717700100101. [DOI] [PubMed] [Google Scholar]
- Brooker S, Jardim-Botelho A, Quinnell RJ, et al. Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in south-eastern Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:146–154. doi: 10.1016/j.trstmh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Medley GF. Immuno-epidemiology of human geohelminhiasis: ecological and immunological determinants of worm burden. Parasitology. 1992;104(Suppl.):105S–119S. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- Cameron N. Human growth, nutrition, and health status in sub-Saharan Africa. Yearbook of Physical Anthropology. 1991;34:211–250. doi: 10.1002/ajpa.1330340611. [DOI] [PubMed] [Google Scholar]
- Cameron N, Gordon-Larsen P, Wrchota EM. Longitudinal analyses of adolescent growth in height, fatness, and fat patterning in rural South African black children. American Journal of Physical Anthropology. 1994;93:307–321. doi: 10.1002/ajpa.1330930304. [DOI] [PubMed] [Google Scholar]
- Carli GA. Parasitologia Clínica — Seleção de Métodos e Técnicas de Laboratório Para Diagnóstico das Parasitoses Humanas. Atheneu; São Paulo: 2001. [Google Scholar]
- CDC Growth charts for the United States: Methods and development. Vital and Health Statistics. 2002;1:246. [PubMed] [Google Scholar]
- Crispim SP, Grillo LP, Santos PFS, et al. Nutritional situation of population in the city with low index of human development, Castro Alves — Bahia. Nutrição Brasil. 2004;3:297–303. [Google Scholar]
- Drachler ML, Andersson MC, Leite JC, et al. Social inequalities and other determinants of height in children: a multi-level analysis. Cadernos de Saúde Pública. 2003;19:1815–1825. doi: 10.1590/s0102-311x2003000600025. [DOI] [PubMed] [Google Scholar]
- Filmer D, Pritchett L. Estimating wealth effects without expenditure data — or tears: an application to educational enrolment in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Fleming FM, Brooker S, Geiger SM, et al. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Tropical Medicine and International Health. 2006;11:56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends in Parasitology. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. American Journal of Clinical Nutrition. 1981;34:2540–2545. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- Hughes RG, Sharp DS, Hughes MC, et al. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. International Journal of Environmental Health Research. 2004;14:163–177. doi: 10.1080/0960312042000218589. [DOI] [PubMed] [Google Scholar]
- Jellife DB. The Assessment of the Nutritional Status of the Community. WHO; Geneva: 1966. (WHO Monograph no. 53). [Google Scholar]
- Katz N, Chaves A, Pelligrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Revue Instituto Medicina Tropical. 1972;14:817–820. [PubMed] [Google Scholar]
- Lwambo NJS, Brooker S, Bundy DAP, et al. Age patterns in stunting and anaemia in African schoolchildren: a cross-sectional study in Tanzania. European Journal of Clinical Nutrition. 2000;54:36–40. doi: 10.1038/sj.ejcn.1600890. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Szarfarc SC, Mondini L. Secular trends in child anemia in S. Paulo city, Brazil (1984–1996) Revista de Saúde Pública. 2000;34:62–72. [PubMed] [Google Scholar]
- Muniz PT, Ferreira MU, Ferreira CS, et al. Intestinal parasitic infections in young children in São Paulo, Brazil: prevalences, temporal trends and associations with physical growth. Annals of Tropical Medicine and Parasitology. 2002;96:503–512. doi: 10.1179/000349802125001311. [DOI] [PubMed] [Google Scholar]
- Neves MBP, Silva EMK, Morais MB. Prevalence and factors associated with iron deficiency in infants treated at a primary care center in Belém, Pará, Brazil. Cadernos de Saúde Pública. 2005;21:1911–1918. doi: 10.1590/s0102-311x2005000600041. [DOI] [PubMed] [Google Scholar]
- Parraga IM, Assis ALO, Prado MS, et al. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. American Journal of Tropical Medicine and Hygiene. 1996;55:150–156. doi: 10.4269/ajtmh.1996.55.150. [DOI] [PubMed] [Google Scholar]
- Picanço MRA. A Idade da Menarca da Menina Brasileira: os Fatores Socioeconômicos e as Diferenças Regionais. Análise dos Dados da ONSN, 1989 [tese] Instituto Oswaldo Cruz; Rio de Janeiro: 1995. [Google Scholar]
- Pinto SL, Franceschini SCC, Priore SE. Nutritional profile, body composition and eating habits in adolescents from Viçosa MG. Nutrição Brasil. 2005;4:251–257. [Google Scholar]
- POF . Pesquisa de Orçamentos Familiares 2002-2003: primeiros resultados: Brasil e grandes regiôes. Coordenaçãode Índices de Preços. IBGE; Rio de Janeiro: 2004. [Google Scholar]
- Quihui L, Valencia ME, Crompton DW, et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:225. doi: 10.1186/1471-2458-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quihui-Cota L, Valencia ME, Crompton DWT, et al. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98:653–659. doi: 10.1016/j.trstmh.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Ridout MS, Demetrio CGB, Firth D. Estimating intraclass correlation for binary data. Biometrics. 1999;55:137–148. doi: 10.1111/j.0006-341x.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- Saldiva SR, Silveira AS, Philippi ST, et al. Ascaris—Trichuris association and malnutrition in Brazilian children. Pediatric and Perinatal Epidemiology. 1999;13:89–98. doi: 10.1046/j.1365-3016.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Sawaya AL, Roberts S. Stunting and future risk of obesity: principal physiological mechanisms. Cadernos de Saúde Pública. 2003;19(Suppl. 1):S21–S28. doi: 10.1590/s0102-311x2003000700003. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121:S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Dreyfuss ML, Chwaya HM, et al. Hookworm control as a strategy to prevent iron deficiency. Nutrition Reviews. 1997;55:223–232. doi: 10.1111/j.1753-4887.1997.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Torres MA, Sato K, Queiroz SS. Anemia em crianças menores de dois anos atendidas nas unidades básicas de saúde no Estado de São Paulo. Revista de Saúde Pública. 1994;28:290–294. doi: 10.1590/s0034-89101994000400008. [DOI] [PubMed] [Google Scholar]
- Tshikuka JG, Gray-Donald K, Scott M, et al. Relationship of childhood protein-energy malnutrition and parasite infections in an urban African setting. Tropical Medicine and International Health. 1997;4:374–382. doi: 10.1111/j.1365-3156.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Ulukanligil M, Seyrek A. Anthropometric status, anaemia and intestinal helminthic infections in shantytown and apartment schoolchildren in the Sanliurfa province of Turkey. European Journal of Clinical Nutrition. 2004;58:1056–1061. doi: 10.1038/sj.ejcn.1601932. [DOI] [PubMed] [Google Scholar]
- WHO . Physical Status: the Use and Interpretation of Anthropometry. WHO; Geneva: 1995. (WHO Technical Report Series no. 854). [PubMed] [Google Scholar]
- WHO . Iron Deficiency Anaemia: Assessment, Prevention, and Control — a Guide for Programme Managers. WHO; Geneva: 2001. [Google Scholar]
- WHO . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. WHO; Geneva: 2002. (WHO Technical Report Series 912). [PubMed] [Google Scholar]