Abstract
The hippocampus is a key brain region regulating complex cognitive and emotional responses, and is implicated in the etiology of depressive and anxiety disorders, many of which exhibit some degree of sex difference. The male rat hippocampus is consistently reported to be slightly but significantly larger than the female. The majority of studies on the development of volumetric sex differences have focused on the effects of estradiol (E2), with relatively few focusing on androgens. We examined the impact of both E2 and androgens on newly born cells in the developing rat hippocampus, and report that neonatal males have significantly more 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU)+ cells than females. Both testosterone (T) and dihydrotestosterone treatment of females significantly increased the number of BrdU+ cells, an effect blocked by the androgen receptor antagonist, flutamide. However, only T significantly increased the number of neuronal nuclear antigen+ neurons in the female rat hippocampus. Interestingly, E2 treatment also increased BrdU+ cells in females, but had no effect on neuron number. Instead, E2 and T significantly increased the number of newly born glial fibrillary acidic protein or glutamine synthetase+ glial cells in females, indicating that androgens and E2 may act independently to achieve distinct endpoints. Quantification of pyknotic cells at two different developmental time points indicates no sex difference in the number of cells dying, suggesting, but not proving, that gonadal steroids are promoting cell genesis.
Keywords: estradiol, glia, neurons, sex difference, testosterone
Introduction
The hippocampus is critically involved in a range of behaviors, including learning, memory, stress, fear, and depressive and anxiety disorders, all of which vary to some degree between males and females (Bale, 2006; Goldstein, 2006). Understanding the etiology of sex differences in the brain could provide insight into the origins of mental illness as well as normal cognitive function, given the central role of the hippocampus in many affective disorders (McEwen, 2007; McCarthy, 2008). Male/female differences in hippocampal morphology tend to be small (reviewed in McCarthy & Konkle, 2005), but are consistent in that males tend to be larger than females, especially the CA1 region (Allen et al., 1989; Allen & Gorski, 1990; Madeira & Lieberman, 1995; Isgor & Sengelaub, 1998, 2003; Rabinowicz et al., 1999; Nunez et al., 2003a,b). The larger hippocampus in males is evident as early as 1 week of life, prior to sex differences in body and brain weight, and correlates with more neurons in males (Hilton et al., 2003; Nunez et al., 2003a,b), as well as more glia (although only in CA3; Conejo et al., 2003).
Sex steroids are potent modulators of hippocampal structure and function (Woolley & McEwen, 1993; Pilgrim & Hutchison, 1994; McEwen, 1999; Ivanova & Beyer, 2000; Rudick & Woolley, 2001). Estrogens have been the focus of most previous studies, with less attention given to the impact of androgens (with exceptions, e.g. Kerr et al., 1995; Isgor & Sengelaub, 1998, 2003; Leranth et al., 2003, 2004; MacLusky et al., 2006; Spritzer & Galea, 2007). The developing male brain, including the hippocampus, is exposed to both estrogens and androgens (Weisz & Ward, 1980), and the levels of estradiol (E2) are similar to those seen in the hypothalamus (Amateau et al., 2004). Both androgen and estrogen receptors are present in the hippocampus, and are actually elevated during development (Sar & Stumpf, 1973; Lieberburg et al., 1977; Handa et al., 1986; Roselli, 1991; Kerr et al., 1995; Ivanova & Beyer, 2000; Solum & Handa, 2001; Brannvall et al., 2005; Wang et al., 2006).
The concordance of high levels of estradiol, testosterone and dihydrotestosterone benzoate, along with their cognate receptors, in the early postnatal period suggests these steroids are essential regulators of rat hippocampal development and may determine the sex difference in hippocampal volume. Hormones could increase the volume of the hippocampus by altering any of three basic developmental processes: (i) increased cell genesis; (ii) decreased cell death; and/or (iii) increased migration of cells into the hippocampus, as well as by increasing axonal and/or dendritic arbors. To date, most of the well-characterized volumetric sex differences reported in other parts of the nervous system employ the common mechanism of differential cell death during a perinatal sensitive period, with more cells dying in one sex than the other (Nordeen et al., 1985; Murakami & Arai, 1989; Arai et al., 1994; Davis et al., 1996; McCarthy et al., 1997; Forger, 2006). Sex differences in the timing of cell proliferation (Jacobson & Gorski, 1981) and in the migratory path of hypothalamic neurons (Wolfe et al., 2005) have been identified, but in neither case have these been implicated as substantially contributing to volumetric sex differences. In the developing hippocampus we found no evidence for a sex difference in cell death (Nunez et al., 2003a), leading us to hypothesize that cell birth may be a contributor to the larger hippocampus in males. The present study investigated the effects of androgens and estrogens on the number of newly born cells in rat hippocampus using 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU) as a marker. We further examined the effects of steroids on cell type in the hippocampus, and found that estrogens and androgens affect the cell types differently. We also confirmed no sex differences in cell death at two early developmental time points at which we did see sex differences in BrdU+ cells.
Materials and methods
Animals
Timed-pregnant Sprague–Dawley rats were purchased from Charles River Laboratory (Wilmington, MA, USA) and allowed to deliver naturally; the day of birth was defined as postnatal day 0 (PND0). Animals were housed under a 12: 12 h light: dark cycle, with food and water freely available. All animal procedures were approved by the University of Maryland at Baltimore Institutional Animal Care and Use Committee and followed National Institutes of Health Guidelines.
Hormonal treatment
On PND0, male and female rat pups were randomly distributed into different experimental groups as illustrated in Table 1, and accordingly marked by subcutaneous ink injection in either the front or hind paws. On both PND0 and PND1, pups were removed from dams and placed on a heating pad (37 °C) to maintain body temperature, then injected subcutaneously with steroids. Testosterone propionate (T) (100 μg/0.1 mL), the non-aromatizable androgen dihydrotestosterone benzoate (DHT) (100 μg/0.1 mL) and estradiol benzoate (E2) (100 μg/0.1 mL) were dissolved in sesame oil vehicle. The doses of steroid are those routinely used by this laboratory to induce sexual differentiation of reproductive parameters (Mong et al., 1999), and are required to overcome the sequestering capacity of alpha-fetoprotein in the neonatal bloodstream (Amateau et al., 2004). The propionate and benzoate moieties allow for slower absorption rates of the steroids, and do not alter hormonal action. Some rats were also injected subcutaneously with the androgen receptor antagonist, flutamide (FLU, 250 μg/0.1 mL, 50 mg/kg). All rats were injected with BrdU i.p. daily from PND1 to PND4 (0.05 mL distilled water containing 50 mg/kg BrdU), except for the experiment in which animals were injected with BrdU only on PND0 and brains collected 24 h later. The extended time course was designed to maximize the potential of detecting new cells following steroid treatment. The injection sites were sealed with cyanoacrylate Vetbond Surgical Adhesive (3M Animal Care Product, St Paul, MN, USA). Pups were randomly distributed to different dams after each treatment.
Table 1.
Experimental treatments and brain weights
| Sex and treatment | Brain weight (g) |
|---|---|
| Female | |
| Vehicle | 0.47 ± 0.01 |
| T | 0.44 ± 0.02 |
| DHT | 0.47 ± 0.01 |
| FLU | 0.46 ± 0.01 |
| Vehicle/Vehicle | 0.47 ± 0.01 |
| T/FLU | 0.46 ± 0.01 |
| E2 | 0.41 ± 0.01 |
| E2/DHT | 0.43 ± 0.01 |
| Male | |
| Vehicle | 0.47 ± 0.01 |
| T | 0.48 ± 0.01 |
| DHT | 0.48 ± 0.01 |
| FLU | 0.47 ± 0.01 |
Pups were collected from four dams on the day of birth, randomly assigned to experimental groups and treated as detailed in the Materials and methods. PND4 brain weights (mean g ± SEM) are not significantly different between groups (ANOVA, P > 0.05). DHT, dihydrotestosterone; E2, estradiol; FLU, flutamide; T, testosterone.
Tissue collection
Two hours after the last PND4 BrdU injection, pups were deeply anesthetized with ketamine/acepromazine (10 mg, i.p.) and transcardially perfused with 0.9% saline until there was no blood trace. Brains were removed, weighed and fixed for 2 h in 4% paraformaldehyde with 2.5% acrolein, followed by 24 h in 4% paraformaldehyde. Brains were stored in 30% sucrose in paraformaldehyde for 72 h and then cryostat-sectioned at 45 μm. Ten–15 sections were collected exclusively from the rostral hippocampus and included the ventromedial nucleus of the hypothalamus.
Immunohistochemistry
Free-floating tissue sections were rinsed with 0.1 M phosphate-buffered saline (PBS) and incubated with 1% sodium borohydride for 20 min, rinsed with PBS, then incubated with 0.04% phenylhydrazine in PBS for 20 min. For BrdU immunohistochemistry, tissue sections were further incubated with 2 N HCl for 60 min at 37 °C to denature DNA, rinsed with PBS, and incubated with 5% goat serum in PBS with 0.4% Triton X-100 (PBS-T) for 60 min, followed by incubation with monoclonal antibody against BrdU (Caltag Laboratories, Carlsbad, CA, USA, 1: 10 000 in PBS-T) at room temperature for 60 min, then for 48 h at 4 °C. Tissue sections were then rinsed and incubated with biotinylated anti-mouse secondary (1: 500, Vector, Burlingame, CA, USA), rinsed with PBS, then incubated in Vectastain Elite ABC reagents (Vector). BrdU-positive cells were detected with nickel-diaminobenzidine (DAB) as a chromogen, giving a deep blue colour to BrdU-positive nuclei. For neuronal nuclear antigen (NeuN) immunohistochemistry, tissue sections were incubated with monoclonal antibody against NeuN (1: 70 000 in PBS-T, Chemicon, Temecula, CA USA) for 60 min at room temperature and then at 4 °C for 48 h. Positive NeuN staining was also visualized via addition of nickel-DAB. Glutamine synthetase (GS; Sigma, 1: 10 000) was visualized with DAB alone. Sections were then rinsed and mounted onto gelatin-subbed slides, dehydrated and coverslipped.
Double-label immunohistochemistry
Some sections were double-labeled with BrdU and a glial specific marker, glial fibrillary acidic protein (GFAP). Cells labeled by both BrdU and GFAP were considered newly differentiated glial cells. The tissue sections were treated as described above, except that after BrdU visualization, sections were thoroughly rinsed overnight then incubated with an antibody against GFAP (Sigma, 1: 10 000), followed by biotinylated secondary antibodies. The GFAP-positive cells were detected with DAB alone (brown color).
Data analysis
BrdU+ cells
BrdU-immunoreactive cells were counted in three different brain areas: the CA1 region and dentate gyrus (DG) of the rostral hippocampus, and the ventromedial hypothalamus (VMH). For each area, the number of BrdU+ cells in a counting frame (5 × 10 μm with 40 × objective) was determined using National Institute of Health (NIH) Image software. Ten–15 sections were collected throughout the rostral hippocampus from each animal. The number of BrdU+ cells was counted bilaterally only in whole sections (free of any tears or other physical defects) that matched anatomically across all animals, resulting in four sections per animal used. The sex and hormonal condition of the animals from which the sections were generated was unknown to the investigator doing the analysis. The total number of BrdU+ cells across all eight counting fields was averaged to give one value per animal.
GFAP+ and GS+ cells
The number of GFAP+ or GS+ cells in the rostral CA1 region was determined using the same counting frame and imaging software as for BrdU+ cells. The number of GFAP+ or GS+ cells was averaged from three–four sections per brain.
NeuN+ cells
The number of NeuN+ cells was counted in three subfields of CA1, the stratum oriens, stratum pyramidale and stratum radiatum, using the Neurolucida program package (Microbrightfield version 2.01). A counting frame (80 × 80 μm with 60 × objective) was used to count NeuN+ cells in each subfield and averaged from four sections per animal. The total number of NeuN+ cells in the rostral CA1 was obtained from combining the NeuN+ cells in all three subfields.
Pyknotic cells
An additional set of tissue sections was stained with Cresyl violet using standard protocols. The number of pyknotic cells, identified by having dark condensed globular nuclei, was determined in a counting frame (5 × 10 μm with 40 × objective) in the CA1 region of the rostral hippocampus using the NIH image software system, and averaged from four sections for each brain.
Statistics
One-way analysis of variance (ANOVA) was applied to measures of brain weight and the number of BrdU+, NeuN+, GFAP+ and GS+ cells, as well as pyknotic and GFAP+/BrdU+ double-labeled cells. In addition, a two-way ANOVA was performed on the number of BrdU+ cells in vehicle-, T- and FLU-treated males and females. Post hoc Tukey test required a level of P < 0.05 to obtain statistical significance.
Results
The number of BrdU+ cells is higher in males and increased by androgen in females in the rostral CA1 and DG, but not VMH
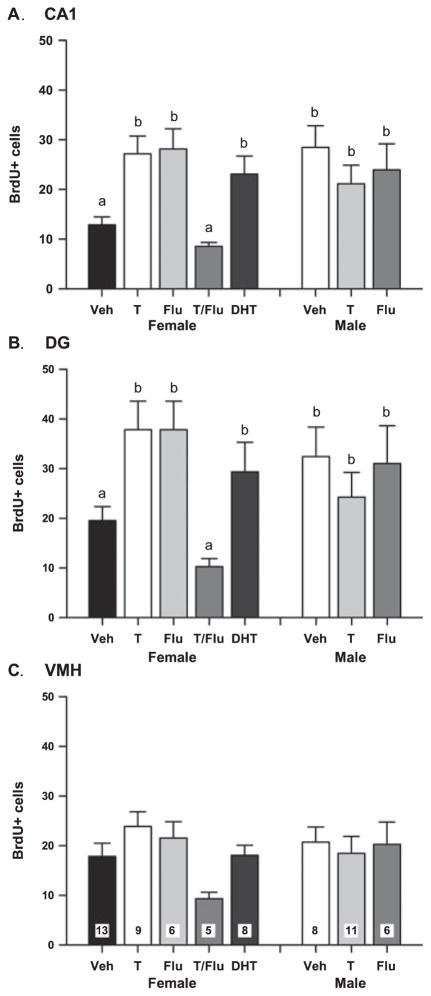
The number of BrdU+ cells in the rostral CA1, DG and VMH was counted in males and females treated with vehicle, DHT, T FLU, or a combination of T and FLU (n = 5–13 per group). A one-way ANOVA indicated a main effect in CA1 (F7,58 = 3.90; P = 0.001), and a two-way ANOVA examining vehicle-, T- and FLU-treated males and females revealed a significant interaction between sex and treatment (F5,47 = 6.05; P = 0.005). Post hoc Tukey tests indicated vehicle-treated males had significantly more BrdU+ cells than their female littermates (P < 0.005), and DHT, T or FLU treatment significantly increased the number of BrdU+ cells in females compared with vehicle-treated females (P < 0.005), raising the number of cells to that seen in males. The same pattern was observed in the DG (one-way ANOVA: F7,58 = 2.41; P = 0.031; two-way ANOVA: F5,47 = 3.82; P < 0.03; Fig. 1B). The ability of FLU to act as an androgen agonist in the absence of androgen has been previously reported (Yeh et al., 1999; MacLusky et al., 2004), and is discussed below. When FLU was administered in combination with T, it completely blocked the effects of the steroid such that the number of BrdU+ cells detected did not differ from control levels (Fig. 1A), implicating the androgen receptor, not the estrogen receptor, as the mediator of the effects of T. In males, there was no effect of either T or FLU treatment on the number of BrdU+ cells in any brain region (Fig. 1A–C).
Fig. 1.
(A) The mean (± SEM) number of 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU)+ cells in the rostral CA1 of PND4 males and females treated with vehicle (Veh), testosterone (T), flutamide (FLU), the combination of T and FLU (T/FLU), or dihydrotestosterone (DHT) on PND0 and 1, and BrdU on PND1–4. Groups with different letters are significantly different from each other (ANOVA, P < 0.05). (B) The mean (± SEM) number of BrdU+ cells in the rostral dentate gyrus (DG) of the same animals as in (A). Groups with different letters are significantly different from each other (ANOVA, P < 0.05). (C) The mean (± SEM) number of BrdU+ cells in the ventromedial hypothalamus (VMH) of the same animals. There were no statistically significant differences between groups. The n/group is indicated in the bar for VMH but applies to all brain regions.
BrdU+ vs pyknotic cells in neonatal rats
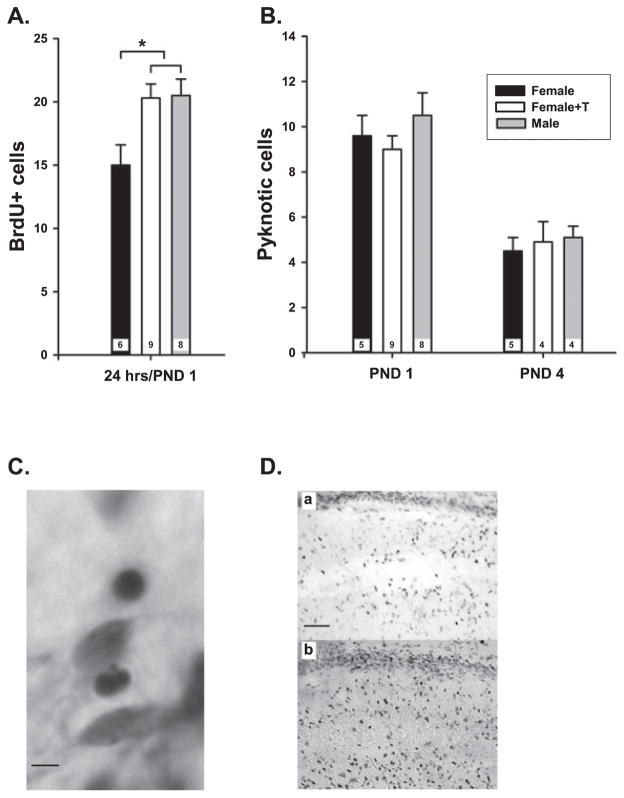
The observation that PND4 males had more BrdU+ cells than females is consistent with a sex difference in cell genesis in the developing hippocampus. However, it is possible that the rate of cell genesis is the same in males and females, but that more cells die in females, leading to an underdetection of new cells selectively in females. To investigate this possibility, pups were injected with BrdU immediately after birth, and the number of BrdU+ cells was examined 24 h later. To assess hormone effects, some pups were also injected with T or vehicle immediately after birth (n = 6–9 per group). There was a sex difference apparent within 1 day of birth, with males having 25% more BrdU+ cells in rostral CA1 compared with females (F2,20 = 5.04; P = 0.016). The ability of T treatment to increase the number of BrdU+ cells was also evident at 24 h after birth (P < 0.05; Fig. 2A), the earliest time point examined after hormone treatment.
Fig. 2.
(A) The mean (± SEM) 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU)+ cells in neonatal CA1 hippocampus of brains collected 24 h after birth. Females were injected with testosterone (T) shortly after birth, and all animals received BrdU at the same time. Males had significantly more BrdU+ cells than females, and T treatment significantly increased the number in females to that of males (ANOVA, *P < 0.05). (B) The number of pyknotic cells detected in Cresyl violet-stained sections from the same animals as in (A) or from animals collected at postnatal day (PND)4 indicated no sex difference or treatment effects on the number of dying cells. The n/group is indicated in each bar. (C) Representative photomicrograph showing the globular condensed nuclei of pyknotic cells in the hippocampus, scale bar: 20 μm. (D) Representative photomicrographs of BrdU+ cells in the neonatal PND4 CA1 hippocampus. (a) female + vehicle; (b) male + vehicle; scale bar: 100 μm.
To gain some insight into whether the sex difference in BrdU+ cells was due to differential rates of cell birth or cell death, adjacent brain sections from the 1-day-old animals used here, as well as sections from earlier experiments at PND4, were stained with Cresyl violet and examined for the number of pyknotic cells in the rostral CA1 region (Fig. 2B; n = 4–9 per group). There was no significant difference in the number of pyknotic cells in the CA1 region either at PND1 or PND4, nor was there an effect of T on the number of pyknotic cells at either time point. There were almost twice as many pyknotic cells at PND1 as PND4 in the CA1 region of the hippocampus (Fig. 2B).
E2 also increases BrdU+ cells in the developing hippocampus
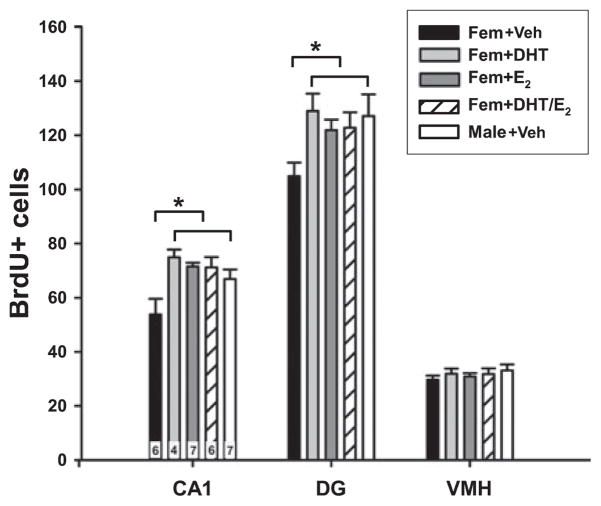
Examination of the data presented in Fig. 1 suggests T has a more potent effect on induction of BrdU+ cells than its 5-alpha-reduced product, DHT, and yet DHT is known to be a more potent androgen. The ability of FLU to block the effects of T on increased BrdU+ cells implicated the androgen receptor, but did not rule out a potential involvement of E2. Therefore, in a separate experiment we explored whether E2 treatment of females also increased the number of BrdU+ cells and whether it acted synergistically with DHT (n = 4–7 per group). The sex difference, with males having more BrdU+ cells in both rostral CA1 and DG, but not the VMH, was once again apparent (F5,29 = 5.47; P = 0.0012; post hoc, P < 0.05). There was also a significant increase in the number of BrdU+ cells in E2-treated females compared with vehicle-treated females, raising them to the level of males (P < 0.05). DHT treatment again increased the number of BrdU+ cells in females to that of males (P < 0.05), as did the combination of DHT + E2 (P < 0.05), but there was no greater effect obtained by combining these two steroids than either one alone (Fig. 3). Thus, E2 treatment increases the number of BrdU+ cells, but does not synergize with DHT. This may be due to a ceiling effect, the maximum number of new cells in a given period has been achieved by either treatment alone, or there may be an antagonism between the effects of E2 and DHT when the two steroids are combined.
Fig. 3.
The mean (± SEM) number of 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU)+ cells in the CA1 and dentate gyrus (DG) regions of the hippocampus and the ventromedial hypothalamus (VMH) in PND4 animals that had been treated with dihydrotestosterone (DHT), estradiol (E2), co-administration of DHT + E2, or vehicle. There were significantly more BrdU+ cells in the CA1 and DG of males, and females treated with E2 or DHT compared with females treated with vehicle (ANOVA; *P < 0.05). There was no effect of sex or hormone treatment on the number of BrdU+ cells in the VMH. The n/group is indicated in the bars for CA1, but applies to all brain regions.
Sex and hormone treatment impact on the number of neurons
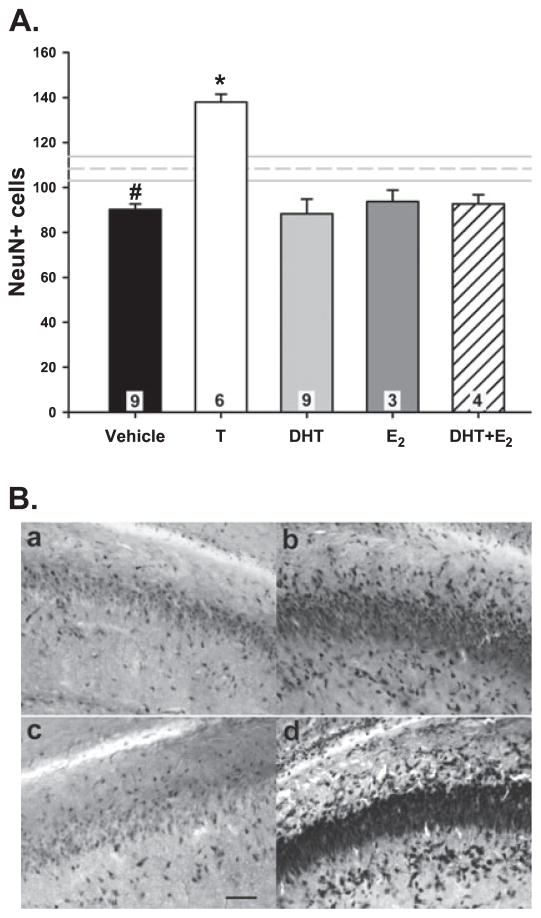
Neurons and glia can be distinguished by detection of cell-specific proteins. NeuN antigen is a protein exclusively expressed by mature neurons that is frequently used as a specific neuronal marker in studies of neurogenesis (Tanapat et al., 1999). We have previously reported that 1-week-old males have more NeuN+ cells in the rostral hippocampus than females (Nunez et al., 2003a,b). In the current study, there were 20% more NeuN+ cells in rostral CA1 of males than females when the animals were only 4 days old (F5,33 = 12.75; P < 0.0001; post hoc, P < 0.05). Treatment of females with T on PND0 and PND1 increased NeuN+ cells by ~50%, which is significantly higher than vehicle-treated females (P < 0.01). There was no effect of treatment with DHT, E2 or E2 + DHT on the number of NeuN+ cells in CA1 (P > 0.05; Fig. 4; n = 3–9 per group). When the effect of androgens on the number of neurons was evaluated separately by CA1 subregion, we observed a significant increase in T-treated females compared with their vehicle-treated female counterparts in the stratum oriens (F5,35 = 11.82; P < 0.001; post hoc, P < 0.001), stratum pyramidale (F5,35 = 10.16; P < 0.001; post hoc, P < 0.001) and stratum radiatum regions (F5,35 = 11.78; P < 0.001; post hoc, P = 0.001; Table 2), as well as a sex difference in stratum oriens (P = 0.01) and stratum radiatum (P = 0.03).
Fig. 4.
(A) Neuron number [neuronal nuclear antigen (NeuN)+ cells] in the CA1 of PND4 female rats treated with vehicle, testosterone (T), dihydrotestosterone (DHT), estradiol (E2), or co-administration of DHT + E2. The mean (± SEM) of vehicle-treated males is depicted by the horizontal dashed and solid lines, respectively. Females had significantly fewer NeuN+ cells compared with males (ANOVA; #P < 0.05). Treatment with T significantly increased neuron number (*P < 0.01 compared with all other groups). The n/group is indicated in each bar. (B) NeuN+ cells in the CA1 region of the hippocampus: (a) female + vehicle; (b) male + vehicle; (c) female + DHT; (d) female + T; scale bar: 100 μm.
Table 2.
NeuN+ cells in subfields of the CA1
| Group | Stratum oriens | Stratum pyramidale | Stratum radiatum |
|---|---|---|---|
| Female: vehicle | 14.7 ± 1.2 | 58.2 ± 2.5 | 17.3 ± 0.9 |
| Male: vehicle | 21.8 ± 2.2* | 64.2 ± 3.5 | 22.7 ± 1.3* |
| Female: T | 30.7 ± 1.3* | 78.3 ± 1.7* | 29.0 ± 1.3* |
| Female: DHT | 18.4 ± 1.1 | 50.4 ± 5.0 | 18.9 ± 1.4 |
| Female: E2 | 20.3 ± 1.9 | 54.0 ± 3.1 | 19.4 ± 2.1 |
| Female: E2 + DHT | 20.4 ± 0.8 | 55.7 ± 3.9 | 21.4 ± 0.6 |
The number of NeuN+ cells was counted in the CA1 subfields: stratum oriens, stratum pyramidale and stratum radiatum (n = 6–9 per group). The data are expressed as mean ± SEM. Asterisks indicate statistical significance compared with vehicle-treated female (ANOVA, *P < 0.01). DHT, dihydrotestosterone; E2, estradiol; T, testosterone.
Sex and hormone treatment impact on the number of new glia
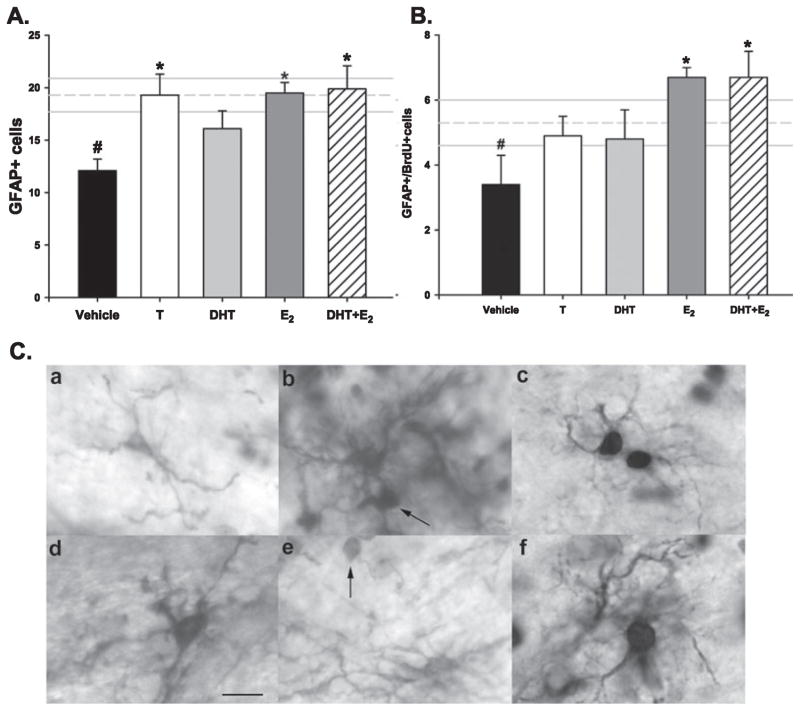
Glial cells can be identified by the expression of the specific glial markers GFAP or GS (Didier et al., 1986; Akimoto et al., 1993; Linser et al., 1997). Both GFAP+ and GS+ cells were detectable immunohistochemically in the CA1 region of the hippocampus on PND4 (Fig. 4C). Because GFAP is a cytoplasmic protein and BrdU is localized to the nucleus, double-label immunohistochemistry can identify cells as newly produced glial cells. By this approach, we were able to identify three different types of cells: single GFAP+ cells; single BrdU+ cells; and BrdU+/GFAP+ cells. We also quantified the number of GS+ cells (Table 3; n = 6–10 per group). Males had significantly more total GFAP+ cells in rostral CA1 than females (F5,49 = 4.19; P = 0.003; post hoc, P < 0.01), and T treatment of females increased the glial cell number to that of males and significantly higher than vehicle-treated females (P < 0.05). E2 treatment, whether given alone or in combination with DHT, also significantly increased the number of GFAP+ cells in females to that seen in males (P < 0.05). However, there was no effect of the non-aromatizable androgen, DHT, on the number of GFAP+ cells (P = 0.20; Fig. 5A). Males also had more GS+ cells than females (F3,20 = 3.86; P = 0.025), and T treatment of females significantly increased the number of GS+ cells to that of males (P < 0.05), but there was no effect of DHT treatment (Table 3). We observed 30% of GFAP-labeled cells were also BrdU+, and males had significantly more BrdU+-labeled glia (BrdU+/GFAP+) than females (F5,41 = 2.376; P = 0.05), but only treatment with E2 or the combination of E2 and DHT significantly increased newly born glia in females (P < 0.05; Fig. 5B). Thus, a consistent requirement for increased glia is E2 or an androgen that can be aromatized to E2.
Table 3.
GS+ cells in the CA1 region of the rostral hippocampus
| Group | GS+ cells (n) |
|---|---|
| Female: vehicle | 11.04 ± 2.1 |
| Female: T | 18.96 ± 1.6* |
| Female: DHT | 13.43 ± 2.6 |
| Male: vehicle | 18.73 ± 1.6* |
The number of GS+ cells was counted in the CA1 region of the rostral hippocampus. The data are expressed as mean ± SEM. Asterisks indicate statistical significance compared with vehicle-treated female (ANOVA, *P < 0.05). DHT, dihydrotestosterone; T, testosterone.
Fig. 5.
Total glial fibrillary acidic protein (GFAP)+ and GFAP+/5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU)+ double-labeled cells in the CA1 region of PND4 female rats treated with testosterone (T), dihydrotestosterone (DHT), estradiol (E2), co-administration of DHT + E2, or vehicle. The mean (± SEM) of vehicle-treated males is depicted by the horizontal dashed and solid lines, respectively. The n/group for both graphs is indicated in each bar of (A). (A) Females had significantly fewer GFAP+ cells compared with males (ANOVA #P < 0.05). Treatment with T, E2 or DHT + E2 significantly increased total GFAP+ number compared with vehicle female (*P < 0.05), while DHT alone had no effect. (B) Females also had significantly fewer GFAP+/BrdU+ double-labeled cells compared with males (ANOVA #P < 0.05), and only treatment with E2 or DHT + E2 significantly increased GFAP+/BrdU+ cell number compared with vehicle-treated females (*P < 0.05). (C) PND4 rat hippocampus double-labeled with BrdU/GFAP (a–c) or BrdU/GS (d–f). Arrows: BrdU+ nuclei. Scale bar: 25 μm.
Sex and hormonal effects on brain weight
Brain weights were obtained on PND4 from male and female neonatal rats treated with vehicle, T, DHT, E2 or FLU, as illustrated in Table 1. No overall difference in brain weight was detected among the groups.
Discussion
The present data demonstrate a sex difference and hormonal modulation of the number of newly born cells in the developing rat hippocampus, with the neonatal male rostral CA1 and DG having more BrdU+ cells than the female. Both androgens and estrogen treatment increased the number of BrdU+ cells in females. There was no sex difference or hormonal modulation of BrdU+ cell number in the VMH, a brain region essential to sexually dimorphic reproductive function and rich in steroid receptors, suggesting the currently observed effects may be specific to the hippocampal formation. Moreover, based on our observation of hormonal modulation of cell type, we hypothesize that the ultimate fate of new cells as neurons or glia depends on the precise hormonal milieu, with androgens favoring differentiation and/or survival of neurons, and estrogens favoring differentiation and/or survival of glia. Because these experiments provide a snapshot view of the dynamic developing hippocampus, hormones may also participate in maturational timing of neurons; for example, T may push a cell already fated to become a neuron faster down that path. Cell-specific effects of androgens vs estrogens have been previously proposed in the context of adult hippocampal cell genesis (Galea, 2007).
Distinguishing the relative contribution of androgens vs estrogens, and the respective roles of cell birth, differentiation and death to the final hippocampal phenotype has proved challenging. Females treated with either DHT or T had more BrdU+ cells than controls, the latter effect being blocked by FLU, an androgen receptor (AR) antagonist widely used to block androgen’s effects in vivo (Naghdi et al., 2001, 2003; MacLusky et al., 2004; Conejo et al., 2005), suggesting that this phenomenon is regulated by androgens. T treatment of males did not further increase BrdU+ cells and there was no effect of FLU either, thus prenatal androgens may be sufficient to initiate the male pattern of new cells. In females, however, FLU alone significantly increased the number of BrdU+ cells, exerting the same magnitude effect as T. There are several published reports of FLU acting as an AR agonist in the absence of T (Yeh et al., 1999; MacLusky et al., 2004). Because we observed that DHT and T both increased the number of BrdU+ cells in females, and that FLU blocked the effect of T treatment, we hypothesized the effects of T were via activation of AR, as opposed to secondary to its aromatization to E2 and activation of estrogen receptor (ER). Contrary to expectation, treatment of females with E2 or E2 combined with the non-aromatizable androgen, DHT, also significantly increased the number of BrdU+ cells in female hippocampus, suggesting ER activation may also be important.
The use of BrdU immunocytochemistry to detect newly born cells has important limitations, the two most critical being: (i) more BrdU+ cells does not necessarily mean more cell genesis; and (ii) new cells does not mean new neurons. To address the first issue, we compared the number of pyknotic, or dying, cells between males and females and females treated with T at the same time points that we compared the number of BrdU+ cells on PND1 and PND4. Given that there is a substantial amount of naturally occurring cell death in the rat hippocampus during the first day of life (Nunez & McCarthy, 2004), and that this is likely to be an important component of the natural progression of hippocampal maturation, it is possible that the greater number of BrdU+ cells in males was a byproduct of more new cells dying in females. In other words, the rate of cell genesis could be identical between the sexes, but more of these cells naturally die off in females, especially because both androgens and estrogens are neuroprotective in the neonatal brain (Hammond et al., 2001; Hsu et al., 2001; Hilton et al., 2006). There was a sex difference and T-induced increase in BrdU+ cells as early as 24 h after birth (PND1), but there was no sex difference or effect of T on the number of pyknotic cells observed in the brains of the same animals. There also was no mean difference in the number of dying cells in PND4 brains. This suggests that differential cell death is not contributing to the sex difference and hormonally induced increase in new cells that we observed at these two developmental time points, and supports a mechanism of differential cell genesis, with more new cells being born in the neonatal male hippocampus. The hippocampus is relatively late maturing, with cell genesis continuing well into the first 2 weeks of life, and synaptogenesis peaking thereafter but not completing until weaning (~PND22, reviewed in McCarthy & Konkle, 2005). Thus, the period of time we are examining is the most likely for hormonally induced organization of the overall cellular profile of the adult hippocampus, which will itself continue to undergo hormonally mediated modifications (Mazzucco et al., 2006).
To investigate the second limitation of BrdU staining, the phenotype of new cells, we employed complementary approaches of counting the total number of neurons using the neuron-specific marker NeuN, the total number of astrocytes labeled with the glia-specific markers GFAP and GS, and the number of cells double-labeled with GFAP and BrdU. We did not determine the fate of specific BrdU+ cells as neurons, as it requires as much as 2 weeks for this process to be complete in the adult brain (Galea, 2007). Instead, taking advantage of the dynamic phase of hippocampal development upon which we are focusing, we used the number of NeuN+ cells at the same time point as BrdU+ cells as a proxy measure for how new cells, born prior to this date, are differentiating. On PND4, males had significantly more NeuN+, GFAP+ and GS+ cells than females. The number of NeuN+ cells was increased in females by treatment with T, which can be aromatized to E2 or reduced to DHT. Neither E2, DHT, nor the combination of E2 and DHT significantly increased NeuN+ cell number in females. It was surprising that the combination of E2 and DHT did not mimic the effect of T, a result that would be predicted if T was acting via its two primary metabolites. Regardless, the fact that E2 had no effect either alone or with DHT implicates androgens as the critical mediator of neuron number, by altering rates of neuronal genesis, phenotypic maturation and/or survival. A much different picture emerged for hormonal effects on the number of cells identified as glia. T treatment of females increased the number of GFAP+ and GS+ cells to the same level as males, but DHT had no effect. In this case, however, E2 treatment also significantly increased the number of GFAP+ cells, regardless of whether DHT was present. Most importantly, the number of new glia (GFAP+/BrdU+) was markedly and significantly increased by E2 treatment, with and without DHT. Thus, E2 action appears to be the critical determinant of differentiation and/or survival of GFAP-expressing astrocytes.
The hippocampus is a target organ for both estrogens and androgens; however, evidence points to androgens playing a more central role in hippocampal development. The density of receptors for androgens is considerably higher than those for estrogens, with the highest AR being consistently detected in the CA1 region of both males and females (Simerly et al., 1990; Roof & Havens, 1992; Brown et al., 1995; Kerr et al., 1995). By contrast, expression of estrogen receptors are at relatively low levels throughout the hippocampus, although like AR, ERα and ERβ expression is elevated during the first week of life and then rapidly falls to adult levels (Ivanova & Beyer, 2000; Solum & Handa, 2001; Wang et al., 2006). The cellular distribution of these receptors varies, with androgen receptors being heavily expressed in the principle cells, although they are also found at low levels in glia (Tabori et al., 2005), whereas estrogen receptors are more common in interneurons and glia of CA1, but are expressed at high levels in the pyramidal cell layer of CA3 of the rat brain (Mehra et al., 2005). In the developing human hippocampus, estrogen receptors are found predominantly in pyramidal neurons as well as radial glia (Gonzalez et al., 2007). Endogenous androgens are important to the development of the male CA1 and CA3 (Isgor & Sengelaub, 1998, 2003), although, interestingly, the relative impact of estrogen vs androgen treatment on these parameters is region specific. For example, CA1 volume is masculinized by T or E2, but not DHT treatment, whereas CA3 volume is masculinized by T or DHT, but not E2 (Isgor & Sengelaub, 1998). Our observation that the number of BrdU+ cells in CA1 was masculinized (increased in females to that of males) by treatment with T or E2, but less so with DHT, and that more neurons and more glia were detected only following T or E2 treatment, respectively, but not DHT, is consistent with these previous findings. It will be of considerable interest to determine what percentage of the sex difference in hippocampal regional volume is contributed by the increased proliferation of neurons and glia or decreased cell death, vs a potential involvement of increased dendritic arborization (Isgor & Sengelaub, 2003). Moreover, whether the steroid-modulated differences in BrdU-labeled cells that we report in the CA1 are also present in the CA3 region remains to be determined. We also reported a sex difference in newly born cells in the DG at 4 days old. Neural stem cells isolated from the fetal DG express AR and proliferate in response to treatment with an androgen analog, an effect blocked by FLU (Brannvall et al., 2005).
Taken together, these results suggest that both androgens and estrogens are responsible for sculpting the sex difference in hippocampal size. Both steroids increase the number of newborn cells, but androgens preferentially support the emergence of neurons, while estrogen promotes the presence of glia in the developing hippocampus. We cannot determine whether the effect of the steroids is predominantly on cell genesis or cell survival, and further experiments will be required to definitively determine the specific role of both steroids. Understanding how both types of hormones impact the developing hippocampus will illuminate the varied roles this brain structure plays in a myriad behavioral and physiological responses that are influenced by the sex of the individual.
Acknowledgments
This work was supported by NIH grant R01 NS050525-02 (M.M.M.).
Abbreviations
- AR
androgen receptor
- BrdU
5-bromo-2′-deoxyuridine-5′-monophosphate
- DAB
diaminobenzidine
- DG
dentate gyrus
- DHT
dihydrotestosterone benzoate
- E2
estradiol benzoate
- ER
estrogen receptor
- FLU
flutamide
- GFAP
glial fibrillary acidic protein
- GS
glutamine synthetase
- NeuN
neuronal nuclear antigen
- PBS
phosphate-buffered saline
- PBS-T
PBS + 0.4% Triton X-100
- PND
postnatal day
- T
testosterone propionate
- VMH
ventromedial hypothalamus
References
- Akimoto J, Itoh H, Miwa T, Ikeda K. Immunohistochemical study of glutamine synthetase expression in early glial development. Brain Res Dev Brain Res. 1993;72:9–14. doi: 10.1016/0165-3806(93)90154-3. [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Arai Y, Murakami S, Nishizuka M. Androgen enhances neuronal degeneration in the developing preoptic area: apoptosis in the anteroventral periventricular nucleus (AVPvN-POA) Horm Behav. 1994;28:313–319. doi: 10.1006/hbeh.1994.1027. [DOI] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Sharma M, Heisler LE, Karsan N, Walters MJ, MacLusky NJ. In vitro labeling of gonadal steroid hormone receptors in brain tissue sections. Steroids. 1995;60:726–737. doi: 10.1016/0039-128x(95)00107-2. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Cimadevilla JM, Arguelles JA, Diaz F, Vallejo-Seco G, Arias JL. Influence of gonadal steroids on the glial fibrillary acidic protein-immunoreactive astrocyte population in young rat hippocampus. J Neurosci Res. 2005;79:488–494. doi: 10.1002/jnr.20372. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Pedraza C, Navarro FF, Vallejo G, Arias JL. Evidence for sexual difference in astrocytes of adult rat hippocampus. Neurosci Lett. 2003;339:119–122. doi: 10.1016/s0304-3940(02)01484-2. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Didier M, Harandi M, Aguera M, Bancel B, Tardy M, Fages C, Calas A, Stagaard M, Mollgard K, Belin MF. Differential immunocytochemical staining for glial fibrillary acidic (GFA) protein, S-100 protein and glutamine synthetase in the rat subcommissural organ, nonspecialized ventricular ependyma and adjacent neuropil. Cell Tissue Res. 1986;245:343–351. doi: 10.1007/BF00213941. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.008. in press. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50:612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Reid DL, Resko JA. Androgen receptors in brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod. 1986;34:293–303. doi: 10.1095/biolreprod34.2.293. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;116:383–391. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen UY, Hsu C. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J Neurophysiol. 2001;86:2374–2380. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberburg I, Maclusky NJ, McEwen BS. 5alpha-Dihydrotestosterone (DHT) receptors in rat brain and pituitary cell nuclei. Endocrinology. 1977;100:598–607. doi: 10.1210/endo-100-2-598. [DOI] [PubMed] [Google Scholar]
- Linser PJ, Trapido-Rosenthal HG, Orona E. Glutamine synthetase is a glial-specific marker in the olfactory regions of the lobster (Panulirus argus) nervous system. Glia. 1997;20:275–283. [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–2398. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145:4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–134. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Besmer HR, Jacobs SC, Keidan GM, Gibbs RB. Influence of maternal grooming, sex and age on Fos immunoreactivity in the preoptic area of neonatal rats: implications for sexual differentiation. Dev Neurosci. 1997;19:488–496. doi: 10.1159/000111246. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Permanence of brain sex differences and structural plasticity of the adult brain. Proc Natl Acad Sci USA. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Kyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal and adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucleus of the amygdala in Morris water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003a;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003b;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Cell death in the rat hippocampus in a model of prenatal brain injury: time course and expression of death-related proteins. Neuroscience. 2004;129:393–402. doi: 10.1016/j.neuroscience.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Pilgrim C, Hutchison JB. Developmental regulation of sex differences in the brain: can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–855. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: more neurons in males; more processes in females. J Child Neurol. 1999;14:98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Autoradiographic localization of radioactivity in the rat brain after the injection of 1,2-3H-testosterone. Endocrinology. 1973;92:251–256. doi: 10.1210/endo-92-1-251. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Nat1 Acad Sci USA. 2006;103:16983–16988. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yeh S, Kang HY, Miyamoto H, Nishimura K, Chang HC, Ting HJ, Rahman M, Lin HK, Fujimoto N, Hu YC, Mizokami A, Huang KE, Chang C. Differential induction of androgen receptor transactivation by different androgen receptor coactivators in human prostate cancer DU145 cells. Endocrine. 1999;11:195–202. doi: 10.1385/endo:11:2:195. [DOI] [PubMed] [Google Scholar]