Abstract
Status hierarchies constitute a fundamental organizing principle of human society. However, little is known about the neural systems that process nonverbal cues that indicate status. Preliminary neuropsychological work has suggested a role for the ventrolateral and ventromedial prefrontal cortex (VLPFC/VMPFC) and the superior temporal cortex (STC). We used functional magnetic resonance imaging to delineate the nature of these roles. Analyses revealed signal changes in the right VLPFC in connection with two primary functions attributed to status cues. Status cues moderate behavior and the right VLPFC showed increased signal for high-status relative to neutral and low-status cues. The VLPFC also showed increased signal for high-status cues displayed by individuals of the opposite gender to the perceiver; this may be relevant to the role status cues play in moderating mate choice behavior. Connectivity results indicated significant positive connectivity between the VLPFC and both the VMPFC and the STC. We suggest that the VLPFC retrieves information from these regions when processing hierarchy cues to facilitate socially adaptive behavior.
INTRODUCTION
Responding to social hierarchy cues is crucial for successful social interactions in humans and other social animals. The perception of hierarchy cues modulates many adaptive behaviors, such as the direction of visual attention (Deaner, Khera, & Platt, 2005), response inhibition (Anderson & Berdahl, 2002; Estep et al., 1988), and mate selection (Miller & Todd, 1998). Clinical reports implicate focal lesions of the ventrolateral prefrontal cortex (VLPFC), the ventromedial prefrontal cortex (VMPFC), and the dorsolateral prefrontal cortex (DLPFC) in impaired processing of social hierarchy information in humans (Karafin, Tranel, & Adolphs, 2004; Mah, Arnold, & Grafman, 2004; Blair & Cipolotti, 2000). Although these findings are suggestive, the specific neural systems involved in processing nonverbal displays of hierarchy are not yet known.
Status is conveyed through nonverbal cues, including gestures and facial cues (Hall, Coats, & LeBeau, 2005). High-status cues reverse undesirable behaviors in subordinates and restrict behaviors such as aggression and approach (Anderson & Berdahl, 2002; Fournier, Moskowitz, & Zuroff, 2002; Estep et al., 1988; Kleinke, 1986). Individuals displaying high-status cues become foci of attention because monitoring high-status individuals yields valuable information for adaptive social behavior (Deaner et al., 2005). Among those behaviors is mate selection: Social dominance is associated with reproductive success in multiple species (Pusey, Williams, & Goodall, 1997; Fedigan, 1983). Given this, individuals who prefer high-status opposite-gender individuals may maximize reproductive fitness. This suggests that mechanisms exist for guiding selective attention to high-status opposite-gender conspecifics.
Little information exists about the neural regions involved in processing status cues. Anecdotal reports suggest that patients with lesions in the VLPFC (Brodmann’s areas 47 and inferior regions of 45) show reduced responsiveness to hierarchy information, indicating this region may be involved (Blair & Cipolotti, 2000). The VLPFC is known to play a role in modulating behavioral responding (Blair, 2004), which is also an important function of status cues (Karafin et al., 2004). Neuropsychological data (Karafin et al., 2004) have also implicated the VMPFC (Brodmann’s areas 11–13, 25 and inferior regions of 10 and 32), a region broadly involved in socioemotional processing (Bechara, Damasio, & Damasio, 2000; Adolphs, 1999). Single-cell recording studies suggest that the superior temporal cortex (STC) may contain “‘dominance hierarchy’ or ‘social status’ cells” (Allison, Puce, & McCarthy, 2000, p. 275; Hasselmo, Rolls, & Baylis, 1989).
On this basis, we predicted first that activity in the VLPFC, the VMPFC, and the STC would increase in response to high-status cues. Second, we predicted that targets’ and perceivers’ gender would modulate neural responses to status cues. Gender provides independent information about status, and status cues are implicated in mate selection for males and females (de Waal, 1986; Nyquist & Spence, 1986). Third, we predicted that the STC and the VMPFC would show significant functional connectivity with the VLPFC during the processing of hierarchy cues. This is based on the hypothesis that the VLPFC modifies behavior in response to status cues by recruiting information from the STC and the VMPFC. To test these hypotheses, we developed a novel stimulus set based on a meta-analysis assessing status perceptions and nonverbal cues (Hall et al., 2005). Participants viewed photographs from this set during fMRI scanning.
METHODS
Stimuli
We created a novel stimulus set depicting high-status, neutral, and low-status nonverbal cues. The cues were selected on the basis of a recent meta-analysis identifying the nonverbal cues that most reliably contribute to perceptions of status (Hall et al., 2005). Four kinds of nonverbal cues varied across poses: brow position (high-status = lowered/low-status = raised), posture (open/closed), gestures (outwardly/inwardly directed, i.e., illustrators and adapters), and gaze (direct/averted). Neutral variants of each cue were also included. Each high-status and low-status pose combined three status cues with one neutral cue (e.g., dominant gesture, posture, and gaze, but neutral brow position). This design permitted us to assess the extent to which each type of cue independently contributed to the perception of status. An equal number of seated and standing poses were shown. Actors were instructed not to alter any variables other than the three cues involved in each pose (e.g., eye width or mouth position). Photos were acquired using a digital camera. The camera was mounted on a tripod in a large room; the actors stood against a bare wall while posing. The resulting photos were digitally cropped and converted to grayscale, and any anomalies (e.g., red eye) resulting from the camera flash were corrected using Adobe Photoshop. The resulting stimulus set consisted of 384 gray-scale photographs of eight male and eight female professional actors (M age = 29.8 ± 5.0 years) showing eight high-status, eight neutral, and eight low-status poses.
We conducted pilot testing to confirm that apparent status varied as a function of the cues we selected, and to assess the extent to which each type of cue independently contributed to status perceptions. Twenty healthy adults participated in the behavioral task (10 women and 10 men, M age = 27.9 ± 5.9 years). Participants were tested individually in a sound-attenuated private room. The task was presented on a laptop using the experimental software E-Studio. The stimulus photographs were presented in random order. Participants used key-presses to rate each stimulus on a 1-to-7 scale anchored by the adjectives dominant (7) and subordinate (1). The instructions specified that, “Dominant people are those who have high status and a great deal of power and authority. In contrast, subordinate people are those who have low status and little power and authority.” Participants were not subjected to time restrictions in rating the photographs. Fixation crosses appeared for 500 msec after each stimulus photo.
Behavioral data were analyzed in order to address two questions. First, we wished to confirm that the poses influenced perceptions of status in the manner intended across participants and actors of both genders. Second, we wished to assess whether each type of cue independently affected attributions of status or whether status attributions were determined by a subset of cues. To address the first question, we conducted a 3 (hierarchy cue) × 2 (subject gender) × 2 (stimulus gender) ANOVA. To address the second question, we created dummy-coded variables for each type of cue, coding each pose “1” if the high-status variant of a given type of cue (e.g., open posture) was incorporated in the pose, “0” if it was not incorporated, and “−1” if the low-status variant (e.g., closed posture) was incorporated. We then ran a simultaneous multiple regression on the 24 poses (8 of each status level, of which 4 were seated and 4 standing) to determine the extent to which each cue (posture, gesture, gaze, brow position, as well as being seated vs. standing) predicted perceptions of status.
fMRI Testing
Participants
Thirty-two healthy right-handed participants from the Washington, D.C., metropolitan area volunteered for the study and were paid for their participation in the fMRI task. The data of two participants could not be included due to scanner error, leaving 30 participants (17 women and 13 men, M age = 26.4 ± 4.7 years). Participants gave written, informed consent before participating in the study, in accordance with the guidelines of the Institutional Review Board at the National Institutes of Health. All participants were in good health, with no history of psychiatric or neurological disease.
Imaging Procedure
Scanning during fMRI task performance used a 1.5-Tesla GE Signa scanner. A total of 156 functional images per participant were taken, with a gradient EPI sequence (repetition time = 2500 msec, echo time = 30 msec, 64 × 64 matrix, flip angle = 90°, FOV = 24 cm). Coverage was obtained with 31 axial slices (thickness, 4 mm, in-plane resolution 3.75 × 3.75 mm). Following the functional scans, a high-resolution anatomical scan was obtained (three-dimension spoiled GRASS with inversion recovery prep pulse; repetition time = 8.1 msec, echo time = 1.8 msec, FOV = 24 cm, flip angle = 20°, 128 axial slices; thickness = 1.5 mm, 256 × 256 matrix) in register with the EPI dataset, covering the whole brain.
During the scan session, four event-related fMRI runs were acquired from each subject. Within each run, 96 gray-scale stimulus photographs were presented for 2000 msec each. Each stimulus photograph was followed by a 500-msec fixation cross. Forty-eight fixation trials (jitters) were presented for 2500 msec at random intervals. Each run began and concluded with six 2500-msec baseline fixation trials. While viewing the stimuli, participants indicated the gender of each stimulus individual by pressing a button with the thumbs of the right or left hand.
Each of the four runs used a separate stimulus and was programmed in E-Studio. Stimuli were presented on a computer display that was projected onto a mirror in the MRI scanner. Participants were placed in a light head restraint within the scanner. (Subsequent analyses indicated that no participant moved more than 4 mm during the scan.)
fMRI Analysis
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI) (Cox, 1996). Both individual and group-level analyses were conducted. The first 4 volumes in each scan series, collected before equilibrium magnetization was reached, were discarded, leaving 730 TRs per subject. fMRI modeling commenced at stimulus onset. Motion correction was performed by registering all volumes in the EPI dataset to a volume that was collected shortly before acquisition of the high-resolution anatomical dataset.
The EPI datasets for each participant were spatially smoothed (using an isotropic 6 mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps when generating group maps. The time-series data were then normalized by dividing the signal intensity at each time point by the mean signal intensity for each run and multiplying the result by 100. As a result, all signal amplitude and regression coefficients represent a percent signal change from the mean.
Regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function to account for the slow hemodynamic response (Cohen, 1997). It will be recalled that our task was a 3 (hierarchy cue) × 2 (stimulus gender) design. We thus created six regressors, one for each combination of pose and target gender. An additional regressor was created for fixation point trials.
Linear regression modeling was performed using the full set of regressors to model baseline drift and residual motion artifact. The baseline was modeled by a first-order function and motion artifacts were modeled using the six estimated rigid-body motion parameters.
Voxelwise group analysis involved transforming single-subject beta coefficients into the standard coordinate space (Talairach & Tournoux, 1988). To identify brain regions responding to the hierarchy and gender cues, a 3 (hierarchy cue) × 2 (subject gender) × 2 (stimulus gender) random-effects ANOVA was performed on the means of all regressors compared to baseline (fixation). This resulted in a group map of areas of differential activation. A subset of clusters showing significant differential activation was selected according to a priori hypotheses about the regions involved in decision making. These clusters were used to define functional ROIs. Average activation levels for individual participants within these ROIs were calculated for each regressor. These data were analyzed using contrast comparisons to determine the pattern of activation across conditions. To correct for multiple comparisons, a spatial clustering operation was performed using AlphaSim with 1000 Monte Carlo simulations taking into account the entire EPI matrix, with a mapwise false-positive probability of p < .05. The labeling of anatomical locations was determined by Talairach—Tournoux Daemon.
We measured functional connectivity by examining covariation across the whole brain with the activation in a specified seed voxel in the VLPFC. We selected the maximally activated voxel within the right VLPFC cluster that resulted from the main effect of status cues in our ANOVA. Then individual participants’ time series were converted to common Talairach space according to their structural dataset. The time series within the seed voxel for each subject was extracted. Baseline plus linear and quadratic trend were removed from each voxel’s time series. The average of the resulting time series inside the brain was treated as a global signal and used as a covariate in the correlation analysis. Then, a voxelwise correlation analysis was conducted between each individual voxel’s time series and that of the identified seed. The proportion of the variation in the signal that could be explained by the correlation with the seed was determined by squaring the resulting correlation coefficient. Correlation coefficients were converted to a Gaussian variable using a Fisher transformation formula in order to reduce the skew and normalize the sampling distribution. To identify regions significantly positively or negatively correlated with the target voxel at group level, a one-sample t test was performed on the transformed correlation coefficients.
Behavioral Testing
Valence-relevant ratings of stimuli were collected following the completion of fMRI testing, using a separate set of study participants. Eighteen healthy participants participated in this task (10 women and 8 men, mean age = 29.1 ± 9.8 years). Participants were tested individually in a sound-attenuated private room. The task was presented on a laptop using the experimental software E-Studio. A representative sampling (including equal numbers of all target individuals and all poses) of 48 of the stimulus photographs presented in the fMRI study were presented. Each stimulus photo appeared over a 1-to-7 scale on which participants rated the photograph. Participants rated all photographs on one scale before moving onto the next scale. The order in which the scales were presented was randomized across participants, as was the order of the photographs within each scale. The scales were shown beneath the statements: “This person grabs my attention,” “I find this person attractive,” and “I find it unpleasant to look at this person.” Scales were anchored by the terms “Definitely no” (1) and “Definitely yes” (7). Participants were not subjected to time restrictions in rating the photographs. For each participant, mean ratings were calculated for each stimulus type (high-status, neutral, or low-status) for male and female actors separately.
RESULTS
Stimulus Validation
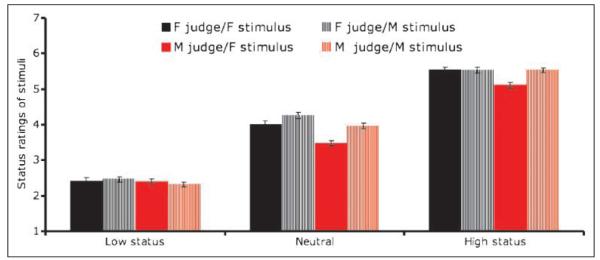
Prior to scanning, we confirmed that apparent status varied as a function of the cues we selected based on a recent meta-analysis (Hall et al., 2005) (for samples, see Figure 1). The results of the ANOVA revealed a highly significant main effect of status cues [F(2, 42) = 997.16, p < .001; Figure 2]. Low-status poses were judged as lower status than neutral poses [t(30) = 19.62, p < .001], which were judged as lower-status than high-status poses [t(30) = 16.90, p < .001]. Results of the regression analysis indicated that each of the four types of cues independently predicted status perceptions [F(5, 18) = 203.44, p < .001; brow position: t = 8.465, p < .001, gaze: t = 5.873, p < .001, posture: t = 4.159, p = .001; gesture: t = 5.486, p < .001]. Whether the pose was seated or standing did not affect status perceptions (t = 1.73, p > .05).
Figure 1.
Sample stimuli showing high (left), neutral (center), and low (right) status poses.
Figure 2.
Ratings of status across male and female stimuli and judges (1 = subordinate; 7 = dominant).
fMRI Data
To identify brain regions responding to the hierarchy and gender cues, a 3 (hierarchy cue) × 2 (subject gender) × 2 (stimulus gender) random-effects ANOVA was performed on the means of all fMRI regressors. The effects directly addressing our hypotheses are reported below: the main effect of hierarchy cues, the interaction of hierarchy cues and stimulus gender, and the interaction among all three factors (hierarchy cues, stimulus gender, and subject gender). The results of these main effects and interactions are reported in Table 1.
Table 1.
Coordinates of Peak Activations and F Values for Regions Demonstrating a Significantly Different BOLD Response for Targets as a Function of Hierarchy Cue, Hierarchy Cue × Stimulus Gender, and Hierarchy Cue × Stimulus Gender × Subject Gender
| Region | L/R | BA | mm3 | F | x | y | z |
|---|---|---|---|---|---|---|---|
| Hierarchy Cue | |||||||
| VLPFC (inferior frontal gyrus)a | R | 47/11 | 933 | 10.02 | 45 | 39 | −16 |
| DLPFC (middle frontal gyrus)b | L | 46 | 589 | 10.42 | −46 | 29 | 24 |
| Middle temporal gyrus | R | 37 | 21,537 | 29.03 | 51 | −67 | 5 |
| Posterior cingulate | L | 30 | 4194 | 16.16 | −10 | −70 | 7 |
| R | 30 | 3408 | 12.55 | 17 | −65 | 2 | |
| Middle occipital gyrus | L | 19 | 19,339 | 26.13 | −50 | −73 | 6 |
| Cuneus | L | 19 | 466 | 9.48 | −27 | −91 | 22 |
| Hierarchy Cue × Stimulus Gender | |||||||
| VMPFC (Orbital gyrus) | R/L | 11 | 408 | 11.03 | 5 | 40 | −22 |
| Amygdala/parahippocampal gyrus | R | 664 | 9.63 | 23 | −7 | −11 | |
| Hierarchy Cue × Stimulus Gender Subject × Gender | |||||||
| VLPFC (inferior frontal gyrus)c | L | 22 | 280 | 7.79 | −52 | 13 | 1 |
| R* | 47 | 213 | 7.40 | 55 | 20 | −1 | |
| Middle frontal gyrus | L | 8 | 676 | 9.30 | −47 | 20 | 45 |
| L* | 6 | 216 | 8.32 | −28 | 18 | 62 | |
| Superior frontal gyrus | L | 6 | 599 | 10.51 | −17 | −13 | 70 |
| STC (Superior temporal gyrus)d | L | 13 | 511 | 7.62 | −53 | −42 | 19 |
| L | 39 | 923 | 9.20 | −59 | −58 | 21 | |
| R | 22 | 319 | 7.65 | 53 | 1 | −1 | |
| R | 41 | 1115 | 8.87 | 57 | −23 | 6 | |
| Inferior temporal gyrus | L | 21 | 1529 | 9.90 | −58 | −22 | −19 |
| L | 20 | 302 | 8.12 | −43 | −6 | −46 | |
| Postcentral gyrus | R | 3 | 260 | 8.85 | 24 | −37 | 55 |
| Fusiform gyrus | R | 37 | 1295 | 11.25 | 44 | −48 | −17 |
| Inferior temporal/middle occipital gyrus | L | 37 | 1553 | 10.32 | −59 | −60 | −13 |
| R | 37 | 2133 | 8.70 | 44 | −69 | −10 | |
| Lingual gyrus | R | 18 | 500 | 7.83 | 14 | −87 | −22 |
| R | 18 | 471 | 8.47 | 29 | −79 | −12 |
All activations significant at p < .005, corrected for multiple comparisons at p < .05; exceptions (*) are significant at p < .005, uncorrected.
For an illustration of the pattern of results in this region, see Figure 3A.
For an illustration of the pattern of results in this region, see Figure 3B.
For an illustration of the pattern of results in this region, see Figure 4A.
For an illustration of the pattern of results in this region, see Figure 4B.
Main Effect of Hierarchy Cues
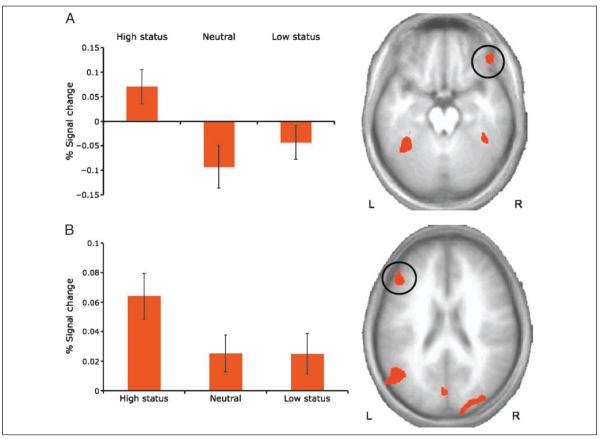
In line with predictions, the main effect of hierarchy revealed significant changes in BOLD responses within the VLPFC (right inferior frontal gyrus, BA 47/11; centered at MNI coordinates x, y, z = 45, 39, −16) (Figure 3A). In addition, significant BOLD responses emerged in regions that included the DLPFC (left middle frontal gyrus, BA 46) (Figure 3B), the bilateral occipito-temporal cortex (including a region of the posterior superior temporal gyri, as well as the middle temporal and middle occipital gyri, extending bilaterally into the fusiform gyrus (BA 37/19), and the bilateral posterior cingulate gyri (BA 30).
Figure 3.
Prefrontal regions obtained from random-effects analysis of the main effect of hierarchy cue (high status, neutral, low status). (A) A region of the right VLPFC showed greater activation during presentation of high-status poses than neutral or low-status poses. (B) A region of the left DLPFC also showed greater activation during presentation of high-status poses than neutral or low-status poses.
Planned t tests indicated that BOLD activation was significantly greater in the VLPFC, the DLPFC, and occipito-temporal clusters for high-status poses relative to neutral poses (ps < .001), but not for low-status poses relative to neutral poses (ps > .05).
Hierarchy Cues × Stimulus Gender
The interaction of hierarchy cues with stimulus gender revealed significant BOLD responses in regions including the right amygdala/parahippocampal gyrus and the VMPFC (right orbital gyrus, BA 11). In both regions, a linear contrast revealed significantly greater BOLD responses as apparent status increased for female actors [t(28) = 5.19 and 2.23, p < .001 and p < .05 for the right amygdala and the VMPFC, respectively]. These contrasts were not significant in either case for male actors.
Hierarchy Cues × Stimulus Gender × Subject Gender
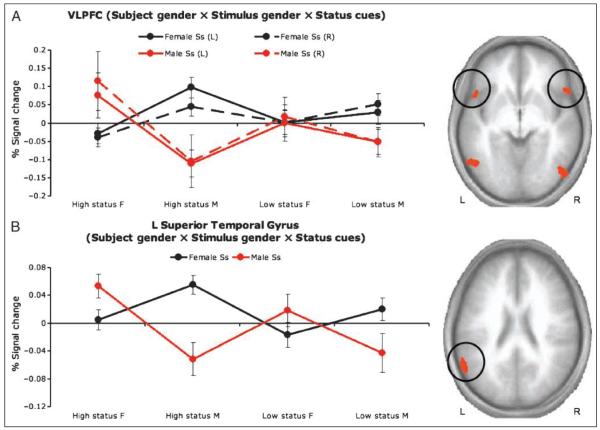
In line with predictions, the interaction among hierarchy cues, stimulus gender, and subject gender revealed significant increases in BOLD activation in bilateral regions of the VLPFC (inferior frontal gyrus, BA 47) and the STC (superior temporal gyrus, BA 41/21). There was also significant interaction within bilateral regions of the occipito-temporal cortex (BA 37).
Across these six regions, consistency emerged in the patterns of BOLD activation. Planned contrast tests (λ = +3 −1 −1 −1) were performed on difference scores calculated by subtracting BOLD activation for neutral poses from those for high-status and low-status poses, respectively. The results of these contrasts indicated that BOLD responses were significantly higher for high-status cues displayed by actors of the opposite gender to the perceiver (i.e., men showed the highest activation when viewing high-status women, and vice-versa; Figure 4) (ps < .05 one-tailed, except the right VLPFC for female participants, p < .10).
Figure 4.
Cortical regions obtained from random-effects analysis of the interaction of Subject gender × Stimulus gender × Hierarchy cue (high status, neutral, low status). (A) Bilateral regions of the VLPFC showed greater activation during presentation of opposite-gender high-status poses than during same-gender poses or opposite-gender low-status poses. This pattern was observed for both female (black lines) and male (red lines) participants. (B) A region of the left STC also showed greater activation during presentation of opposite-gender high-status poses than during same-gender poses or opposite-gender low-status poses.
Table 2 presents the other main effects (subject gender; stimulus gender) and interactions (Subject gender × Hierarchy cue; Subject gender × Stimulus gender) resulting from our ANOVA.
Table 2.
Coordinates of Peak Activations and F Values for Regions Demonstrating a Significantly Different BOLD Response for Targets as a Function of Subject Gender, Stimulus Gender, Subject Gender × Stimulus Gender, and Subject Gender × Hierarchy Cue
| Region | L/R | BA | mm3 | x | y | z |
|---|---|---|---|---|---|---|
| Subject Gendera | ||||||
| Inferior occipital gyrus | R | 18 | 1081 | 31 | −98 | −16 |
| L | 17/18 | 756 | −29 | −101 | −15 | |
| Fusiform | L | 18 | 579 | −44 | −80 | −19 |
| Middle temporal gyrus | L | 21 | 539 | −39 | 7 | −41 |
| Lingual gyrus/Declive | L | 18 | 232 | −11 | −81 | −18 |
| Stimulus Genderb | ||||||
| Middle occipital gyrus | R | 19 | 8210 | 27 | −90 | 4 |
| Lingual gyrus | L | 18 | 6382 | −20 | −98 | −10 |
| Inferior temporal gyrus | R | 37 | 2614 | 52 | −60 | −13 |
| VLPFC (inferior frontal gyrus) |
R | 47 | 920 | 27 | 25 | −12 |
| Posterior cingulate | R | 29 | 865 | 8 | −43 | 7 |
| Caudate | L | 712 | −9 | −10 | 22 | |
| Precuneus | R | 31 | 255 | 27 | −69 | 21 |
| Inferior frontal gyrus | R | 38 | 247 | 48 | 21 | −14 |
| VLPFC (inferior frontal gyrus) |
L | 47 | 230 | −34 | 32 | −13 |
| Subject Gender × Stimulus Gender | ||||||
| Middle temporal gyrus | R | 39 | 1412 | 57 | −72 | 23 |
| Subject Gender × Hierarchy Cue | ||||||
| Cuneus | R | 17 | 812 | 20 | −90 | 6 |
| Middle temporal gyrus | L | 21 | 721 | −64 | −8 | −8 |
| Uncus | R | 28 | 581 | 20 | 2 | −30 |
All activations significant at p < .005 and corrected at p < .05 for multiple comparisons.
All regions show greater activity for male subjects than female subjects with the exception of lingual gyrus/declive.
All regions show greater activity for female stimuli than male stimuli.
Connectivity
We hypothesized that the VLPFC may recruit information from the STC and the VMPFC when responding to status cues (Blair, 2004; Karafin et al., 2004). In line with this prediction, we found significant positive connectivity between our VLPFC seed voxel (x, y, z = 45, 39, −16) and the right STC (BA 22/38) and the left VMPFC (BA 11) (t = 3.643, p < .001, r = .56). This seed was the maximally activated voxel in the VLPFC cluster found for the main effect for status cue (see Table 3 for additional connectivity information).
Table 3.
Regions in Which Activation Varies as a Function of Signal Change in the VLPFC
| Region | +/− | L/R | BA | t | x | y | z |
|---|---|---|---|---|---|---|---|
| VLPFC (inferior frontal gyrus) |
+ | L | 47 | 4.14 | −33 | 25 | −18 |
| VMPFC (middle frontal gyrus) |
+ | L | 11 | 9.47 | −33 | 41 | −10 |
| STC (superior temporal gyrus) |
+ | R | 22/38 | 5.80 | 56 | 14 | −9 |
| Middle temporal gyrus | + | R | 21 | 4.22 | 64 | −7 | −10 |
| Middle temporal gyrus | − | R | 21/37 | 4.05 | 58 | −53 | 2 |
| Medial frontal gyrus | − | L | 32 | 3.93 | −3 | 3 | 48 |
| Cuneus/Lingual gyrus | − | L/R | 18 | 5.27 | 1 | −78 | 5 |
| Superior occipital gyrus | − | R | 19 | 4.60 | 31 | −79 | 28 |
| Precuneus | − | L | 31 | 4.09 | −13 | −68 | 29 |
| Precentral gyrus | − | L | 6 | 4.23 | −43 | −9 | 47 |
| Middle frontal gyrus | − | L | 6 | 3.99 | −21 | 15 | 60 |
Behavioral Data
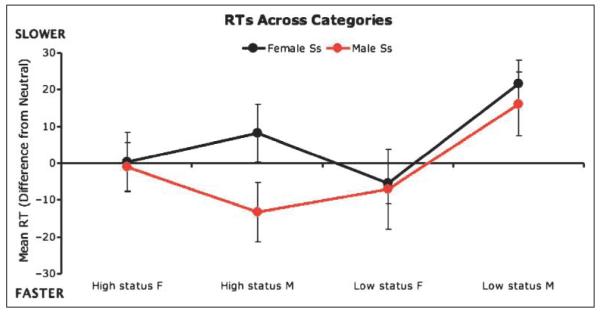
Our fMRI data indicated that activity in brain regions involved in response modulation was most influenced by opposite-gender relative to same-gender high-status cues. To assess whether task performance responses were consistent with this pattern (i.e., that opposite-gender high-status cues influenced responding more than same-gender high-status cues), we analyzed participants’ behavioral responses during the scanning task. Near-ceiling accuracy levels (M = 98.3%, SD = 2.3%) precluded viable analyses of response accuracy. However, analysis of RT data revealed a 3 (hierarchy cue) × 2 (subject gender) × 2 (same-gender/opposite-gender stimulus) interaction among these three variables [F(2, 56) = 7.06, p < .005]. An examination of the means indicated a response pattern similar to that revealed by fMRI data (Figure 5). Subjects responded more slowly to opposite-gender high-status cues than to same-gender high-status cues, but both genders responded similarly to male and female low-status cues.
Figure 5.
Response times (RTs) to high-status, neutral-status, and low-status cues across male and female stimuli and participants.
Additional behavioral testing examined participants’ ratings of the degree to which the stimuli were attention-eliciting, attractive, and unpleasant (Table 4). In line with prior work (Deaner et al., 2005; Gangestad, Simpson, Cousins, Garver-Apgar, & Christensen, 2004; Hawley, 1999), repeated measures ANOVAs showed that high-status cues were rated as the most attention-eliciting cues, and as more attractive than low-status cues (although not, in this sample, neutral cues). High-status cues were not rated as more unpleasant than low-status cues (although they were relative to neutral cues), which is important to note given the role of the VLPFC in this study and previous suggestions that the VLPFC responds to aversive cues (Kringelbach, 2005; O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001).
Table 4.
Behavioral Responses to Hierarchy Cues on Three Indices
| Mean Scores |
|||||
|---|---|---|---|---|---|
| Variable | Effects | t | High Status |
Neutral | Low Status |
| Attention | HS > N | 5.93* | 4.76 | 3.25 | 3.50 |
| HS > LS | 5.86* | ||||
| Attractive | LS < N | 3.57** | 3.06 | 3.32 | 2.93 |
| LS < HS | 2.05*** | ||||
| Unpleasant | HS > N | 4.53* | 4.13 | 3.46 | 3.88 |
| LS > N | 4.26** | ||||
HS = high status; N = neutral; LS = low status.
p < .001.
p < .05.
p < .06.
DISCUSSION
This is the first fMRI study, to our knowledge, to identify the important role that the VLPFC (BA 47/11) plays in the response to hierarchy information. The VLPFC showed greater activity to high-status cues than neutral or low-status cues. Moreover, our connectivity analysis indicated that the VLPFC may orchestrate the response to high-status cues through interaction with the VMPFC (BA 11) and the right STC. Previous neuropsychological (Karafin et al., 2004; Mah et al., 2004; Allison et al., 2000) and animal work (Hasselmo et al., 1989) has implicated these two regions in processing socioemotional cues such as status. We also determined that activity in the VLPFC was particularly enhanced to opposite-gender high-status cues. This occurred despite status cues being identical across male and female targets.
Previous work has suggested that the VLPFC is involved in the representation of punishment information (Kringelbach, 2005; O’Doherty et al., 2001). On this basis, the increased VLPFC response could be interpreted as a response to the aversiveness of high-status cues. Social dominance cues carry implications of aggressive behavior (Hawley, 1999). In the current study, participants rated individuals showing high-status cues as more unpleasant than individuals showing neutral cues. However, activity in the VLPFC can also increase to reward-related stimuli (Budhani, Richell, & Blair, 2006; Zald, Curtis, Chernitsky, & Pardo, 2005). Moreover, participants rated low-status cues as unpleasant and unattractive, but the VLPFC did not show a differential response to these stimuli. A slightly superior region of the VLPFC to the one identified here has been consistently implicated in studies examining reappraisal (Goldin, McRae, Ramel, & Gross, 2008; Phan et al., 2005; Ray et al., 2005; Ochsner, Bunge, Gross, & Gabrieli, 2002). Reappraisal has been conceptualized as a “cognitive—linguistic strategy that alters the trajectory of emotional responses by reformulating the meaning of a situation” (Goldin et al., 2008). It is possible that participants in our study were engaging in reappraisal in response to individuals displaying high status [anonymous reviewer’s suggestion]. However, it is not clear why reappraisal would particularly occur in response to high-status cues of opposite-gender individuals.
An aversive-response interpretation is also incompatible with increased VLPFC responses to high-status opposite-gender cues. Socially dominant individuals are considered attractive social partners and are liked more than lower-status individuals (Hawley, 1999), and dominance is correlated with health, fertility, and reproductive success in humans and other species (Sapolsky, 2005; Gangestad et al., 2004; Pusey et al., 1997; Fedigan, 1983). Taken together, the evidence suggests the VLPFC response in the current study to individuals displaying high-status cues is unlikely to reflect perception of these cues as aversive.
Patterns of VLPFC activation may instead reflect a role in modifying behavioral responding (Budhani et al., 2006; Zald et al., 2005). It has been suggested that the VLPFC modifies behavior through behavioral inhibition (Aron, Robbins, & Poldrack, 2004; Cools, Clark, Owen, & Robbins, 2002; Elliott, Friston, & Dolan, 2000). Compatible with this suggestion, high-status cues are thought to inhibit unwanted behavior in subordinates (Anderson & Berdahl, 2002; Drea & Wallen, 1999). However, recent work assessing the VLPFC’s role in behavior modification suggests that the VLPFC facilitates the activation of alternative motor responses (Budhani et al., 2006; Zald et al., 2005). Following this, and given the present findings, we suggest that the VLPFC orchestrates a change in behavior proportional to the social context, such as the social dominance of other individuals. This may include, but is not limited to, behavioral inhibition.
The region of the VLPFC responding to high-status cues in the current study (x, y, z = 45, 39, −16) is proximal to that shown previously to respond to facial expressions, particularly angry expressions: x, y, z = 42, 42, −16 (LaBar, Crupain, Voyvodic, & McCarthy, 2003; Blair, Morris, Frith, Perrett, & Dolan, 1999); x, y, z = 38, 26, −11 (LaBar et al., 2003); x, y, z = −48, 24, −14 (Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998). This is consistent with suggestions that the VLPFC may use both social hierarchy and emotional cue information to influence appropriate selection of behavioral responses in social contexts (Blair, 2004). For example, anger cues shown by lower-status individuals may prompt antagonistic responding, whereas anger cues shown by higher-status individuals may prompt submissive responding (Blanchard & Blanchard, 2003).
Prior neuropsychological work has suggested a role for the VMPFC in responding to status cues (Karafin et al., 2004). Karafin et al. (2004) found that patients with VMPFC lesions made less use of gender and age information in their dominance judgments although they showed no overall impairment in dominance judgments. Our connectivity analysis indicated that the VLPFC orchestrates the response to high-status individuals through interaction with the VMPFC (BA 11) and the right STC (BA 22). Thus, the VMPFC may be involved in responding to status cues and may interact with the VLPFC, but may not play a primary role. A significant BOLD response within the VMPFC was only seen for the Hierarchy cue × Stimulus gender interaction and not the main effect of status. The VMPFC, like the amygdala, showed a linear increase in BOLD response as status increased for female, but not male, stimuli. A main effect of status in the VMPFC would have been predicted on the basis of prior data. The main effect may simply not have been significant in this study. Future studies may find that the VMPFC responds similarly to male and female stimuli; preliminary data we have collected for a follow-up study show consistent effects of status (high > low) across both genders in the VMPFC.
Prior animal work suggests that the VLPFC retrieves information from the temporal lobes, particularly the STC (Miyashita & Hayashi, 2000; Petrides, 1996). This region is involved in the perception of biological motion (Puce, Allison, Bentin, Gore, & McCarthy, 1998; Oram, Perrett, & Hietanen, 1993), particularly when socially relevant objects are observed (Martin & Weisberg, 2003). Previous work has suggested the involvement of the STC in processing information regarding status cues (Allison et al., 2000; Adolphs, 1999; Hasselmo et al., 1989). The current data support this contention. The main effect of hierarchy cues revealed large bilateral regions of activation that, although centered in BA 19, extended into the posterior STC. Moreover, the connectivity results suggest that the VLPFC may make use of information represented by the STC to orchestrate an appropriate response to status-relevant cues.
The left middle frontal gyrus (DLPFC), in which we observed a main effect of status cues, has frequently been implicated in top—down attentional control (Miller & Cohen, 2001; Desimone & Duncan, 1995). Greater attention is generally directed toward high-status than low-status individuals in a social group, a finding seen across primate species, including humans (Shepherd, Deaner, & Platt, 2006; Kleinke, 1986). Individuals across the social hierarchy will forfeit food in order to view high-status group members (Deaner et al., 2005). Participants in our study also rated high-status cues as more attention grabbing than low-status or neutral cues. Activation in the DLPFC in response to high-status cues may reflect the recruitment of top—down control to increase attention to individuals displaying these cues.
Limitations
One limitation of this study is the absence of explicit behavioral data aimed at paralleling the effects observed in the analyses of neural activation. The fMRI task was designed to minimize explicit task demands, limiting available information about explicit behavioral responses to status cues. Reaction time data for the fMRI did reveal a three-way interaction among stimulus gender, subject gender, and pose, such that both male and female responses were slowed by opposite-gender but not same-gender high-status cues, but no differences were found between male and female subjects looking at low-status cues. This interaction is consistent with VLPFC activation patterns and our hypotheses. However, explicit behavioral data measuring response modification to status cues would be informative. The explicit behavioral data we collected assessed the relationship between status cues and affective valence rather than behavioral response modifications relevant to VLPFC activation. Future studies assessing the relation between explicit behavioral and neural responses would provide valuable information on the nature of the neural responses to status cues.
Another limitation of this study is that the combinations of cues used to create status-related poses limited our ability to determine independent effects of each separate cue on neural activation. However, no data from prior studies indicate that the cues we used (gaze, gesture, posture, or brow position) independently generate VLPFC activation patterns like those we observed. Gaze direction affects activity in the intraparietal sulcus, the fusiform gyrus, the STC, and the VMPFC (Schilbach et al., 2006; Garrett, Menon, MacKenzie, & Reiss, 2004; Kingstone, Tipper, Ristic, & Ngan, 2004; George, Driver, & Dolan, 2001; Hoffman & Haxby, 2000) but not the VLPFC. Nonverbal gestures and postures activate the STC and the primary motor cortex as well as neurons in the “mirror” systems of the ventral premotor and inferior parietal cortex (Blake & Shiffrar, 2007). Changes in object size (relevant to posture changes) activate primarily occipital and parietal regions (Cavina-Pratesi, Goodale, & Culham, 2007). No fMRI studies have examined responses to brow cues in isolation. We conclude that the patterns of VLPFC activation observed in the present study were in response to changes in apparent status created by a combination of cues rather than to any one of the cues in isolation.
Conclusion
The organization of human social groups depends heavily on hierarchical structures. Monitoring the status of other group members and effectively modulating behavior in response to status cues is crucial for an individual’s adaptive functioning. Neurological or psychiatric disorders that impair these processes generally result in serious social deficits (Karafin et al., 2004; Mah et al., 2004; Blair & Cipolotti, 2000). Little prior information exists regarding the neural structures that subserve the processing of status cues. The present data indicate that the VLPFC, in interaction with the STC and the VMPFC, plays a crucial role in monitoring such cues. We suggest that the VLPFC recruits information from these regions during the processing of status cues to facilitate modulation of socially adaptive behavior.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health: National Institute of Mental Health. The authors thank Ellen Condon and Elizabeth Finger for their assistance with data collection, and the anonymous reviewers of this article for their helpful comments.
Footnotes
National Institute of Mental Health
REFERENCES
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Anderson C, Berdahl JL. The experience of power: Examining the effects of power on approach and inhibition tendencies. Journal of Personality and Social Psychology. 2002;83:1362–1377. [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy”. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. What can animal aggression research tell us about human aggression? Hormones and Behavior. 2003;44:171–177. doi: 10.1016/s0018-506x(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: Probabilistic response reversal deficits in adult individuals with psychopathy. Journal of Abnormal Psychology. 2006;115:552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Goodale MA, Culham JC. FMRI reveals a dissociation between grasping and perceiving the size of real 3D objects. Public Library of Science ONE. 2007;2:e424. doi: 10.1371/journal.pone.0000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Waal FB. The integration of dominance and social bonding in primates. Quarterly Review of Biology. 1986;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Drea CM, Wallen K. Low-status monkeys “play dumb” when learning in mixed social groups. Proceedings of the National Academy of Sciences, U.S.A. 1999;96:12965–12969. doi: 10.1073/pnas.96.22.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep DQ, Nieuwenhuijsen K, Bruce KEM, De Neef KJ, Walters PAIII, Baker SC, et al. Inhibition of sexual behaviour among subordinate stumptail macaques Macaca arctoides. Animal Behaviour. 1988;36:854–864. [Google Scholar]
- Fedigan LM. Dominance and reproductive success in primates. Yearbook of Physical Anthropology. 1983;26:91–129. [Google Scholar]
- Fournier MA, Moskowitz DS, Zuroff DC. Social rank strategies in hierarchical relationships. Journal of Personality and Social Psychology. 2002;83:425–433. doi: 10.1037//0022-3514.83.2.425. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA, Cousins AJ, Garver-Apgar CE, Christensen PN. Women’s preferences for male behavioral displays change across the menstrual cycle. Psychological Science. 2004;15:203–207. doi: 10.1111/j.0956-7976.2004.01503010.x. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you, kid: Neural systems underlying face and gaze processing in fragile X syndrome. Archives of General Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Coats EJ, LeBeau LS. Nonverbal behavior and the vertical dimension of social relations: A meta-analysis. Psychological Bulletin. 2005;131:898–924. doi: 10.1037/0033-2909.131.6.898. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behavioural Brain Research. 1989;32:203–218. doi: 10.1016/s0166-4328(89)80054-3. [DOI] [PubMed] [Google Scholar]
- Hawley PH. The ontogenesis of social dominance: A strategy-based evolutionary perspective. Developmental Review. 1999;19:97–132. [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Karafin MS, Tranel D, Adolphs R. Dominance attributions following damage to the ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2004;16:1796–1804. doi: 10.1162/0898929042947856. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: An fMRI investigation. Brain & Cognition. 2004;55:269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: A research review. Psychological Bulletin. 1986;100:78–100. [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cognitive Neuropsychology. 2003;20:575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller GF, Todd PM. Mate choice turns cognitive. Trends in Cognitive Sciences. 1998;2:190–198. doi: 10.1016/s1364-6613(98)01169-3. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Hayashi T. Neural representation of visual objects: Encoding and top—down activation. Current Opinions in Neurobiology. 2000;10:187–194. doi: 10.1016/s0959-4388(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Nyquist LV, Spence JT. Effects of dispositional dominance and sex role expectations on leadership behaviors. Journal of Personality and Social Psychology. 1986;50:87–93. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oram MW, Perrett DI, Hietanen JK. Directional tuning of motion-sensitive cells in the anterior superior temporal polysensory area of the macaque. Experimental Brain Research. 1993;97:274–294. doi: 10.1007/BF00228696. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1996;351:1455–1461. doi: 10.1098/rstb.1996.0130. discussion 1461–1462. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah NJ, Fink GR, et al. Being with virtual others: Neural correlates of social interaction. Neuropsychologia. 2006;44:718–730. doi: 10.1016/j.neuropsychologia.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16:R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Zald DH, Curtis C, Chernitsky LA, Pardo JV. Frontal lobe activation during object alternation acquisition. Neuropsychology. 2005;19:97–105. doi: 10.1037/0894-4105.19.1.97. [DOI] [PubMed] [Google Scholar]