Abstract
Background
Corneal grafting is by far the most common form of transplantation. Many grafts suffer from immune rejection and current therapies are associated with many side effects, requiring more effective and safe therapies. Alpha–melanocyte stimulating hormone (α–MSH) is a neuropeptide that suppresses host inflammatory defense mechanisms. The purpose of this study was to determine the role of local therapy with α-MSH on corneal allograft survival, and the mechanisms by which it may influence graft outcome.
Methods
Orthotopic corneal transplantation was performed, with recipients receiving subconjunctival α-MSH or sham injections twice weekly. Grafts were followed for 70 days and graft inflammation/opacification was compared between the two groups in a masked fashion. Graft infiltration and ocular gene expression of select inflammatory cytokines was evaluated at different timepoints. Additionally, allospecific delayed type hypersensitivity (DTH) was compared among the groups 3 weeks post transplantation.
Results
Results showed a significant increase in corneal graft survival in α-MSH-treated recipients as compared to controls. While 75% of allografts in α-MSH-treated hosts survived at 70 days, 43% survived in controls (p=0.04). Graft infiltration studies demonstrated a significant decrease in the number of mononuclear and polymorphonuclear cells in α-MSH-treated mice as compared to controls at days 7 and 14 after transplantation. Further, allospecific DTH and gene expression of interferon-γ and interleukin-2 showed a significantly reduced expression in α-MSH-treated mice as compared to controls.
Conclusions
In this study, we provide for the first time, in vivo evidence that treatment with local α-MSH may significantly reduce allorejection of orthotopic transplants.
Keywords: alpha-melanocyte-stimulating hormone, neuropeptide, corneal transplantation, dendritic cells, allograft rejection
INTRODUCTION
The eye is an immunologically privileged site; therefore, it is perhaps not surprising that corneal transplantation, the only therapy for many corneal disorders leading to blindness, is the most successful form of solid tissue transplantation, with a 2-year success rate of as high as 90% in uncomplicated non-vascularized and uninflamed host beds (1). In the United States alone, nearly 40,000 grafts are performed each year, making it the most common form of solid tissue transplantation. However, immune rejection remains the most frequent cause of graft failure (2), and in vascularized and inflamed hosts beds, or in eyes with previously failed grafts, graft rejection can exceed 70–90%, even with maximal local and systemic immune suppression (3). In fact, more corneal allografts fail per year in the United States than any other form of solid-tissue transplant, with no improvement in transplant survival over the past several decades. Corticosteroids remain the mainstay of therapy; and the recent usage of cyclosporin A (CsA) and tacrolimus have shown unconvincing benefits on graft survival (4), producing severe ocular and systemic side effects (1, 5). Therefore, the development of less toxic and targeted immunosuppressive methods is a priority in corneal transplantation.
α-MSH is an evolutionarily conserved neuropeptide of 13-amino-acids long with equally conserved melanocortin family of receptors (6). This neuropeptide is produced predominately by the central nervous system but is also produced by monocytes, keratinocytes, epithelial cells, and Langerhans cells (LCs) (7). It is found in the skin, intestinal tract, the lung and the eye (8, 9). While it was initially known for its control of melanogenesis in pigmentary cells, its role as an immunomodulatory and anti-inflammatory peptide has now been established (7, 10, 11). α-MSH has been shown to modulate the activation of NF-kappa B (12), regulating the production of pro-inflammatory cytokines and chemokines, the expression of co-stimulatory factor B7-1 (CD86) and CD40 on dendritic cells (DCs) (13), as well as the migration of neutrophils and macrophages. Further, it inhibits the activation of adhesion molelcues (14), and suppresses macrophage reactive oxygen intermediates and nitric oxide (15). Melanocortins exert their activities via a group of five melanocortin receptors (MC-R) that stimulate pigmentation (MC-1R), inhibit food intake (MC-4R), and inhibit inflammatory responses (MC-1R, MC-3R, and MC-5R) (16). MC-1R, with the highest affinity for α-MSH, is expressed on immune and inflammatory cells, including macrophages, DCs, monocytes, neutrophils, B cells, CD8+ T cells, and natural killer cells, and is upregulated by pro-inflammatory cytokines (10, 13, 17).
Recently, systemic application of α-MSH has been shown to improve rheumatoid arthritis, inflammatory bowel disease, encephalitis, and experimental autoimmune uveitis (18, 19). In addition, the systemic administration of α-MSH has been shown to improve heart transplantation survival (20), although the exact mechanism remains unclear. In the eye, α-MSH is constitutively expressed in the aqueous humor (9), where it is able to suppress T cell activation and production of interferon (IFN)-γ (21). Further, α-MSH treatment in experimental uveitis, induced the expansion of CD4+CD25+ regulatory T cells in the aqueous humor, and suppressed pro-inflammatory cytokines and chemokines (22). Moreover, systemic application of α-MSH in a traumatic ocular inflammation model has been shown to decrease the aqueous humor protein level and the influx of inflammatory cells (23–25).
In the present study, we investigated the possible role of α-MSH on orthotopic corneal allograft survival. We demonstrate, for the first time, that local application of α-MSH, leads to significant allograft survival. The increased survival is associated with a decreased expression of pro-inflammatory cytokines, a decreased influx of inflammatory cells into the graft site, and suppressed delayed type hypersensitivity (DTH) response in grafted hosts. Our results demonstrate not only the therapeutic effects of α-MSH, but also begin to elucidate its mechanistic actions in the transplantation model.
METHODS
Experimental Animals
Seven to 14-week-old male BALB/c and C57BL/6 mice (Taconic Farms, Germantown, NY or from our own breeding facility) were used in these experiments. All protocols were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Animals were housed under specific pathogen-free conditions and were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal Transplantation
Our standard protocol for murine orthotopic corneal transplantation was used for these studies (25). Briefly, the center of the allogeneic donor cornea (C57BL/6 mice) was marked with a 2-mm diameter microcurette, and excised with Vannas scissors (Storz Instruments Co., St. Louis, MO) and placed into chilled phosphate-buffered saline (PBS). The recipient graft bed (BALB/c) was prepared by excising a 1.5-mm site in the central cornea. The donor button was than transplanted into the corneal bed of BALB/c recipients and secured with 8 interrupted 11-0 nylon sutures (Sharpoint, Vanguard, TX). Graft sutures were removed in all cases on day 7. Recipients either received a sham injection (n=12) or were treated with 2.0 μg subconjunctival injections of α-MSH twice weekly (n=16). A third group was initially treated with 2.0 μg subconjunctival injections of α-MSH twice weekly, but treatment was stopped at week 4 (n = 12).
Grafts were evaluated biomicroscopically in a masked fashion for 70 days every 2–3 days, and graft opacity and neovascularization were measured according to a standardized scheme in which grafts with an opacity score of 2+ or greater after 3 weeks were considered rejected (27). Transplants with an opacity score of 3+ or greater, after 2 weeks, which never cleared by 8 weeks, were also regarded as rejected. Mice with surgical complications (cataract, significant anterior synechiae or failure to form an anterior chamber by day 2) were excluded from the study. Both isografts as well as normal (ungrafted) host mice were used as controls in some experiments.
Subconjunctival injection of α-MSH
BALB/c (H-2d) mice were used as recipients of minor H- and MHC- mismatched C57BL/6 (H-2b) corneal transplants. After general anesthesia, subconjunctival injections of 5.0 μl (2.0 μg each) synthesized α-MSH (Peninsula Laboratories, Belmont, CA) in phosphate-buffered saline (PBS) was performed with a 100μl glass syringe (Hamilton Company, Reno, NE) and a 30-gauge needle, that was then repeated twice weekly at rotating conjunctival sites. Transplanted mice received α-MSH treatment indefinitely (n=16) or for 4 weeks only (n=12). The same amount of PBS was used as a control for sham injections (n=12).
RNA Preparation and Ribonuclease Protection Assay (RPA) of Cytokine Expression
At select time points after corneal transplantation (n = 6–10 corneas per experiment for cytokine template), total RNA was extracted by the single-step method using RNA-STAT-60 (Tel-Test Inc., Friendswood, TX). Briefly, whole corneas were homogenized and centrifuged to remove cellular debris. The RNA pellet was resuspended in nuclease-free water and processed together as a group. Detection and quantification of murine cytokine mRNAs were performed with a multiprobe RPA system (BD Bioscience, Franklin Lake, NJ) as recommended by the supplier and as we have previously described (28). Briefly, a mixture of [α-32P] UTP-labeled antisense riboprobe was generated from the cytokine template set mCK1b (2 weeks post transplantation; to analyze the cytokine profile pre-rejection) (BD Bioscience). Ten microgram of total RNA was used in each sample. Total RNA was hybridized overnight at 56°C with 300 pg of the 32P-anti-sense riboprobe mixture. After purification by ethanol precipitation, the samples were resolved on 5% polyacrylamide sequencing gels. The gels were dried and subjected to autoradiography. Protected bands were observed after exposure of gels to x-ray film. The bands were quantitated by densitometric analysis and normalized to glyceraldehyde-3- phosphate dehydrogenase (GAPDH). Naïve mice were used as controls and all samples were analyzed in duplicates.
Histopathological Evaluation of Graft Infiltration
To measure leukocytic infiltration of grafts before any clinical signs of rejection, sham-treated and α-MSH-treated mice were euthanized at day 7 (n=5) and day 14 (n=5) after transplantation and their grafted eyes were enucleated. The enucleated eyes were fixed with 10% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. At least 10 sections per eye were evaluated morphologically by light microscopy in a masked fashion and the total number of leukocytes, polymorphonuclear cells and mononuclear cells counted per section.
Antibodies & Immunohistochemistry
The immunohistochemical staining procedures were performed using FITC-conjugated hamster anti-mouse CD3-e (145-2C11, T lymphocyte marker, BD PharMingen, San Diego, CA). Hamster IgG (BD PharMingen) was used as the isotype control. Grafted corneas were excised from α-MSH-treated and sham-treated BALB/c mice at days 7 and 14. Eight-μm frozen sections were fixed in acetone for immunofluorescence staining as previously described (29). In brief, sections were immunostained with the conjugated primary antibody or isotype-matched control antibody for 2 hours. Thereafter, the samples were incubated with secondary antibodies for 1 hour if necessary. All staining procedures were performed at room temperature, and each step was followed by three thorough washings in PBS for 5 minutes each. The samples were covered with mounting medium (Vector, Burlingame, CA) and analyzed using confocal laser scanning microscope (Leica TCS 4D, Lasertechnik, Heidelberg, Germany). At least 5 sections per eye (n=3) were analyzed for each experiment.
Assessment of Donor-Specific DTH
At three weeks after corneal transplantation, 1 × 106 irradiated (2000 R) spleen cells from donors syngeneic with the corneal grafts (C57BL/6), in 10 μl Hanks’ balanced salt solution, were injected into the right pinnae of sham-treated or α-MSH-treated corneal hosts. As a control, a similar number of spleen cells were injected into the ear pinnae of BALB/c mice that had been immunized 1 week earlier by subcutaneous injection of 10×106 spleen cells of C57BL/6 mice. All experiments and controls consisted of 5 animals per group. After 24 and 48 hours, ear thickness was measured in a masked fashion with a low-pressure micrometer (Mitutoyo, MTI Corporation, Paramus, NJ). Allospecific ear swelling was expressed as follows: specific ear swelling = (24-hr measurement of right ear − 0 hr measurement of right ear) − (24-hr measurement of left ear − 0 hr measurement of left ear) × 10−3 mm. Ear-swelling responses are presented as mean ± SE. Because results at 24 and 48 hours were similar, only 24-hr data are presented. Naïve BALB/c mice were used as controls.
Statistical Analyses
Rates of corneal graft survival were plotted as Kaplan-Meier survival curves and were compared using the log-rank (Mantel-Cox) or Student’s t-test for specific time point for α-MSH-treated mice as compared to the sham-treated controls. mRNA expression values are given as mean ± SE. Student’s t-test was used for comparison of DTH responses and graft infiltration. Statistical significance was defined as p<0.05.
RESULTS
Corneal graft survival in local α-MSH-treated mice
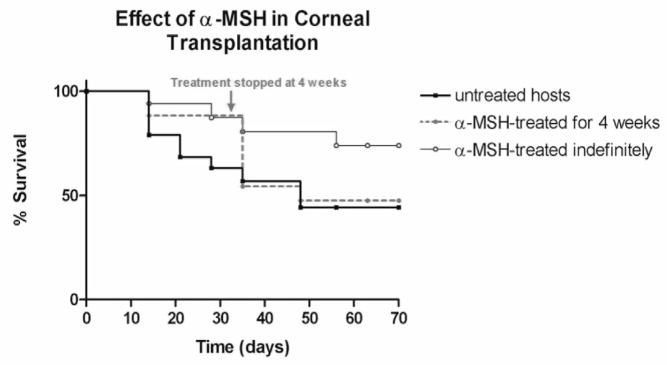
In order to evaluate the corneal graft survival in α-MSH-treated mice as compared with no therapy, we treated recipients with a twice a week local subconjunctival injection of 2.0 μg (5μl) α-MSH and compared the graft survival rate in these mice to sham treatment (Fig. 1). Corneal allografts in α-MSH-treated recipients showed a significant increase in graft survival as compared to sham-treated hosts (p=0.04). 87.5% of allografts treated with α-MSH (n=16) survived at 4 weeks and 75% of allografts in α-MSH-treated hosts survived at 70 days. Recipients treated with sham injections (n=12), however, had a graft survival of 58% at 4 weeks and 43% of sham-treated allografts survived at 70 days. The third group, in which the α-MSH treatment was discontinued at four weeks (n=12), had a similar graft survival as the untreated group, with 47% graft survival by the end of the study at 70 days. Graft survival in this group dropped dramatically within 6 days of stopping the α-MSH treatment. Moreover, we did not observe a difference in the degree of neovasculatization, between the α-MSH-treated and sham-treated group. We conclude that α-MSH treatment significantly increases graft survival and that elevated levels of α-MSH are required beyond 4 weeks.
Figure 1. Increased graft survival with α-MSH treatment.
Kaplan-Meier graft survival curves are shown for allogeneic corneal transplants. Grafts are shown for hosts treated with sham injections (solid bold line, n=12), hosts treated indefinitely with α-MSH (solid line, n=16), and hosts that were treated with α-MSH for 4 weeks only (dotted line, n=12). There is a delay in rejection in α-MSH-treated hosts with a significantly higher survival rate for α-MSH-treated mice as compared to sham treated/untreated control mice after 70 days. When α-MSH treatment was suspended after 4 weeks, there was an increase in graft rejection, with a final survival rate similar to control mice.
Profile of corneal leukocyte infiltration
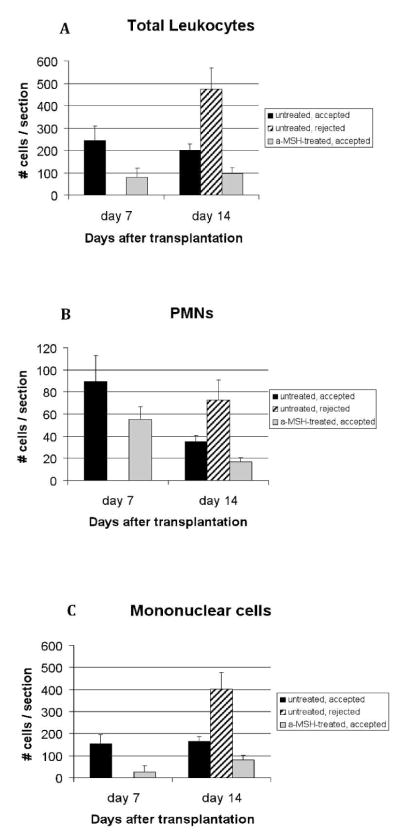
To elucidate the potential mechanisms by which the α-MSH treatment influences corneal graft outcome, we compared the graft infiltration profile between α-MSH-treated hosts and controls. Corneas were excised at either 7 or 14 days after corneal transplantation and sections were subjected to H&E staining for morphological enumeration of mononuclear and polymorphonuclear (PMN) cells (Fig. 2). The results demonstrated a 70% and 51% decrease in leukocyte infiltration in α-MSH-treated hosts as compared to sham-treated hosts at day 7 (p=0.01) and day 14 (p=0.005) respectively (Fig 2A). In addition, the leukocyte infiltration in sham-treated rejected grafts, was increased by 194.6% over the non-rejected sham-treated grafts at day 14. While the decrease in leukocyte infiltration on day 7 in the α-MSH-treated eyes, was partially due to a decrease in PMN cells (Fig 2B), and mainly due to a significant reduction of mononuclear cells (Fig 2C; p=0.004 between α-MSH and sham-treated goups), on day 14 it was both due to a 52.2% reduction of PMN (Fig 2B; p=0.01 between α-MSH and sham-treated goups) and a 51.2% reduction in mononuclear cells (p=0.04).
Figure 2. Leukocyte infiltration into corneal allografts is decreased with α-MSH treatment.
Allogeneic corneal grafts were performed in BALB/c mice, treated with α-MSH (n=10) or sham injections (n=10). At days 7 (n=5 per group, all grafts still accepted as rejection occurs at day 10–14) and 14 (n=5 per group, grafts divided into accepted and rejected), corneas were harvested and the leukocytic infiltration quantified (A) and subtyped morphologically with light microscopy for polymorphonuclear cells (PMN) (B) and mononuclear cells (C). Data depicted as mean ± SE. Significantly decreased infiltration of total leukocytes, PMN and mononuclear cells was observed in α-MSH-treated hosts as compared sham-treated hosts at both time points studied.
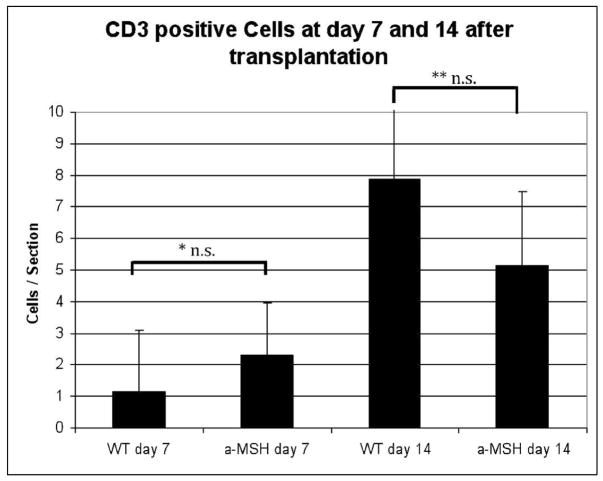
We further evaluated the effect of α-MSH treatment on the number of infiltrating CD3+ T cells based on membrane expression of CD3 on days 7 and 14 after transplantation (Fig. 3). At day 7 after transplantation, only a few CD3+ cells were present in both groups (Fig. 3), with no significant difference between the sham-treated WT mice as compared to α-MSH-treated recipients at either time point (day 7, p=0.19; day 14, p=0.2). In conclusion, the decreased infiltration of PMN and mononuclear cells, but not T cells, suggests a reduced innate immune response.
Figure 3. α-MSH does not alter T cell infiltration into corneal allografts.
Allogeneic corneal grafts were performed in BALB/c mice (n=3/group). At days 7 and 14, corneas were harvested and T cell infiltration quantified based on immunohistochemical staining with anti-CD3. At day 7 after transplantation (A), few CD3+ cells were present in both groups. By day 14, there was an increase in CD3+ cells into both groups (B), while the numbers were lower in α-MSH-treated mice. The difference between both groups was not significant for both time points. Data depicted as mean ± SE. * p=0.20, ** p=0.19, n.s. = not significant, WT= sham-treated wild type mice.
Suppression of delayed-type hypersensitivity (DTH) with α-MSH treatment
To assess the level of sensitization of α-MSH-treated transplant recipients against donors, allospecific DTH studies were performed among sham-treated and α-MSH-treated recipients (Fig. 4). Naïve mice served as negative controls and mice that were previously sensitized to donor cells served as positive controls. The DTH studies were performed at 3 weeks post transplantation, when graft recipients are known to have a positive DTH response. Compared to sham-treated hosts, α-MSH-treated hosts demonstrated a significant (p = 0.01) decrease in DTH alloreactivity, indicating suppressed sensitization in α-MSH-treated mice (Fig. 4).
Figure 4. Donor-specific delayed-type hypersensitivity (DTH) is decreased in α-MSHtreated hosts.
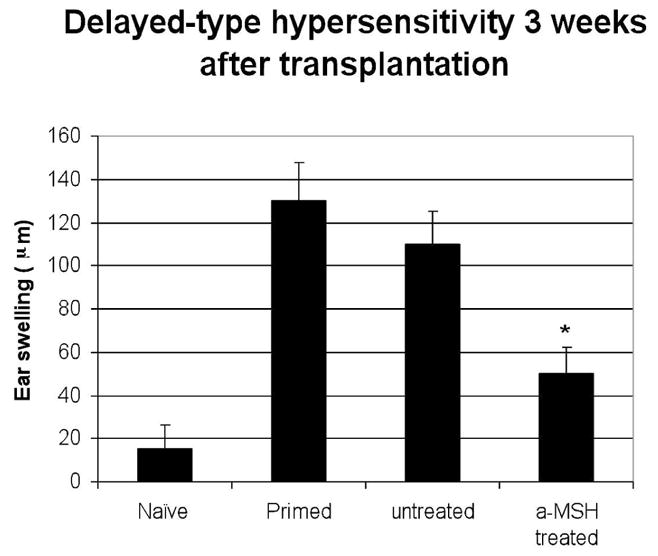
Ear swelling of naïve (negative control), subcutaneously primed (positive control) BALB/c hosts, sham-treated/untreated hosts and α-MSH-treated hosts were measured by a micrometer, 24 and 48 hours after ear challenge by donor cells at 3 weeks post corneal transplantation (n=5/group). α-MSH-treated hosts demonstrated a significantly suppressed degree of donor-specific ear swelling (DTH) as compared to control sham-treated hosts (*p = 0.008).
Suppression of Th1 cytokines interleukin (IL)-2 and interferon (IFN)-γ in response to α-MSH treatment
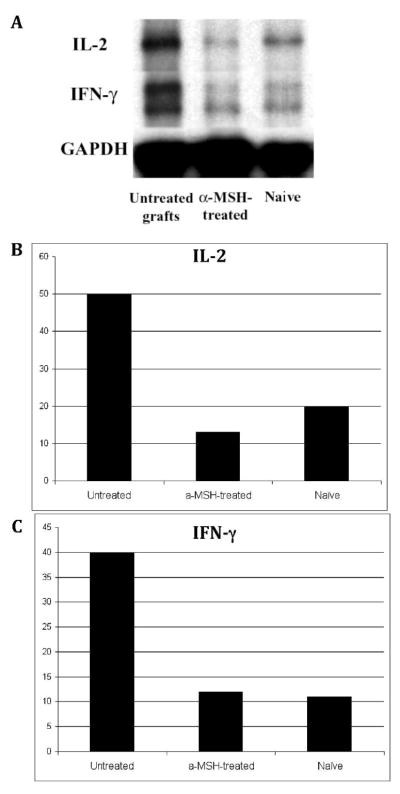
To evaluate the effect of α-MSH treatment on the production of Th1 cytokines before rejection of allografts at 3 weeks post transplantation, corneas were excised 2 weeks post transplantation (before onset of allorejection) and examined for Th1 cytokine gene expression by RPA, in both α-MSH-treated and sham-treated recipients. Results demonstrated a suppression of Th1 cytokines IL-2 and IFN-γ in α-MSH-treated mice as compared to sham-treated hosts (Fig. 5). The level of IL-2 and IFN-γ expression in α-MSH-treated recipients 2 weeks post transplantation was negligible and comparable to that in naïve mice, and was 76% and 70% lower than in sham-treated recipients for IL-2 and IFN-γ respectively. We conclude that a-MSH treatment suppressed inflammation and was associated with decreased expression of Th1 cytokines.
Figure 5. Th1 cytokines are decreased in α-MSH-treated hosts.
Corneas of sham-treated and α-MSH-treated hosts were excised pre-rejection at 2 weeks post transplantation, and RPA was performed to detect gene expression levels of Th1 cytokines in the transplanted corneas. Autoradiography data are shown in (A). On the basis of the migration pattern of the undigested probes, specific bands were identified for each cytokine. Densitometric analyses normalized to GAPDH are shown in (B, C) for untreated/sham-treated and α-MSH-treated recipients. Results demonstrate suppression of both IL-2 (B) and IFN-γ (C) in α-MSH-treated as compared to shamtreated mice, similar to the level of naïve mice.
DISCUSSION
Despite the considerable advancements in the field of corneal immunology over the past 30 years, there have been no major alterations in the pharmacotherapy of corneal transplantation since the advent of corticosteroids half a century ago. Patients are often retained long-term on topical corticosteroids for prevention of allograft rejection, in spite of the well-known risks of glaucoma and cataracts with this therapy. In our previous work, we have had variable success in achieving a significant improvement in graft survival through neutralization or gene deletion of selective cytokines, chemokines, co-stimulatory factors, vascular endothelial growth factors, and their respective receptors (30–34). Further, systemic blockade of soluble factors and receptors is not easily applicable in patients due to safety concerns. Here, the treatment with an endogenous anti-inflammatory mediator, offers the possibility of a local and safe regulation of corneal alloimmunity with no or minimal side-effects. While the use of systemic α-MSH has been shown to be partially successful in an experimental heterotopic heart transplantation model (19, 35), our results demonstrate substantially that local α-MSH treatment significantly reduces allorejection of orthotopic transplants.
We focused on the local application and functional role of α-MSH in corneal alloimmunity and examined the effects of α-MSH treatment on transplant survival. The prolonged survival of these graft under cover of α-MSH alone, was in sharp contrast to the only 3 day delay in rejection shown in a heart transplantation model (20). However, corneal grafts started to reject soon after cessation of α-MSH treatment at 4 weeks, suggesting that inhibition of allosensitization alone may not be sufficient to maintain long-term corneal graft survival, and that long-term α-MSH treatment may be needed. It could also suggest that conjunctival injections are not an effective method of delivering α-MSH. Although α-MSH treatment suppressed the activation of a Th1 cell response to alloantigens, our results do not suggest that α-MSH induced regulatory immunity in this case as suggested by its ability to promote regulatory T cell activation under other experimental conditions (11). It is possible that part of the effects of α-MSH seen in this study, are due to regulation of NF kappa B, suppression of inflammatory cytokines and chemokines, as well as a direct regulation on the expression of MHC class II and and co-stimulatory molecules on corneal antigen-presenting cells. Nevertheless, some CD4-deficient mice have been shown to reject corneal allografts within ten weeks after surgery, suggesting involvement of CD8+ T cells (36). However, these rejected grafts did not generate a significant DTH response, as shown here (14).
Corneal allograft rejection is primarily mediated by CD4+ Th1 cells. This study demonstrates that α-MSH mediates the Th1 response by inhibiting the production of IL-2 and IFN-γ, coinciding with the suppression of allograft-specific DTH. Our data is in accordance with previous data from experiments examining the effects of α-MSH in the skin. Injection of α-MSH into the skin, where a hapten was applied, induced IL-10 production by DCs, therefore resulting in a failure to prime T cells that mediate contact hypersensitivity (37). Our previous work indicated that IL-1 and TNF-αare important cytokines in corneal alloimmunity (31, 32, 38). This is related to our current data, as IFN-γ can synergize with IL-1 and tumor necrosis factor (TNF)-αto stimulate Th1-attracting chemokine production, while antagonizing Th2-attracting chemokines. The abolished expression of the Th1 cytokines IL-2 and IFN-γ in α-MSH treated corneal allografts herein, was not due to a decrease in T cell infiltration, but rather due to a suppression of T cell activation. This is evident by the fact that the number of T cells in α-MSH treated and untreated allografts was not statistically significantly. These results are in accordance with previous studies by Taylor et al., demonstrating the suppression of T cell activation by α-MSH, without prevention of T cell proliferation (9).
The differentiation of CD4+ T cells into Th1 or Th2 effector cells is mainly controlled by the cytokine environment and the nature of corneal antigen presenting cells (APCs). There is substantial evidence that α-MSH is able to regulate the function of APCs, through upregulation of IL-10 production (39), possibly suppressing differentiation of CD4+ T cell towards Th1 cells. Further, α-MSH has been shown to downregulate the expression of the co-stimulatory molecules CD86 and CD40, as well as ICAM-1 on DCs in a dose dependant manner (13, 40). These effects of α-MSH are partially mediated through the inhibition of endotoxin activation of transcription factors such as NFκB (41), resulting in its broad anti-inflammatory properties.
A major component of the early alloimmune response is the leukocyte recruitment into the corneal graft. α-MSH has been shown to suppress the bacterial endotoxin-mediated inflammatory activity and chemotaxis of macrophages and neutrophils (42–44), as well as to downregulate adhesion molecules (45). Similarly, there was a profound decrease in the number of PMN and mononuclear cells in α-MSH-treated eyes. Taken together, α-MSH is able to decrease the inflammatory response after transplantation through different mechanisms, including a decrease in inflammatory cells.
In summary, this study demonstrates that treatment with α-MSH alone, or in conjunction with other immunosuppressive therapy, may prove to be an effective strategy to suppress the rejection of corneal transplants, reducing the risk of the myriad toxic side effects of alternative non-specific immunosuppressives. Neuropeptides, and in particular α-MSH, are attractive compounds to be used as therapeutic targets in transplantation due to their molecular structure, their behavior and their endogenous nature.
Acknowledgments
The authors acknowledge the contribution of our colleagues at the Schepens Eye Research Institute, Dr. Douglas Faunce, for his technical assistance, Dr. Jacqueline Doherty and Marie Ortega for managerial support and Ms. Jian Gu for her help in the histopathological studies.
This work was supported by grant R01-EY-012963 from the National Institutes of Health, a research grant from the Massachusetts Lions Eye Research Fund, and a Research to Prevent Blindness Physician-Scientist Merit Award (MRD).
References
- 1.The Collaborative Corneal Transplantation Studies Research Group. The Collaborative Corneal Transplantation Studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392. [PubMed] [Google Scholar]
- 2.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology. emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;9:625. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hamrah P, Djalilian AR, Stulting RD. Immunologic high-risk penetrating keratoplasty. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. Vol. 2. Mosby; St. Louis: 2005. p. 1619. [Google Scholar]
- 4.Hill JC. The use of cyclosporine in high-risk keratoplasty. Am J Ophthalmol. 1989;107:506. doi: 10.1016/0002-9394(89)90494-7. [DOI] [PubMed] [Google Scholar]
- 5.Raizman M. Corticosteroid therapy of eye disease. Fifty years later Arch Ophthalmol. 1996;114:1000. doi: 10.1001/archopht.1996.01100140208016. [DOI] [PubMed] [Google Scholar]
- 6.Eberle AN. The melanotropins. S. Karger; Basel: 1988. [Google Scholar]
- 7.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 8.Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of α-MSH peptides. J Leukoc Biol. 2000;67:233. doi: 10.1002/jlb.67.2.233. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AW, Streilein JW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 10.Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18:140. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AW. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell Mol Biol. 2003;49:143. [PubMed] [Google Scholar]
- 12.Bohm M, Schulte U, Kalden H, Luger TA. Alpha-melanocyte-stimulating hormone modulates activation of NF-kappa B and AP-1 and secretion of interleukin-8 in human dermal fibroblasts. Ann N Y Acad Sci. 1999;885:277. doi: 10.1111/j.1749-6632.1999.tb08685.x. [DOI] [PubMed] [Google Scholar]
- 13.Becher E, Mahnke K, Brzoska T, Kalden D-H, Grabbe S, Luger TA. Human peripheral blood-derived dendritic cells express functional melanocortin receptor MC-1R. Ann NY Acad Sci. 1999;885:188. doi: 10.1111/j.1749-6632.1999.tb08676.x. [DOI] [PubMed] [Google Scholar]
- 14.Morandini R, Boeynaems JM, Hadley SJ, MacNeil S, Ghanem G. Modulation of ICAM-1 expression by α-MSH in human melanoma cells and melanocytes. J Cell Physiol. 1998;175:276. doi: 10.1002/(SICI)1097-4652(199806)175:3<276::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Hiltz ME, Catania A, Lipton JM. Inhibition of IL-1β-induced peripheral inflammation by peripheral and central administration of analogs of the neuropeptide α-MSH. Brain Res Bull. 1993;32:311. doi: 10.1016/0361-9230(93)90192-e. [DOI] [PubMed] [Google Scholar]
- 16.Wikberg JE, Mucience R, Mandrika I, Pruris P, Lindblom J, Post C, et al. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000;42:393. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- 17.Neumann Andersen G, Nagaeva O, Mandrika I, Petrovska R, Mucience R, Minchera- Nilsson L, et al. MC1 receptors are constitutively expressed on leukocyte subpopulations with antigen presenting and cytotoxic functions. Clin Exp Immunol. 2001;126:441. doi: 10.1046/j.1365-2249.2001.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of α-melanocyte stimulating hormone (α-MSH) Ann NY Acad Sci. 2000;917:239. doi: 10.1111/j.1749-6632.2000.tb05389.x. [DOI] [PubMed] [Google Scholar]
- 19.Getting SJ. Melanocortin peptides and their receptors: new targets for anti-inflammatory therapy. Trends Pharmacol Sci. 2002;23:447. doi: 10.1016/s0165-6147(02)02103-x. [DOI] [PubMed] [Google Scholar]
- 20.Gatti S, Colombo G, Buffa R, Turcatti F, Gabofalo L, Carboni N, et al. α-melanocyte stimulating hormone protects the allograft in experimental heart transplantation. Transplantation. 2002;74:1678. doi: 10.1097/00007890-200212270-00005. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol. 1999;73:72. doi: 10.1159/000058738. [DOI] [PubMed] [Google Scholar]
- 22.Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines α-melanocyte stimulating hormone and transforming growth factor-β2. J Leukoc Biol. 2002;72:946. [PubMed] [Google Scholar]
- 23.Naveh N, Marshall J. Melanocortins are comparable to corticosteroids as inhibitors of traumatic ocular inflammation in rabbits. Graefe’s Arch Clin Exp Ophthalmol. 2001;239:840. doi: 10.1007/s00417-001-0379-1. [DOI] [PubMed] [Google Scholar]
- 24.Shiratori K, Ohgami K, Illieva IB, Koyama Y, Yoshida K, Ohno S. Inhibition of endotoxin-induced uveitis and potentiation of cyclooxygenase-2 protein expression by α-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci. 2004;45:159. doi: 10.1167/iovs.03-0492. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Miyata S, Itoh Y, Mizuki N, Ohgami K, Shiratori K, et al. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int Immunopharmacol. 2004;4:1059. doi: 10.1016/j.intimp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639. [PubMed] [Google Scholar]
- 27.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice-evidence that the immunogenic rules of rejection do not apply. Transplantation. 1992;54:694. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Yamagami S, Hamrah P, Miyamoto K, Miyazaki D, Dekaris I, Dawson T, et al. CCR5 chemokine receptor mediates recruitment of MHC-class II-positive Langerhans cells in the corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:1201. doi: 10.1167/iovs.04-0658. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 31.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 32.Yamada J, Dana MR, Zhu SN, Alard P, Streilein JW. Interleukin 1 receptor antagonist suppresses allosensitization in corneal transplantation. Arch Ophthalmol. 1998;116:1351. doi: 10.1001/archopht.116.10.1351. [DOI] [PubMed] [Google Scholar]
- 33.Hamrah P, Yamagami S, Liu Y, Zhang Q, Vora SS, Lu B, et al. Deletion of the chemokine receptor CCR1 prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2007;48:1228. doi: 10.1167/iovs.05-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y, Dana MR. Blockade of CD40-CD154 ligand costimulatory pathway promotes survival of allogeneic corneal transplants. Invest Ophthalmol Vis Sci. 2001;42:987. [PubMed] [Google Scholar]
- 35.Colombo G, Gatti S, Turcatti F, Sordi A, Fassati LR, Bonino F, et al. Gene expression profiling reveals multiple protective influences of the peptide alpha-melanocyte-stimulating hormone in experimental heart transplantation. J Immunol. 2005;175:3391. doi: 10.4049/jimmunol.175.5.3391. [DOI] [PubMed] [Google Scholar]
- 36.Haskova Z, Usiu N, Pepose JS, Ferguson TA, Stuart PM. CD4+ T cells are critical for corneal, but not skin, allograft rejection. Transplantation. 2000;69:483. doi: 10.1097/00007890-200002270-00004. [DOI] [PubMed] [Google Scholar]
- 37.Grabbe S, Bhardwaj RS, Mahnke K, Simon MM, Schwarz T, Luger TA. α-Melanocyte stimulating hormone induces hapten-specific tolerance in mice. J Immunol. 1996;156:473. [PubMed] [Google Scholar]
- 38.Qian Y, Dekaris I, Yamagami S, Dana MR. Topical soluble tumor necrosis factor receptor type I suppresses ocular chemokine gene expression and rejection of allogeneic corneal transplants. Arch Ophthalmol. 2000;118:1666. doi: 10.1001/archopht.118.12.1666. [DOI] [PubMed] [Google Scholar]
- 39.Bhardwaj RS, Schwarz A, Becher E. Pro-opiomelanocortin-derived peptides induce IL- 10 production in human monocytes. J Immunol. 1996;156:2517. [PubMed] [Google Scholar]
- 40.Luger TA, Scholzen TE, Brzoska T, Bohm M. New insights into the function of α-MSH and related peptides in the immune system. Ann NY Acad Sci. 2003;994:133. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- 41.Manna SK, Aggarwal BB. α-melanocyte-stimulating-hormone inhibits the nuclear transcription factor NF-κB activation induced by various inflammatory agents. J Immunol. 1998;161:2873. [PubMed] [Google Scholar]
- 42.Catania A, Rajora N, Capsoni F, Minonzo F, Star RA, Lipton JM. The neuropeptide α-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1999;17:675. doi: 10.1016/0196-9781(96)00037-x. [DOI] [PubMed] [Google Scholar]
- 43.Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM. α-MSH production, receptors, and influence on neopetrin in human monocytes/macrophage cell line. JAMA. 1996;59:248. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- 44.Taylor AW. The immunomodulating neuropeptide alpha-melanocyte stimulating hormone (a-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J Neuroimmunol. 2005;162:43. doi: 10.1016/j.jneuroim.2005.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalden D-H, Fastrich M, Brzoska T, Scholzen T, Hartmeyer M, Schwarz T, et al. Alpha-melanocyte- stimulating hormone reduces endotoxin-induced activation of nuclear NF-κB in endothelial cells. Ann NY Acad Sci. 1999;885:254. [Google Scholar]