Summary
Docosahexaenoic acid (DHA) and arachidonic acid (AA) are found in high concentrations in brain cell membranes and are important for brain function and structure. Studies suggest that AA and DHA are hydrolyzed selectively from the sn-2 position of synaptic membrane phospholipids by Ca2+-dependent cytosolic phospholipase A2 (cPLA2) and Ca2+-independent phospholipase A2 (iPLA2), respectively, resulting in increased levels of the unesterified fatty acids and lysophospholipids. Cell studies also suggest that AA and DHA release depend on increased concentrations of Ca2+, even though iPLA2 has been thought to be Ca2+-independent. The source of Ca2+ for activation of cPLA2 is largely extracellular, whereas Ca2+ released from the endoplasmic reticulum can activate iPLA2 by a number of mechanisms. This review focuses on the role of Ca2+ in modulating cPLA2 and iPLA2 activities in different conditions. Furthermore, a model is suggested in which neurotransmitters regulate the activity of these enzymes and thus the balanced and localized release of AA and DHA from phospholipid in brain, depending on the primary source of the Ca2+ signal.
Keywords: Phospholipase A2, calcium, arachidonic acid, docosahexaenoic acid, endoplasmic reticulum, signaling
1. Overview of the phospholipase A2 families
Although PLA2 enzymes have been known for over 100 years, only within the last 15 years has their importance for neurochemical processes been widely recognized [1]. These enzymes catalyze the hydrolysis of esterified fatty acids at the stereospecifically numbered (sn)-2 position of membrane phospholipids to release unesterified fatty acids and form lysophospholipids [2–9]. The fatty acids and their metabolic products are important for normal brain function and can alter neuropathological processes [1, 10–15].
PLA2s are classified according to their sequence homology and Ca2+ dependence. They belong to three main families having different catalytic sequences, and these sequences are conserved among PLA2s in the same family. The three most described families are: low molecular mass secretory PLA2 (sPLA2), higher molecular mass Ca2+-dependent cytosolic PLA2 (cPLA2), and “Ca2+-independent” PLA2 (iPLA2) [24](See Table 1). Other less studied PLA2 families have been described, such as platelet-activating factor-acetyl hydrolase (PAF-AH) [8, 21] and lysosomal PLA2 [22, 23].
Table 1.
PLA2 enzymes and their subcellular localization, and Ca2+-dependence of their activity [1, 8, 16–23]
| Enzyme | sPLA2 | cPLA2 | iPLA2 | Lysosomal- PLA2 |
PAF-AH |
| No catalytic effect. |
No effect | No effect | |||
| Effect of Ca2+ |
Stimulated (mM) |
Translocation | Activated by Ca2+ release from ER* |
||
| Expression profile |
Ubiquitous | Ubiquitous | Heart, brain, placenta, islets, testes, skeletal muscle |
Heart, lung, liver, kidney, spleen, brain, thymus, inflammatory cells (macrophages) |
Ubiquitous |
| Fatty acid preference |
None | AA | DHA | oleate and linoleate are preferred relative to arachidonate |
DHA and AA |
This effect may be achieved by Ca2+ release itself and not by Ca2+ from the ER binding the enzyme
The sPLA2 family is subdivided into groups: sPLA2-IB, sPLA2-IIA, sPLA2-IIC, sPLA2-IID, sPLA2-IIE, sPLA2-IIF, sPLA2-III, sPLA2-V, sPLA2-X, and sPLA2-XII [1]. The genes for human sPLA2–IB map to chromosome 12q23–24, those for group II subfamily sPLA2s (IIA, IIC, IID, IIE, IIF) and for sPLA2-V map to chromosome 1p34–36, and the gene for sPLA2-X maps to chromosome 16p12–13 [1]. sPLA2s are Ca2+-dependent for catalytic activity (generally requiring millimolar Ca2+ concentrations) [1, 25, 26], consistent with their extracellular activity and millimolar Ca2+ concentrations in the extracellular space [27]. Evidence also points to an intracellular functional role sPLA2 in caveolae-containing compartments around the nucleus of immune cells and in the nucleus of astrocytes and neurons [28–30].
The cPLA2 family is subdivided into cPLA2α, cPLA2β, cPLA2γ, cPLA2δ, cPLA2ɛ and cPLA2ζ, which also are referred to as groups IVA, IVB, IVC, IVD, IVE and IVF, respectively. They are coded by gene on different human chromosomes, chromosome 1 (cPLA2α), 15 (cPLA2β, cPLA2δ, cPLA2ɛ, cPLA2ζ) and chromosome 19 (cPLA2γ), which are not the same as the chromosomes that code for the sPLA2 family [31–38]. cPLA2 becomes activated after translocating to the plasma membrane from the cytosol, and is constitutively membrane-bound [1, 38]. Although Ca2+ is not necessary for cPLA2 catalytic activity, nanomolar Ca2+ concentrations are needed for its binding to the membrane, where other enzymes that regulate the arachidonic acid (AA) cascade, including cyclooxygenases (COXs) and lipoxygenases, also are located [16].
The iPLA2s have extremely different structures compared to the cPLA2 and sPLA2 families [39]. They occur in numerous splicing variants, only some of which are functional. iPLA2s are classified as group VI and are subdivided into VIA and VIB, or into iPLA2β and iPLA2γ, respectively. Each iPLA2 is coded by a different chromosome, iPLA2β is coded by chromosome 23q13 [40] and iPLA2γ is coded by chromosome 7q31 [1, 41]. iPLA2β is unique among PLA2s, as it has multiple ankyrin repeats, which are common motifs found in more than 400 proteins, including cell cycle regulators and transcription factors. Ankyrin repeats have been implicated in protein–protein interactions and can influence many physiological functions [39]. A site near the C-terminus of some iPLA2β variants can bind to calmodulin with the help of Ca2+ [1, 42–44].
The PAF-AHs are classified as VIIA (lipoprotein-associated- PLA2), VIIB (PAF-AH II) VIIIA (PAF-Ib) and VIIIB (PAF-Ib or VIIIB forms a heterodimer with VIIIA) [5]. PAF-AH was first named for its ability to cleave the acetyl group from the sn-2 position of platelet activating factor (PAF); however, this enzyme can cleave oxidized lipids in the sn-2 position up to 9 carbons long, not just PAF. This enzyme also has been shown to access substrate in the aqueous phase, unlike the other PLA2s studied [21], in a Ca2+-independent fashion [6]. Type I PAF-AH is a complex of two catalytic subunits, alpha1 and alpha2, a regulatory beta subunit, and a gamma subunit [45]. Its structure is very similar to the structure of G proteins [46], whereas type II PAF-AH is a single polypeptide [47]. Type II PAF-AH is myristoylated at the N-terminus and distributed in both the cytosol and membranes. Plasma PAF-AH has a closed structure when compared to II PAF-AH, and exists in association with plasma lipoproteins [21].
Lysosomal or group XV PLA2 is a calcium-independent PLA2 enzyme of 25 kDa [23], which has a distinguishing characteristic of having its maximal activity in an acidic medium, and accordingly, it also is called acidic iPLA2 [23, 48].
This review will focus on the roles of the cPLA2 and iPLA2 in mammalian brain. We propose a model for receptor-mediated regulation of PLA2 enzyme activities by Ca2+ signals originating from intracellular and extracellular sources, leading to the controlled, balanced and localized release of specific fatty acids as signaling molecules.
2. cPLA2 and iPLA2 in the brain
cPLA2 is found in mammalian brain in three different forms: cPLA2α (85-kDa), cPLA2β (114-kDa), and cPLA2γ (61-kDa). cPLA2β is found mainly in the cerebellum, whereas cPLA2α and cPLA2γ are uniformly distributed in rat brain [16, 31, 33]. cPLA2α is found both in astrocytes and neurons [16]. In neurons of rat brain, cPLA2 has been localized at post-synaptic sites, suggesting that it is important in neurotransmission (see table 2) [17].
Table 2.
| cPLA2 | iPLA2 | |
| Localization | Cytosol | Cytosol, ER 80-kDa (iPLA2β) rat |
| Mol. Mass | 85-kDa (cPLA2α) 114-kDa (cPLA2β) 61 -kDa (cPLA2γ) |
85-kDa (subtype unknown) monkey 110-kDa (subtype unknown) bovine 30-kDa (subtype unknown) bovine |
iPLA2 is believed to account for more than 70% of brain PLA2 activity [25]. iPLA2β (80-kDa) was purified from rat brain [49] and found in all brain regions, with the highest activity in striatum, hypothalamus, and hippocampus [16, 49]. Similarly, two different iPLA2s (110-kDa and 39-kDa) were purified from bovine brain [51, 52]. iPLA2 (85-kDa) also was identified in monkey brain, in the neocortex, amygdala, hippocampus, caudate nucleus, putamen, and nucleus accumbens, whereas in the thalamus, hypothalamus and globus pallidus it was lightly labeled (see table 2). On the other hand, the midbrain, vestibular, trigeminal and inferior olivary nuclei, and the cerebellar cortex were densely labeled [18]. Individual iPLA2 subtypes have not been investigated in the bovine or monkey brain. The enzyme is present in astrocytes [53] and in neurons, where its localization in dendrites and axon terminals suggests that it plays a role in neuronal signaling [18].
3. The cPLA2 product: Arachidonic acid and brain function
Arachidonic acid (AA, 20:4n-6), an n-6 polyunsaturated fatty acid (PUFA), comprises approximately 5–15% of total fatty acids in most tissue phospholipids [54]. It can be synthesized in the liver from dietary derived linoleic acid, or obtained directly from dietary sources [55]. AA is preferentially released from phospholipid by cPLA2 [1, 4, 56]. It and its metabolites (prostaglandins, leukotrienes, thromboxanes, lipoxins) in brain influence synaptic signaling [57, 58], neuronal firing [59], neurotransmitter release [60], activation of intracellular receptors [61], hypothalamic-pituitary function [62], nociception [63], gene expression [11], cerebral blood flow [64], circadian rhythm [65], and appetite [66], among other targets. Altered AA metabolism has been implicated in neuronal death [67] and in a number of neurological, neurodegenerative, and psychiatric disorders, including epilepsy [68], ischemia, stroke [69], HIV-associated dementia [13], amyotrophic lateral sclerosis [70], Alzheimer disease [71], Parkinson disease [72], schizophrenia [73], bipolar disorder [74–76] and depression [77, 78].
4. The iPLA2 product: Docosahexaenoic acid and brain function
Docosahexaenoic acid (DHA, 22:6n-3) is the most abundant n-3 PUFA in brain and is necessary for normal brain function [54]. Esterified DHA within membrane phospholipids is hydrolyzed preferentially by iPLA2 [79].
Humans can obtain DHA by eating fish or fish products, or can synthesize it in the liver from circulating α-linolenic acid, through serial steps of desaturation, elongation and oxidation [55, 80]42]. Dietary restriction of n-3 PUFAs in animal models decreased brain DHA content while increasing concentrations of the elongation product of AA, docosapentaenoic acid (22:5n-6) [80–82]. Rats deprived of dietary n-3 PUFAs for 15 weeks had increased brain mRNA, protein and activity levels of cPLA2, sPLA2 and COX-2, but decreased expression of iPLA2 and COX-1 [83].
DHA has been found important for membrane function and fluidity, photoreceptor function [54], memory [84, 85], problem solving [86], and developmental visual and sensory functions [54]. Some studies but not others indicate that dietary DHA supplementation is beneficial in a number of brain disorders [87, 88]. In rats, dietary n-3 PUFA deprivation for 15 weeks increased aggression and depression scores on behavioral tests [89]. DHA, like AA, can be metabolized by COX and lipoxygenase enzymes and generate active compounds [90, 91]. DHA products, in contrast to AA products, seem to have a beneficial effect in inflammatory and neurodegenerative conditions. Resolvins of the D series and docosatrienes, which are bioactive DHA products that possess potent anti-inflammatory, immunoregulatory [92, 93], and neuroprotective actions [94], are termed neuroprotectins [90].
5. Docosahexaenoic acid as a signaling molecule: competition with arachidonic acid?
AA is well recognized as a signaling molecule [10, 95–97] and there are a number of lines of evidence indicating that DHA is a signaling molecule as well [12, 79, 98–100].
1) DHA can be released from phospholipid following activation of neuroreceptors
Activation of serotonin (5-HT) 5-HT2A receptors in astroglioma cells caused DHA release from membrane phospholipids [101]. Muscarinic receptor activation by arecoline also caused DHA release and plasma-derived DHA incorporation into rat brain synaptic membranes, consistent with its participation in neuronal signaling [102, 103].
2) DHA exerts intracellular downstream effects
DHA increased N-methyl-D-aspartate (NMDA) function in neurons. Both DHA and AA increased entry of extracellular Ca2+ into neurons via glutamatergic NMDA receptors, and DHA also increased the probability of NMDA channel opening [104]. In another study, AA increased while DHA inhibited glutamate-induced prostaglandin release from astrocytes; DHA inhibited the AA effect [105].
Unesterified DHA can promote Ca2+ release from intracellular stores in the endoplasmic reticulum (ER) [106]. Ca2+ released from the ER can be a continuous source of Ca2+ for mitochondria, can activate dehydrogenases and mitochondrial ATP synthesis and energy production, and can stimulate neuroprotective signals [107]. On the other hand, Ca2+ uptake into mitochondria can modulate the activity of Ca2+ channels in the ER [108, 109]. If the balance between mitochondria and the ER is disturbed, a Ca2+ overload in the mitochondria can trigger necrosis or apoptosis [110].
Release of Ca2+ from intracellular stores via purinergic [111] or nicotinic [112] receptors can be neuroprotective. Intracellular Ca2+ release induced by isoflurane [113] or fructose-1,6-biphosphate was shown to be neuroprotective [114, 115]. Ca2+ release from the ER, induced by AA or DHA, reduced the peak of subsequent Ca2+ release induced by G protein activation, showing a complex relation between these two PUFAs and intracellular Ca2+ [100].
Neuroprotectin D1 is a metabolite of DHA that may account for part of the opposite effects of DHA and AA. The synthesis of neuroprotectin D1 was increased in cells exposed to the Ca2+ ionophore A23187 or to interleukin-1β, and depended on PLA2 activity [98]. Both A23187 and interleukin-1β can induce Ca2+ release from intracellular stores [116]. However, regulation by ER Ca2+ of neuroprotectin D1 synthesis induced by A23187 or interleukin-1β has not been studied. Neuroprotectin D1 also can inhibit β-amyloid production [117] and COX-2 expression [99]. COX-2, which appears involved in neuroinflammation and is overexpressed in the Alzheimer disease brain [118, 119], converts AA to prostaglandins that also can increase β-amyloid production [120]. COX-2 also has been implicated in multiple sclerosis, amyotrophic lateral sclerosis, Parkinson disease, and Creutzfeldt-Jakob disease [14]. DHA in these conditions may modulate COX-2 activity and AA metabolite production via COX-2.
A DHA neuroprotective effect also is supported by evidence that iPLA2 inhibition increased neuronal death induced by reactive oxygen species [121]. The data together suggest that increased iPLA2 activity and DHA release can be neuroprotective.
In other contexts, iPLA2 activation may have different consequences, related to its different subtypes, their location, and substrate availability in brain. In apoptotic cells, iPLA2γ seems necessary for mitochondrial membrane pore transition formation [122]. iPLA2 is a substrate for caspase-3 (or other caspases), and is cleaved during the apoptotic process at the consensus Asp183. The resulting fragment, iPLA2(184-C), possesses the entire catalytic domain and seven of eight ankyrin repeats, and is functionally more active than intact iPLA2 in cells [123]. On the other hand, inhibition of iPLA2β induced apoptosis in one study [124]. The role of DHA in apoptosis related to iPLA2 inhibition is not known.
6. Ca2+ signaling as control mechanism of iPLA2 and cPLA2 activities
Preferential release of DHA and AA by iPLA2 and cPLA2, respectively [49, 74, 79], raises the possibility that the enzymes are controlled by separate mechanisms and serve different or even opposing functions [75]. For example, pharmacological studies with chronic lithium, carbamazepine and valproate in unanesthetized rats, when combined with in vivo kinetic fatty acid modeling and molecular biology measurements, confirmed in vitro findings that cPLA2 is specific for AA release, whereas iPLA2 is comparatively specific for DHA release from phospholipid in brain synaptic membranes [74, 125, 126]
cPLA2 requires Ca2+ for its translocation and arachidonic acid release [36]. Therefore, Ca2+ entry through ligand-gated ion channels, such as NMDA receptors, can activate this enzyme [127, 128]. In neurons, NMDA receptor activation induces Ca2+ entry from the extracellular space and activates cPLA2, and this activation is reversed by MK-801 (an NMDA antagonist), by removing calcium from the extracellular space, or by chronically administering lithium, valproic acid or carbamazepine, mood stabilizers effective in bipolar disorder, to rats [129, 130] [131, 132]. The cPLA2 coupling to Ca2+ entry through the membrane seems to depend on its location in the cell [133]. The localization of Ca2+ entry appears more important than the pattern of Ca2+ entry. In this sense, single transients, repetitive oscillationrs or sustained plateaus of Ca2+ activate cPLA2. Under these circumstances, cPLA2 translocates to the nucleus where it promotes AA release. On the other hand, potassium-induced Ca2+ entry can induce depolarization and the opening of voltage-dependent Ca2+ channels located at sites close to neurotransmitter release. However, potassium depolarization does not activate AA release, probably because cPLA2 is not located close to releasing sites [133]. In agreement with this evidence of Ca2+ dependence of neurotransmitter-induced cPLA2 activity, 5-HT receptor activation in hippocampal neurons induced an increase in PLA2-mediated AA release that was reversed by removing extracellular Ca2+ [134].
iPLA2 has been considered to be Ca2+-independent, because its catalytic activity was found to be independent of Ca2+ when studied in an incubation medium [135]. However, in fractionated cytosol, Ca2+ later was shown to decrease iPLA2β activity, probably by modifying its interaction with calmodulin [43]. Multiple contact points in the 15-kDa C-terminal portion of iPLA2β have been identified as determinants of its Ca2+-dependent interaction with calmodulin [44]. Interaction between calmodulin and iPLA2 inhibits iPLA2 activity and is modulated by Ca2+ [43].
iPLA2β is highly localized in the ER in areas around the cell nucleus [20] and in the cytosol; it is less expressed near plasma membranes and mitochondria [18]. iPLA2β immunoreactivity has been detected around the nuclear envelope in cortical and limbic areas of the rhesus brain [18], as well in dendrites and terminals, suggesting a local roles.
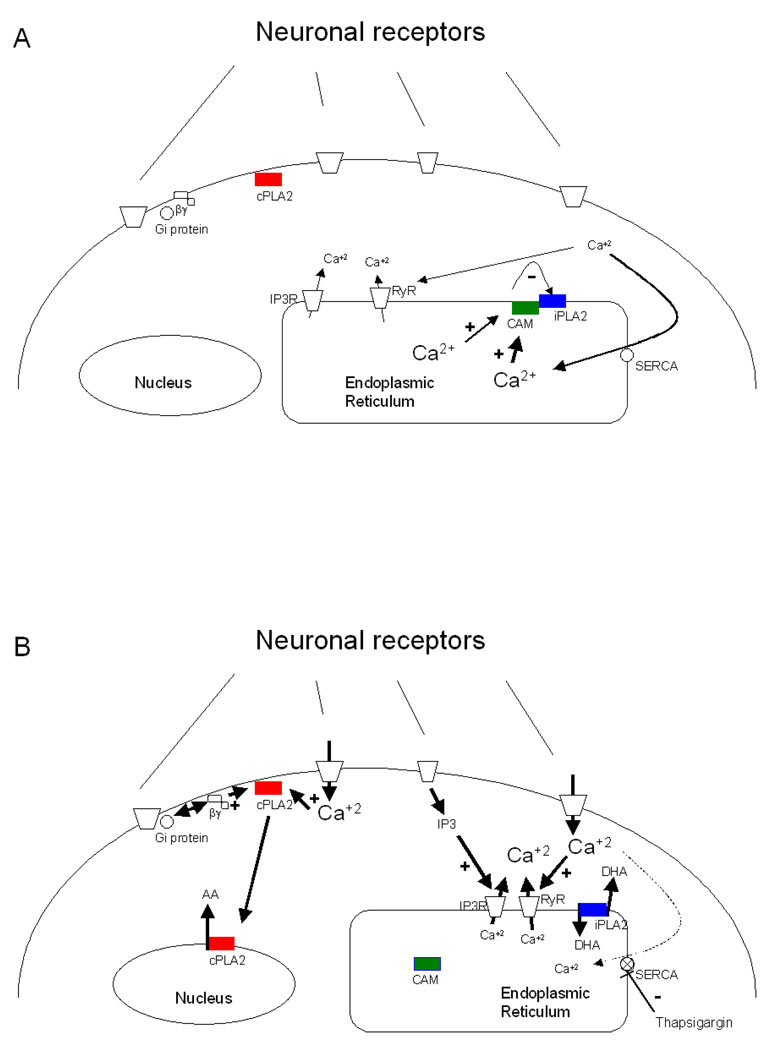
As illustrated in Figure 1, Ca2+ can be released from intracellular ER stores in several ways. The ER has two types of membrane receptors, an inositol-1,4,5-phosphate receptor (InsP3R), and a ryanodine receptor (RyR) that can receive information from the cytosol and release Ca2+ from the ER lumen. This signaling can be evoked by purinergic [136, 137], muscarinic [138], or metabotropic glutamatergic receptors [139], so as to produce receptor-mediated inositol-3-phosphate (InsP3) release and activate the InsP3R. Activation of NMDA receptors [140, 141], AMPA receptors [142], or even InsP3Rs [143] can release Ca2+ by “Ca2+-induced Ca2+ release” (CICR) through activation of RyRs. Inhibition of the reticular ATPase pump (SERCA) by thapsigargin, DBHQ (2,5-di(tert-butyl)hydroquinone), or Br(2)-TITU (1,3-dibromo-2,4,6-tris (methyl-isothio-uronium) benzene), also can cause a sustained release of Ca2+ from the ER by a different mechanism. Ca2+ is believed to be released on a continuous basis from the ER and refilled by SERCA. Blocking SERCA activity may, as a result, increase cytosolic Ca2+ concentrations (see Figure 1) [144]. The Ca2+ released from intracellular stores by ATP, A23187, thapsigargin, or arginine vasopressin may activate iPLA2 [42, 145], even in the presence of intracellular or extracellular Ca2+ chelators such as BAPTA or EGTA, which suggests that ER calcium emptying can transiently modulate the enzyme availability by releasing iPLA2 from calmodulin binding [145].
Figure 1.
Hypothetical regulation of cPLA2 (red) and iPLA2 (blue) activities by activation of neuronal receptors. Receptor activation can stimulate cPLA2 though Ca2+ entry from extracellular space or via Gi protein coupling. iPLA2 can be activated by Ca2+ release from intracellular stores through activation of InsP3 or Ca2+ -induced Ca2+ release from the ER. (A) cPLA2 is not stimulated by Ca2+ and do not release AA. SERCA works continuously to pump Ca2+ into the ER. In this circumstance, calmodulin inhibits iPLA2 in a Ca2+ dependent manner. (B) Neuronal receptors are stimulated and permeate Ca2+ or stimulate the production of IP3. cPLA2 is translocated to the nucleus in presence of µM concentration of Ca2+ and release AA. When Ca2+ concentration is decreased inside ER, either by an increase in ER receptors stimulation or by SERCA inhibition, probably calmodulin (green, represented as CAM) is not interacting with iPLA2, allowing its activity. Differences in Ca2+ symbols size represent difference in concentrations of this ion, whereas small letters represents decreased calcium concentrations and big letters represents increased calcium concentrations.
Smooth muscle cells treated with the calmodulin inhibitor W-7 or with thapsigargin release AA from the same pool, suggesting that iPLA2β is bound to a calmodulin, and that calmodulin acts as a sensor of reticular Ca2+ to regulate iPLA2 activity [42, 44]. The PLA2 family is formed by ankyrins, and ankyrins are related to protein-protein interactions and may modulate regulation of iPLA2 activity by calmodulin [39]. Moreover, thapsigargin increases iPLA2β expression in purified ER [146]. Strokin et al. [79], who demonstrated the exclusive release of DHA by iPLA2, also showed that the ionophore A23187 increased DHA release 11-fold and AA release only 3.9 -fold compared to their respective baselines. In those studies, extracellular Ca2+ may have stimulated cPLA2 directly by increasing Ca2+ close to the plasma membrane, and have activated iPLA2 indirectly by releasing Ca2+ from intracellular stores by a CICR. ATP-induced AA release was suppressed by removing extracellular Ca2+ in that study, but DHA release was unchanged. Thus ATP could have induced Ca2+ release from intracellular stores to selectively activate iPLA2 [79, 147].
This putative modulation of iPLA2 activity and DHA release by ER Ca2+ release is further suggested by experimental studies of ischemia or oxygen and glucose deprivation (OGD). In mouse C2C12 myotubes, OGD increased 4-bromoenol lactone (BEL, an iPLA2 inhibitor)-sensitive iPLA2 activity that could be blocked by an siRNA against iPLA2β. iPLA2β protein in these cells was identified mainly at the endoplasmic reticulum, where it accumulated further during OGD, whereas the mRNA level was unchanged [148]. In agreement with these results, DHA is released during decapitation-induced ischemia [149]. Ca2+ entry from the extracellular space is a well-known source of intracellular Ca2+ during OGD. However, an important component of the Ca2+ increase is due to ER Ca2+ release, and particularly SERCA dysfunction is an important mechanism for ischemic Ca2+ overload [150, 151]. These results suggest that OGD may activate iPLA2 in the ER after ER Ca2+ release by the physical dissociation of iPLA2 from calmodulin.
Other evidence for the activation of iPLA2 by Ca2+ derived from the ER comes from a study of the iPLA2 effect on store operated calcium entry (SOCE). SOCE is another regulatory mechanism of Ca2+ homeostasis. It consists in the entry of Ca2+ from extracellular space that is induced by Ca2+ released from intracellular stores [152, 153]. In rat cerebellar astrocytes, iPLA2 is a major regulator of SOCE. During depletion of ER Ca2+ stores, iPLA2 is believed to be activated, resulting in opening channels in plasma membrane by the formation of lysophospholipids, which may affect the lipid environment of the channels or directly interact with them. In this regard, both depletion of ER Ca2+ stores and inhibition of calmodulin increased BEL-sensitive iPLA2 activity in cultured cerebellar astrocytes. Furthermore, the specific antisense inhibition of iPLA2 reduces the SOCE [153]. This study, using antisense to inhibit iPLA2 and SOCE currents as the physiological outcome, is strong evidence that iPLA2 is indeed the PLA2 subtype that can be dissociated from calmodulin either by an inhibitor of calmodulin or by Ca2+ release from ER.
These results suggest Ca2-controlled activation of iPLA2 in vivo and different possibilities for neurotransmitter regulation of AA and DHA release and signaling, since many neurotransmitters are able to stimulate InsP3 formation or CICR. Given that Ca2+ in the ER lumen is believed to be regulated by specific proteins, changes in intracellular Ca2+ concentrations can transmit signals both to the ER surface and the lumen. For example, calreticulin is an ER chaperone that can detect changes in Ca2+ inside the ER and interact with other proteins to integrate Ca2+ release with different cell functions [154]. Calmodulin, and possibly other Ca2+ sensitive proteins, may link ER Ca2+ release to iPLA2 activation. Ca2+ from these different sources, thus, may couple neurotransmission in different ways to iPLA2 and cPLA2 activities, thereby regulating DHA and AA signaling.
Agonist activation of dopaminergic D2 [155–157], serotonergic 5-HT2A/2C [158], NMDA[130] or cholinergic muscarinic receptors [102, 103] has been shown to increase AA release from rat brain membrane phospholipids in vivo. Muscarinic and 5-HT2A receptor activation also increased DHA release in vivo or in vitro [101–103]. D2 and 5-HT2A/2C agonists can activate cPLA2 by a G protein mechanism [134, 159], whereas 5-HT agonists and NMDA agonists can do so by increasing cell entry of extracellular Ca2+, since cPLA2 is Ca2+-dependent [133, 134]. 5-HT2A receptor activation also can release Ca2+ from InsP3R stores [160], and muscarinic agonists can increase Ca2+ influx into the cell to also release Ca2+ from intracellular stores [161]. Thus, Ca2+ from the two sources can separately control the activities of both cPLA2 and iPLA2, thereby regulating AA and DHA release from membrane phospholipids.
7. Calcium modulation of PLA2 activity and brain pathology
In many neurodegenerative, psychiatric diseases and in conditions having increased neuronal death, the modulation exerted by DHA in docosanoid production could reduce neuronal damage (see [99] for review). Inhibition by DHA of Ca2+ entry through L-type calcium channels could be a beneficial effect of DHA [162]. Although a description of how differential activation of cPLA2 and iPLA2 by Ca2+ could affect pathological process is beyond the scope of this review, here we briefly present data suggesting their roles.
Alzheimer Disease
Alzheimer disease is a progressive neurodegenerative disease that can involve changes brain PLA2 and PUFA metabolism, with evidence for marked disturbances in brain Ca2+ homeostasis including excess Ca2+ release from the ER [163–165]. AA metabolism was shown to be increased in brains of Alzheimer disease patients using positron emission tomography, presumably in relation to neuroinflammation and activation of cPLA2 and sPLA2 via astrocytic cytokine receptors [166, 167] [168–170], whose immunoreactivity have both been reported to be increased in the postmortem Alzheimer brain [50, 170]. cPLA2 can be activated by entry of extracellular Ca2+ into cells through ionotropic NMDA receptors [127], and NMDA binding sites were decreased in the postmortem Alzheimer disease brain [171]. cPLA2 mediated AA release can be increased by the β-amyloid peptide that accumulates in the Alzheimer disease brain, probably via mediation of NMDA receptors [172]. Additionally, a low plasma DHA level has been associated with Alzheimer disease [173], but controlled supplementation studies have not been performed. β-amyloid can induce membrane-associated oxidative stress or form an oligomeric pore in the membrane [174], which could affect cPLA2 activity. DHA may be beneficial in Alzheimer disease by decreasing β-amyloid release [175].
Cerebral Ischemia
Depolarization caused by energy failure in cerebral ischemia will lead to opening of voltage-gated Ca2+ channels in the membrane and excessive release of neurotransmitters [176], including glutamate. Glutamate activation of NMDA receptors normally is limited by magnesium blocking the channel, but depolarization releases magnesium and the receptor can then be opened to Ca2+ by the excessive glutamate [177]. In ischemia, a sustained depolarization and excess glutamate can maintain the NMDA channel open and load Ca2+ into the cell [176], thereby activating Ca2+-sensitive enzymes including cPLA2 [178–181], and causing inflammatory mediator production [178, 182]. Hypoxia was shown to release both AA and DHA in rat brain, at a ratio of 3.6 to 1 [149]. In ischemia, neuroprotectin D1 and DHA were protective [183–185]. DHA can block currents of L-type of calcium channels[162], and inhibit AA induced prostaglandin production [105].
Depression
Depression has been proposed to be related to NMDA receptor overactivation [186–188], with resultant neuronal damage [189]. Thus, NMDA receptor antagonists induce an antidepressant effect in animal models [190–193] and in humans [188], and classical antidepressants can regulate NMDA receptor function [186]. Neuroinflammation likely is involved in depression [194]. Decreased plasma DHA levels were found in depressed individuals with coronary problems [195], but n-3 PUFA supplementation in depressed patients produced contradictory results [196, 197].
8. iPLA2 and brain disease
Neurodegeneration associated with brain iron accumulation (NBIA) comprises a heterogeneous group of disorders, and includes patients with mutations in the PLA2G6 gene encoding iPLA2β [198]. Children with PLA2G6 mutations show progressive cognitive and motor skill regression, with cerebellar ataxia and dystonia, as well as cerebellar cortical atrophy and gliosis. Mice with a targeted disruption of the iPLA2β gene show severe motor dysfunction, associated with widespread degeneration of brain axons and/or synapses, accompanied by the swollen axons and vacuoles [199].
9. Conclusions
cPLA2 and iPLA2 are important lipid regulatory enzymes that selectively release AA and DHA, respectively, from brain membrane phospholipids. The two PUFAs and their metabolites have many physiological effects and their relative rates and brain sites of release, controlled by the two enzymes and their concentrations in phospholipid, may help to fine-tune brain physiology and metabolism and when disrupted, lead to psychiatric and neurological disease. cPLA2 has been shown to depend on extracellular-derived Ca2+ in both in vitro and in vivo studies. Although Ca2+ was shown not to be necessary for iPLA2 activation in some in vitro studies, Ca2+ released from the intracellular stores of the ER can activate iPLA2 in cells, which suggests that activation can depend on Ca2+ in vivo. Neurotransmitters coupled to InsP3R activation or to CICR modulate iPLA2 activity by regulating intracellular Ca2+ stores. Further experiments are needed to elaborate the in vivo conditions under which this occurs.
Acknowledgments
We thank J. Bell, Dr. M. Basselin, Dr. J. Rao, Dr. M. Igarashi, Dr. F. Gao, Dr. J.Y. Park and the NIH Fellows Editorial Board for reviewing the manuscript. We also thank Kathy Benjamin for correcting the language of this manuscript. This study was entirely supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. The authors have no conflict of interest.
Abbreviations
- AA
arachidonic acid
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- BEL
4-bromoenol lactone (inhibitor of iPLA2)
- DHA
docosahexaenoic acid
- ER
endoplasmic reticulum
- CICR
Ca2+-induced Ca2+ release
- COX
cyclooxygenase
- InsP3R
inositol 1,4,5-trisphosphate receptor
- LOX
lipoxygenase
- NMDA
N-methyl-D-aspartate
- OGD
oxygen and glucose deprivation
- PAF-AH
Plasma activated factor-acetyl hydrolase PLA2, phospholipase A2
- cPLA2
cytosolic phospholipase A2
- iPLA2
Ca2+-independent phospholipase A2
- sPLA2
secretory phospholipase A2
- PUFA
polyunsaturated fatty acid
- 5-HT
5-hydroxytrypamine (serotonin)
- RyR
ryondine receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+ ATPase
- sn-2 position
position 2 in a 3-carbon glycerol backbone
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 2.Kramer RM, Hession C, Johansen B, Hayes G, McGray P, Chow EP, Tizard R, Pepinsky RB. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–5775. Scopus. [PubMed] [Google Scholar]
- 3.Karnauchow TM, Chan AC. Characterization of human placental blood vessel phospholipase A2, demonstration of substrate selectivity for arachidonyl-phosphatidylcholine. Int J Biochem. 1985;17:1317–1319. doi: 10.1016/0020-711x(85)90054-0. Scopus. [DOI] [PubMed] [Google Scholar]
- 4.Diez E, Chilton FH, Stroup G, Mayer RJ, Winkler JD, Fonteh AN. Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate. Biochem J. 1994;301(Pt 3):721–726. doi: 10.1042/bj3010721. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. Scopus. [DOI] [PubMed] [Google Scholar]
- 6.Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovasc Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. Scopus. [DOI] [PubMed] [Google Scholar]
- 7.Farooqui AA, Horrocks LA. Plasmalogens: workhorse lipids of membranes in normal and injured neurons and glia. Neuroscientist. 2001;7:232–245. doi: 10.1177/107385840100700308. Scopus. [DOI] [PubMed] [Google Scholar]
- 8.Derewenda ZS, Derewenda U. The structure and function of platelet-activating factor acetylhydrolases. Cell Mol Life Sci. 1998;54:446–455. doi: 10.1007/s000180050172. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe A, Hiraoka M, Shayman JA. Positional specificity of lysosomal phospholipase A2. J Lipid Res. 2006;47:2268–2279. doi: 10.1194/jlr.M600183-JLR200. Scopus. [DOI] [PubMed] [Google Scholar]
- 10.Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol. 2005;32:89–103. doi: 10.1385/MN:32:1:089. Scopus. [DOI] [PubMed] [Google Scholar]
- 12.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. Scopus. [DOI] [PubMed] [Google Scholar]
- 14.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. Scopus. [DOI] [PubMed] [Google Scholar]
- 15.Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS, pathologies. BMB Rep. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqui AA, Horrocks LA. Brain phospholipases A2: a perspective on the history. Prostaglandins Leukot Essent Fatty Acids. 2004;71:161–169. doi: 10.1016/j.plefa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J Hirnforsch. 1999;39:391–400. [PubMed] [Google Scholar]
- 18.Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA 2) in monkey brain. J Neurocytol. 2005;34:447–458. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- 19.Abe A, Hiraoka M, Wild S, Wilcoxen SE, Paine R, 3rd, Shayman JA. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J Biol Chem. 2004;279:42605–42611. doi: 10.1074/jbc.M407834200. Scopus. [DOI] [PubMed] [Google Scholar]
- 20.Bao S, Jin C, Zhang S, Turk J, Ma Z, Ramanadham S. Beta-cell calcium-independent group VIA phospholipase A(2) (iPLA(2)beta): tracking iPLA(2)beta movements in response to stimulation with insulin secretagogues in INS-1 cells. Diabetes. 2004;531 Suppl 1:S186–S189. doi: 10.2337/diabetes.53.2007.s186. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism and signaling. J Lipid Res. 2008 doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinozaki K, Waite M. A novel phosphatidylglycerol-selective phospholipase A2 from macrophages. Biochemistry. 1999;38:1669–1675. doi: 10.1021/bi982123q. [DOI] [PubMed] [Google Scholar]
- 23.Kim TS, Sundaresh CS, Feinstein SI, Dodia C, Skach WR, Jain MK, Nagase T, Seki N, Ishikawa K, Nomura N, Fisher AB. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J Biol Chem. 1997;272:2542–2550. doi: 10.1074/jbc.272.4.2542. Scopus. [DOI] [PubMed] [Google Scholar]
- 24.Turk J, Ramanadham S. The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2beta) in beta-cells. Can J Physiol Pharmacol. 2004;82:824–832. doi: 10.1139/y04-064. Scopus. [DOI] [PubMed] [Google Scholar]
- 25.Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. Scopus. [DOI] [PubMed] [Google Scholar]
- 26.Laychock SG. Phospholipase A2 activity in pancreatic islets is calcium-dependent and stimulated by glucose. Cell Calcium. 1982;3:43–54. doi: 10.1016/0143-4160(82)90036-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 27.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 28.Nardicchi V, Macchioni L, Ferrini M, Goracci G. The presence of a secretory phospholipase A2 in the nuclei of neuronal and glial cells of rat brain cortex. Biochim Biophys Acta. 2007;1771:1345–1352. doi: 10.1016/j.bbalip.2007.08.007. Scopus. [DOI] [PubMed] [Google Scholar]
- 29.Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, Kudo I. Functional association of type IIA secretory phospholipase A(2) with the glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan in the cyclooxygenase-2-mediated delayed prostanoid-biosynthetic pathway. J Biol Chem. 1999;274:29927–29936. doi: 10.1074/jbc.274.42.29927. Scopus. [DOI] [PubMed] [Google Scholar]
- 30.Mathisen GH, Thorkildsen IH, Paulsen RE. Secretory PLA2-IIA and ROS generation in peripheral mitochondria are critical for neuronal death. Brain Res. 2007;1153:43–51. doi: 10.1016/j.brainres.2007.03.067. Scopus. [DOI] [PubMed] [Google Scholar]
- 31.Underwood KW, Song C, Kriz RW, Chang XJ, Knopf JL, Lin LL. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J Biol Chem. 1998;273:21926–21932. doi: 10.1074/jbc.273.34.21926. Scopus. [DOI] [PubMed] [Google Scholar]
- 32.Chiba H, Michibata H, Wakimoto K, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y. Cloning of a gene for a novel epithelium-specific cytosolic phospholipase A2, cPLA2delta, induced in psoriatic skin. J Biol Chem. 2004;279:12890–12897. doi: 10.1074/jbc.M305801200. Scopus. [DOI] [PubMed] [Google Scholar]
- 33.Pickard RT, Strifler BA, Kramer RM, Sharp JD. Molecular cloning of two new human paralogs of 85-kDa cytosolic phospholipase A2. J Biol Chem. 1999;274:8823–8831. doi: 10.1074/jbc.274.13.8823. [DOI] [PubMed] [Google Scholar]
- 34.Song C, Chang XJ, Bean KM, Proia MS, Knopf JL, Kriz RW. Molecular characterization of cytosolic phospholipase A2-beta. J Biol Chem. 1999;274:17063–17067. doi: 10.1074/jbc.274.24.17063. Scopus. [DOI] [PubMed] [Google Scholar]
- 35.Sharp JD, White DL, Chiou XG, Goodson T, Gamboa GC, McClure D, Burgett S, Hoskins J, Skatrud PL, Sportsman JR, et al. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J Biol Chem. 1991;266:14850–14853. Scopus. [PubMed] [Google Scholar]
- 36.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. Scopus. [DOI] [PubMed] [Google Scholar]
- 37.Ohto T, Uozumi N, Hirabayashi T, Shimizu T. Identification of novel cytosolic phospholipase A(2)s, murine cPLA(2)δ, ε, and ζ, which form a gene cluster with cPLA(2)β. J Biol Chem. 2005;280:24576–24583. doi: 10.1074/jbc.M413711200. Scopus. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. Scopus. [DOI] [PubMed] [Google Scholar]
- 39.Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 40.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A(2) J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. Scopus. [DOI] [PubMed] [Google Scholar]
- 42.Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. Scopus. [DOI] [PubMed] [Google Scholar]
- 43.Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. Implications for cardiac cycle-dependent alterations in phospholipolysis. J Biol Chem. 1996;271:20989–20992. doi: 10.1074/jbc.271.35.20989. Scopus. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2beta. implications for structure and function. J Biol Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. Scopus. [DOI] [PubMed] [Google Scholar]
- 45.Moro F, Arrigo G, Fogli A, Bernard L, Carrozzo R. The beta and gamma subunits of the human platelet-activating factor acetyl hydrolase isoform Ib (PAFAH1B2 and PAFAH1B3) map to chromosome 11q23 and 19q13.1, respectively. Genomics. 1998;51:157–159. doi: 10.1006/geno.1998.5322. [DOI] [PubMed] [Google Scholar]
- 46.Ho YS, Swenson L, Derewenda U, Serre L, Wei Y, Dauter Z, Hattori M, Adachi T, Aoki J, Arai H, Inoue K, Derewenda ZS. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature. 1997;385:89–93. doi: 10.1038/385089a0. Scopus. [DOI] [PubMed] [Google Scholar]
- 47.Rice SQ, Southan C, Boyd HF, Terrett JA, MacPhee CH, Moores K, Gloger IS, Tew DG. Expression, purification and characterization of a human serine-dependent phospholipase A2 with high specificity for oxidized phospholipids and platelet activating factor. Biochem J. 1998;330(Pt 3):1309–1315. doi: 10.1042/bj3301309. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackermann EJ, Dennis EA. Mammalian calcium-independent phospholipase A2. Biochim Biophys Acta. 1995;1259:125–136. doi: 10.1016/0005-2760(95)00143-z. Scopus. [DOI] [PubMed] [Google Scholar]
- 49.Yang HC, Mosior M, Ni B, Dennis EA. Regional distribution, ontogeny, purification, and characterization of the Ca2+-independent phospholipase A2 from rat brain. J Neurochem. 1999;73:1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- 50.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer's disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 51.Hirashima Y, Farooqui AA, Mills JS, Horrocks LA. Identification and purification of calcium-independent phospholipase A2 from bovine brain cytosol. J Neurochem. 1992;59:708–714. doi: 10.1111/j.1471-4159.1992.tb09426.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 52.Yang HC, Farooqui AA, Horrocks LA. Effects of sialic acid and sialoglycoconjugates on cytosolic phospholipases A2 from bovine brain. Biochem Biophys Res Commun. 1994;199:1158–1166. doi: 10.1006/bbrc.1994.1352. Scopus. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Yu S, Sun AY, Sun GY. Oxidant-mediated AA release from astrocytes involves cPLA(2) and iPLA(2) Free Radic Biol Med. 2003;34:1531–1543. doi: 10.1016/s0891-5849(03)00152-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 54.Neuringer M, Anderson GJ, Connor WE. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu Rev Nutr. 1988;8:517–541. doi: 10.1146/annurev.nu.08.070188.002505. Scopus. [DOI] [PubMed] [Google Scholar]
- 55.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 56.Shikano M, Masuzawa Y, Yazawa K, Takayama K, Kudo I, Inoue K. Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim Biophys Acta. 1994;1212:211–216. doi: 10.1016/0005-2760(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 57.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 59.Scuri R, Mozzachiodi R, Brunelli M. Role for calcium signaling and arachidonic acid metabolites in the activity-dependent increase of AHP amplitude in leech T sensory neurons. J Neurophysiol. 2005;94:1066–1073. doi: 10.1152/jn.00075.2005. Scopus. [DOI] [PubMed] [Google Scholar]
- 60.Ojeda SR, Urbanski HF, Junier MP, Capdevila J. The role of arachidonic acid and its metabolites in the release of neuropeptides. Ann N Y Acad Sci. 1989;559:192–207. doi: 10.1111/j.1749-6632.1989.tb22609.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 61.Striggow F, Ehrlich BE. Regulation of intracellular calcium release channel function by arachidonic acid and leukotriene B4. Biochem Biophys Res Commun. 1997;237:413–418. doi: 10.1006/bbrc.1997.7152. Scopus. [DOI] [PubMed] [Google Scholar]
- 62.Cowell AM, Buckingham JC. Eicosanoids and the hypothalamo-pituitary axis. Prostaglandins Leukot Essent Fatty Acids. 1989;36:235–250. doi: 10.1016/0952-3278(89)90136-1. Scopus. [DOI] [PubMed] [Google Scholar]
- 63.Smith HS. Arachidonic acid pathways in nociception. J Support Oncol. 2006;4:277–287. Scopus. [PubMed] [Google Scholar]
- 64.Pickard JD. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab. 1981;1:361–384. doi: 10.1038/jcbfm.1981.41. Scopus. [DOI] [PubMed] [Google Scholar]
- 65.Vanecek J, Vollrath L. Melatonin modulates diacylglycerol and arachidonic acid metabolism in the anterior pituitary of immature rats. Neurosci Lett. 1990;110:199–203. doi: 10.1016/0304-3940(90)90811-m. Scopus. [DOI] [PubMed] [Google Scholar]
- 66.Baile CA, Simpson CW, Bean SM, McLaughlin CL, Jacobs HL. Prostaglandins and food intake of rats: a component of energy balance regulation? Physiol Behav. 1973;10:1077–1085. doi: 10.1016/0031-9384(73)90191-1. Scopus. [DOI] [PubMed] [Google Scholar]
- 67.Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104:1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 68.McCown TJ, Knapp DJ, Crews FT. Inferior collicular seizure generalization produces site-selective cortical induction of cyclooxygenase 2 (COX-2) Brain Res. 1997;767:370–374. doi: 10.1016/s0006-8993(97)00773-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol. 2001;49:176–185. [PubMed] [Google Scholar]
- 71.Bazan NG, Colangelo V, Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat. 2002;68–69:197–210. doi: 10.1016/s0090-6980(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 72.Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2003;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- 73.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Moller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. Scopus. [DOI] [PubMed] [Google Scholar]
- 74.Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. Scopus. [DOI] [PubMed] [Google Scholar]
- 75.Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. Scopus. [DOI] [PubMed] [Google Scholar]
- 76.Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, Russ MJ, Rapoport SI. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31 Suppl:S157–S161. doi: 10.1007/BF02637069. Scopus. [DOI] [PubMed] [Google Scholar]
- 78.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. Scopus. [DOI] [PubMed] [Google Scholar]
- 79.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 82.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 83.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. Scopus. [DOI] [PubMed] [Google Scholar]
- 84.Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot Essent Fatty Acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Y, Wang L, Xu RJ, Chen ZY. DHA depletion in rat brain is associated with impairment on spatial learning and memory. Biomed Environ Sci. 2006;19:474–480. Scopus. [PubMed] [Google Scholar]
- 86.Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr. 2007;85:1572–1577. doi: 10.1093/ajcn/85.6.1572. Scopus. [DOI] [PubMed] [Google Scholar]
- 87.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12:207–227. Scopus. [PubMed] [Google Scholar]
- 88.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. Scopus. [DOI] [PubMed] [Google Scholar]
- 89.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. Scopus. [DOI] [PubMed] [Google Scholar]
- 90.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 91.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. Scopus. [DOI] [PubMed] [Google Scholar]
- 92.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. Scopus. [DOI] [PubMed] [Google Scholar]
- 94.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. Scopus. [DOI] [PubMed] [Google Scholar]
- 95.Okuda S, Saito H, Katsuki H. Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience. 1994;63:691–699. doi: 10.1016/0306-4522(94)90515-0. Scopus. [DOI] [PubMed] [Google Scholar]
- 96.Katsuki H, Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. Scopus. [DOI] [PubMed] [Google Scholar]
- 97.Nishizaki T, Nomura T, Matsuoka T, Enikolopov G, Sumikawa K. Arachidonic acid induces a long-lasting facilitation of hippocampal synaptic transmission by modulating PKC activity and nicotinic ACh receptors. Brain Res Mol Brain Res. 1999;69:263–272. doi: 10.1016/s0169-328x(99)00117-5. Scopus. [DOI] [PubMed] [Google Scholar]
- 98.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. Scopus. [DOI] [PubMed] [Google Scholar]
- 100.Sergeeva M, Strokin M, Reiser G. Regulation of intracellular calcium levels by polyunsaturated fatty acids, arachidonic acid and docosahexaenoic acid, in astrocytes: possible involvement of phospholipase A2. Reprod Nutr Dev. 2005;45:633–646. doi: 10.1051/rnd:2005050. Scopus. [DOI] [PubMed] [Google Scholar]
- 101.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. Scopus. [DOI] [PubMed] [Google Scholar]
- 102.Jones CR, Arai T, Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem Res. 1997;22:663–670. doi: 10.1023/a:1027341707837. Scopus. [DOI] [PubMed] [Google Scholar]
- 103.DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–355. doi: 10.1111/j.1471-4159.1991.tb02603.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 104.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem. 2007;102:1771–1782. doi: 10.1111/j.1471-4159.2007.04663.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 106.Aires V, Hichami A, Filomenko R, Ple A, Rebe C, Bettaieb A, Khan NA. Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase C gamma and -delta via phosphatidylserine binding site: implication in apoptosis in U937 cells. Mol Pharmacol. 2007;72:1545–1556. doi: 10.1124/mol.107.039792. [DOI] [PubMed] [Google Scholar]
- 107.Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. Scopus. [DOI] [PubMed] [Google Scholar]
- 108.Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34:365–383. doi: 10.1016/s0143-4160(03)00139-8. Scopus. [DOI] [PubMed] [Google Scholar]
- 109.Paschen W. Endoplasmic reticulum dysfunction in brain pathology: critical role of protein synthesis. Curr Neurovasc Res. 2004;1:173–181. doi: 10.2174/1567202043480125. Scopus. [DOI] [PubMed] [Google Scholar]
- 110.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. Scopus. [DOI] [PubMed] [Google Scholar]
- 111.Wu J, Holstein JD, Upadhyay G, Lin DT, Conway S, Muller E, Lechleiter JD. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci. 2007;27:6510–6520. doi: 10.1523/JNEUROSCI.1256-07.2007. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Egea J, Rosa AO, Cuadrado A, Garcia AG, Lopez MG. Nicotinic receptor activation by epibatidine induces heme oxygenase-1 and protects chromaffin cells against oxidative stress. J Neurochem. 2007;102:1842–1852. doi: 10.1111/j.1471-4159.2007.04665.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 113.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–615. doi: 10.1097/00000542-200503000-00020. Scopus. [DOI] [PubMed] [Google Scholar]
- 114.Bickler PE, Buck LT. Effects of fructose-1,6-bisphosphate on glutamate release and ATP loss from rat brain slices during hypoxia. J Neurochem. 1996;67:1463–1468. doi: 10.1046/j.1471-4159.1996.67041463.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 115.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–429. doi: 10.1213/01.ane.0000223671.49376.b2. table of contents. Scopus. [DOI] [PubMed] [Google Scholar]
- 116.Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol. 2007;293:C1103–C1111. doi: 10.1152/ajpcell.00249.2007. Scopus. [DOI] [PubMed] [Google Scholar]
- 117.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. Scopus. [DOI] [PubMed] [Google Scholar]
- 119.Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer's disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. Scopus. [DOI] [PubMed] [Google Scholar]
- 120.Kadoyama K, Takahashi Y, Higashida H, Tanabe T, Yoshimoto T. Cyclooxygenase-2 stimulates production of amyloid beta-peptide in neuroblastoma × glioma hybrid NG108–15 cells. Biochem Biophys Res Commun. 2001;281:483–490. doi: 10.1006/bbrc.2001.4357. [DOI] [PubMed] [Google Scholar]
- 121.Peterson B, Knotts T, Cummings BS. Involvement of Ca2+-independent phospholipase A2 isoforms in oxidant-induced neural cell death. Neurotoxicology. 2007;28:150–160. doi: 10.1016/j.neuro.2006.09.006. Scopus. [DOI] [PubMed] [Google Scholar]
- 122.Kinsey GR, McHowat J, Patrick KS, Schnellmann RG. Role of Ca2+-independent phospholipase A2gamma in Ca2+-induced mitochondrial permeability transition. J Pharmacol Exp Ther. 2007;321:707–715. doi: 10.1124/jpet.107.119545. [DOI] [PubMed] [Google Scholar]
- 123.Atsumi G, Murakami M, Kojima K, Hadano A, Tajima M, Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J Biol Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. Scopus. [DOI] [PubMed] [Google Scholar]
- 124.Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochim Biophys Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 125.Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem Res. 1999;24:399–406. doi: 10.1023/a:1020989701330. Scopus. [DOI] [PubMed] [Google Scholar]
- 126.Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. Scopus. [DOI] [PubMed] [Google Scholar]
- 127.Taylor AL, Bonventre JV, Uliasz TF, Hewett JA, Hewett SJ. Cytosolic phospholipase A2 alpha inhibition prevents neuronal NMDA receptor-stimulated arachidonic acid mobilization and prostaglandin production but not subsequent cell death. J Neurochem. 2008;106:1828–1840. doi: 10.1111/j.1471-4159.2008.05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pellerin L, Wolfe LS. Release of arachidonic acid by NMDA-receptor activation in the rat hippocampus. Neurochem Res. 1991;16:983–989. doi: 10.1007/BF00965841. Scopus. [DOI] [PubMed] [Google Scholar]
- 129.Taylor M, Shajahan P, Lawrie SM. Comparing the use and discontinuation of antipsychotics in clinical practice: an observational study. J Clin Psychiatry. 2008;69:240–245. doi: 10.4088/jcp.v69n0210. Scopus. [DOI] [PubMed] [Google Scholar]
- 130.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. Scopus. [DOI] [PubMed] [Google Scholar]
- 131.Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic administration of valproic acid reduces brain NMDA signaling via arachidonic acid in unanesthetized rats. Neurochem Res. 2008;33:2229–2240. doi: 10.1007/s11064-008-9700-2. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Basselin M, Villacreses NE, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007;62:934–943. doi: 10.1016/j.biopsych.2007.04.021. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanfeliu C, Hunt A, Patel AJ. Exposure to N-methyl-D-aspartate increases release of arachidonic acid in primary cultures of rat hippocampal neurons and not in astrocytes. Brain Res. 1990;526:241–248. doi: 10.1016/0006-8993(90)91228-9. Scopus. [DOI] [PubMed] [Google Scholar]
- 134.Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolf RA, Gross RW. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985;260:7295–7303. Scopus. [PubMed] [Google Scholar]
- 136.Basheer R, Arrigoni E, Thatte HS, Greene RW, Ambudkar IS, McCarley RW. Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons. J Neurosci. 2002;22:7680–7686. doi: 10.1523/JNEUROSCI.22-17-07680.2002. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Doengi M, Deitmer JW, Lohr C. New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. Faseb J. 2008 doi: 10.1096/fj.07-101782. [DOI] [PubMed] [Google Scholar]
- 138.Yamamoto K, Hashimoto K, Isomura Y, Shimohama S, Kato N. An IP3-assisted form of Ca2+-induced Ca2+ release in neocortical neurons. Neuroreport. 2000;11:535–539. doi: 10.1097/00001756-200002280-00022. [DOI] [PubMed] [Google Scholar]
- 139.Arce C, Del Campo AB, Figueroa S, Lopez E, Aranguez I, Oset-Gasque MJ, Gonzalez MP. Expression and functional properties of group I metabotropic glutamate receptors in bovine chromaffin cells. J Neurosci Res. 2004;75:182–193. doi: 10.1002/jnr.10824. Scopus. [DOI] [PubMed] [Google Scholar]
- 140.Hayashi T, Kagaya A, Takebayashi M, Oyamada T, Inagaki M, Tawara Y, Yokota N, Horiguchi J, Su TP, Yamawaki S. Effect of dantrolene on KCl- or NMDA-induced intracellular Ca2+ changes and spontaneous Ca2+ oscillation in cultured rat frontal cortical neurons. J Neural Transm. 1997;104:811–824. doi: 10.1007/BF01285550. Scopus. [DOI] [PubMed] [Google Scholar]
- 141.Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. Scopus. [DOI] [PubMed] [Google Scholar]
- 142.Morton-Jones RT, Cannell MB, Housley GD. Ca2+ entry via AMPA-type glutamate receptors triggers Ca2+-induced Ca2+ release from ryanodine receptors in rat spiral ganglion neurons. Cell Calcium. 2008;43:356–366. doi: 10.1016/j.ceca.2007.07.003. Scopus. [DOI] [PubMed] [Google Scholar]
- 143.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Inesi G, Hua S, Xu C, Ma H, Seth M, Prasad AM, Sumbilla C. Studies of Ca2+ ATPase (SERCA) inhibition. J Bioenerg Biomembr. 2005;37:365–368. doi: 10.1007/s10863-005-9472-1. [DOI] [PubMed] [Google Scholar]
- 145.Nowatzke W, Ramanadham S, Ma Z, Hsu FF, Bohrer A, Turk J. Mass spectrometric evidence that agents that cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology. 1998;139:4073–4085. doi: 10.1210/endo.139.10.6225. Scopus. [DOI] [PubMed] [Google Scholar]
- 146.Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koizumi S, Saito Y, Nakazawa K, Nakajima K, Sawada JI, Kohsaka S, Illes P, Inoue K. Spatial and temporal aspects of Ca2+ signaling mediated by P2Y receptors in cultured rat hippocampal astrocytes. Life Sci. 2002;72:431–442. doi: 10.1016/s0024-3205(02)02273-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 148.Poulsen KA, Pedersen SF, Kolko M, Lambert IH. Induction of group VIA phospholipase A2 activity during in vitro ischemia in C2C12 myotubes is associated with changes in the level of its splice variants. Am J Physiol Cell Physiol. 2007;293:C1605–C1615. doi: 10.1152/ajpcell.00012.2007. Scopus. [DOI] [PubMed] [Google Scholar]
- 149.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Larsen GA, Skjellegrind HK, Moe MC, Vinje ML, Berg-Johnsen J. Endoplasmic reticulum dysfunction and Ca2+ deregulation in isolated CA1 neurons during oxygen and glucose deprivation. Neurochem Res. 2005;30:651–659. doi: 10.1007/s11064-005-2753-6. Scopus. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab. 2002;22:206–214. doi: 10.1097/00004647-200202000-00008. Scopus. [DOI] [PubMed] [Google Scholar]
- 152.Kachoei BA, Knox RJ, Uthuza D, Levy S, Kaczmarek LK, Magoski NS. A store-operated Ca(2+) influx pathway in the bag cell neurons of Aplysia. J Neurophysiol. 2006;96:2688–2698. doi: 10.1152/jn.00118.2006. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singaravelu K, Lohr C, Deitmer JW. Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes. J Neurosci. 2006;26:9579–9592. doi: 10.1523/JNEUROSCI.2604-06.2006. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25:307–311. doi: 10.1016/s0968-0004(00)01588-7. Scopus. [DOI] [PubMed] [Google Scholar]
- 155.Bhattacharjee AK, Chang L, White L, Bazinet RP, Rapoport SI. Imaging apomorphine stimulation of brain arachidonic acid signaling via D2-like receptors in unanesthetized rats. Psychopharmacology (Berl) 2008;197:557–566. doi: 10.1007/s00213-008-1073-3. Scopus. [DOI] [PubMed] [Google Scholar]
- 156.Bhattacharjee AK, Chang L, White L, Bazinet RP, Rapoport SI. D-Amphetamine stimulates D2 dopamine receptor-mediated brain signaling involving arachidonic acid in unanesthetized rats. J Cereb Blood Flow Metab. 2006;26:1378–1388. doi: 10.1038/sj.jcbfm.9600290. Scopus. [DOI] [PubMed] [Google Scholar]
- 157.Bhattacharjee AK, Chang L, Lee HJ, Bazinet RP, Seemann R, Rapoport SI. D2 but not D1 dopamine receptor stimulation augments brain signaling involving arachidonic acid in unanesthetized rats. Psychopharmacology (Berl) 2005;180:735–742. doi: 10.1007/s00213-005-2208-4. Scopus. [DOI] [PubMed] [Google Scholar]
- 158.Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. Scopus. [DOI] [PubMed] [Google Scholar]
- 159.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 160.Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Ca(v)1.2 L type Ca(2+) currents via a PLCbeta/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. Scopus. [DOI] [PubMed] [Google Scholar]
- 161.Fisher SK, Domask LM, Roland RM. Muscarinic receptor regulation of cytoplasmic Ca2+ concentrations in human SK-N-SH neuroblastoma cells: Ca2+ requirements for phospholipase C, activation. Mol Pharmacol. 1989;35:195–204. Scopus. [PubMed] [Google Scholar]
- 162.Ferrier GR, Redondo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc Res. 2002;54:601–610. doi: 10.1016/s0008-6363(02)00275-4. Scopus. [DOI] [PubMed] [Google Scholar]
- 163.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Green KN, Smith IF, Laferla FM. Role of calcium in the pathogenesis of Alzheimer's disease and transgenic models. Subcell Biochem. 2007;45:507–521. doi: 10.1007/978-1-4020-6191-2_19. [DOI] [PubMed] [Google Scholar]
- 165.Mattson MP, Chan SL. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003;34:385–397. doi: 10.1016/s0143-4160(03)00128-3. Scopus. [DOI] [PubMed] [Google Scholar]
- 166.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, Rapoport SI. Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49:1414–1421. doi: 10.2967/jnumed.107.049619. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 168.Dinarello CA. Biology of interleukin 1. Faseb J. 1988;2:108–115. [PubMed] [Google Scholar]
- 169.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 170.Sun LL, Cheng C, Liu HO, Shen CC, Xiao F, Qin J, Yang JL, Shen AG. Src suppressed C kinase substrate regulates the lipopolysaccharide-induced TNF-alpha biosynthesis in rat astrocytes. J Mol Neurosci. 2007;32:16–24. doi: 10.1007/s12031-007-0003-x. Scopus. [DOI] [PubMed] [Google Scholar]
- 171.Scheuer K, Maras A, Gattaz WF, Cairns N, Forstl H, Muller WE. Cortical NMDA receptor properties and membrane fluidity are altered in Alzheimer's disease. Dementia. 1996;7:210–214. doi: 10.1159/000106881. Scopus. [DOI] [PubMed] [Google Scholar]
- 172.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 173.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 174.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pringle AK. In, out, shake it all about: elevation of [Ca2+]i during acute cerebral ischaemia. Cell Calcium. 2004;36:235–245. doi: 10.1016/j.ceca.2004.02.014. Scopus. [DOI] [PubMed] [Google Scholar]
- 177.Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. Scopus. [DOI] [PubMed] [Google Scholar]
- 178.Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008;49:162–168. doi: 10.1194/jlr.M700406-JLR200. Scopus. [DOI] [PubMed] [Google Scholar]
- 180.Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007;102:1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. Scopus. [DOI] [PubMed] [Google Scholar]
- 181.Lazarewicz JW, Salinska E, Wroblewski JT. NMDA receptor-mediated arachidonic acid release in neurons: role in signal transduction and pathological aspects. Adv Exp Med Biol. 1992;318:73–89. doi: 10.1007/978-1-4615-3426-6_7. Scopus. [DOI] [PubMed] [Google Scholar]
- 182.Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002;35:67–86. doi: 10.5483/bmbrep.2002.35.1.067. Scopus. [DOI] [PubMed] [Google Scholar]
- 183.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 184.Shimazawa M, Nakajima Y, Mashima Y, Hara H. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res. 2009;1251:269–275. doi: 10.1016/j.brainres.2008.11.031. Scopus. [DOI] [PubMed] [Google Scholar]
- 185.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations and Alzheimer's disease. J Lipid Res. 2008 doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. Scopus. [DOI] [PubMed] [Google Scholar]
- 187.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. Scopus. [DOI] [PubMed] [Google Scholar]
- 188.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. Scopus. [DOI] [PubMed] [Google Scholar]
- 189.Petrie RX, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder, A critical review. Pharmacol Ther. 2000;87:11–25. doi: 10.1016/s0163-7258(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 190.Rosa AO, Lin J, Calixto JB, Santos AR, Rodrigues AL. Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav Brain Res. 2003;144:87–93. doi: 10.1016/s0166-4328(03)00069-x. Scopus. [DOI] [PubMed] [Google Scholar]
- 191.Cardoso CC, Lobato KR, Binfare RW, Ferreira PK, Rosa AO, Santos AR, Rodrigues AL. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:235–242. doi: 10.1016/j.pnpbp.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 192.Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport. 2002;13:387–391. doi: 10.1097/00001756-200203250-00005. Scopus. [DOI] [PubMed] [Google Scholar]
- 193.Almeida RC, Felisbino CS, Lopez MG, Rodrigues AL, Gabilan NH. Evidence for the involvement of L-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of memantine in mice. Behav Brain Res. 2006;168:318–322. doi: 10.1016/j.bbr.2005.11.023. Scopus. [DOI] [PubMed] [Google Scholar]
- 194.Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327. doi: 10.1038/sj.mp.4000586. Scopus. [DOI] [PubMed] [Google Scholar]
- 195.Parker GB, Heruc GA, Hilton TM, Olley A, Brotchie H, Hadzi-Pavlovic D, Friend C, Walsh WF, Stocker R. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Res. 2006;141:279–286. doi: 10.1016/j.psychres.2005.08.005. Scopus. [DOI] [PubMed] [Google Scholar]
- 196.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA, Ness AR. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–431. doi: 10.1017/S0007114507801097. Scopus. [DOI] [PubMed] [Google Scholar]
- 197.Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, Papakostas GI, Dording CM, Sonawalla SB, Nierenberg AA, Alpert JE, Fava M. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:639–645. doi: 10.1016/j.euroneuro.2008.04.011. Scopus. [DOI] [PubMed] [Google Scholar]
- 198.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JE, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. Scopus. [DOI] [PubMed] [Google Scholar]
- 199.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. Scopus. [DOI] [PMC free article] [PubMed] [Google Scholar]